Abstract
Purpose
Skin-sparing mastectomy (SSM) enables a radical cure of breast cancer while overcoming the cosmetic issues related to surgery. We review our experience of performing SSMs and assess whether preservation of the nipple–areola complex (NAC) could have been an option for some patients who underwent SSM.
Methods
The subjects of this retrospective study were women who underwent SSM that utilized four incision types; namely, the so-called tennis racket incision, a periareolar and midaxillary incision, an areola-sparing and midaxillary incision, and a small transverse elliptical incision. We assessed whether preservation of the NAC would have been an option in SSM, based on histologic examination of three serial cut surfaces of the specimen around the nipple, ruling out the option when evidence of the malignant lesion/s was found in at least one of the following locations: in the nipple, within a 1-cm radius from the base of the nipple, or within 1 cm from the surface of the NAC.
Results
We performed 193 SSMs. The cumulative 10-year local disease-free survival rate was 98%, with 89% of patients reporting levels of satisfaction with the reconstructed breast, of excellent, very good, or good. We evaluated that 70 of the 193 procedures could have been performed as nipple-sparing mastectomy (NSM).
Conclusions
The outcomes of SSM in this series were excellent and NSM might have been an option for about one-third of the patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In systemic therapy for breast cancer, local control is the major objective of surgery [1], and breast-conserving surgery is the mainstream treatment. However, based on the size or site of the lesion and the extent of intraductal involvement, about one-third of women with breast cancer still choose mastectomy [2]. Skin-sparing mastectomy (SSM) with immediate breast reconstruction (IBR), as first described by Toth and Lappert in 1991, is the method of choice for radical cure of breast cancer with optimal cosmetic results [3]. We have been achieving excellent outcomes with this procedure at Jikei University Kashiwa Hospital since July, 2003 [4], and after some trial and error, we have adopted four types of skin incision chosen according to the needs of the patient [5, 6].
Cense’s team first used “nipple-sparing mastectomy” (NSM) as a technical term in 2001 to describe SSM that preserves the skin tissue of the nipple–areola complex (NAC). They reviewed the literature and concluded that there was limited indication for the procedure because of an unacceptably high risk of local relapse [7]. We present an overview of our experience of performing SSM and assess retrospectively whether NSM might have been an option for these patients.
Patients and methods
The subjects of our retrospective study were Japanese women who underwent SSM with IBR at Jikei University Kashiwa Hospital between July 1, 2003 and March 31, 2017. Breast surgeons performed the mastectomy procedures, and plastic surgeons performed the breast reconstructions. All patients were advised of their choices for adjuvant therapy based on the findings of postoperative pathological examination and provided informed consent for implementation of the selected treatment.
SSM was planned so as to remove the nipple, with or without the areola complex, biopsy scars (excluding the core needle biopsy scar), and the entire breast parenchyma [3]. Four types of incisions were used for SSM: Type A, a periareolar incision with lateral extension (the so-called tennis-racket incision); Type B, a periareolar incision with a midaxillary line incision; Type C, a straight incision with a small elliptical incision at the baseline of the nipple, within the areola complex in a mediolateral (C1) or craniocaudal (C2) direction (the so-called areola-sparing incision, with caudal extension added if needed for Type C2) and a midaxillary line incision; and Type D, a small transverse elliptical incision encompassing the entire NAC with a transverse axillary incision (Fig. 1).
We assessed the cumulative local and distant disease-free survival rates and the cumulative overall survival rate using the Kaplan–Meier method; the level of satisfaction of the patients and their breast surgeons with the reconstructed breast according to their responses on a 5-point questionnaire (excellent/very good/good/not very good/poor); and the suitability of preservation of the NAC as an option in SSM based on histopathologic examination of three serial cut surfaces of the specimen, including the section through the nipple and the two cut surfaces adjacent to the nipple (Fig. 2a). We considered NSM as an option for all patients, except those in whom malignant lesion/s were identified in at least one of the following locations: the nipple, within a 1-cm radius from the base of the nipple in the section through the nipple, or within 1 cm from the NAC surface or surgical margin of the skin side (Fig. 2b). We analyzed statistics using the Chi-square test and t test, with P < 0.05 considered significant.
a Three-slice cutting line of serial sections of a specimen from skin-sparing mastectomy, including a section through the nipple and two cut surfaces adjacent to the nipple. b Three cut surfaces were used to assess involvement of the nipple–areola complex (NAC), to identify the presence of malignant lesion/s (1) in the nipple, (2) within a 1-cm radius from the base of the nipple in the section through the nipple, or (3) within 1 cm from the NAC surface
Results
We performed SSM in 193 of 666 mastectomies. Table 1 delineates the characteristics of the patients, their tumors, the operative procedures, and the type of axillary management, according to the incision types. Tumors were staged based on the system of the American Joint Committee on Cancer. Types C and D incisions were used for device-based reconstructions (tissue expander [TE] or silicone breast implant [SBI]).
We applied Type A for all patients during the first 4 years, and less frequently thereafter until 2010. Type B and Type C were introduced in 2007 and 2008, respectively, to achieve better cosmetic outcomes. Type D was introduced in 2011 and then Type C2 was introduced in 2015 in response to the increase in device-based breast reconstruction. In accordance with that increase, we have adopted the Type C2 incision even for patients with a small areola, often extending the incision to the caudal side to better expose the operative field. The number of device-based breast reconstructions has increased remarkably since 2011.
The mean follow-up period was 64.5 (5–171) months. The cumulative 5-year local disease-free survival rate was 99.3%, the cumulative 10-year local disease-free survival rate was 98.0%, and the cumulative overall survival rates were 97.0% at 5 years and 89.2% at 10 years (Fig. 3).
Postoperative complications included hemorrhage that required reoperation (n = 1); deep vein thrombosis (n = 1); insufficient circulation (n = 8), resulting in skin necrosis (n = 6) and flap loss (n = 2); and infection at the surgical site requiring removal of the tissue expander (n = 3).
Surveys about satisfaction with the reconstructed breast were returned by 168 patients (87.0%) and 190 breast surgeons (98.4%). Most patients (88.7%) and breast surgeons (95.3%) expressed that their level of satisfaction with the cosmetic results of the reconstructed breast was excellent, very good, or good (Fig. 4). Most patients who expressed levels of satisfaction that were not very good, or were awful, were unhappy with the size and/or shape of the reconstructed breast. Only one patient expressed dissatisfaction about malposition of the NAC.
We assessed the option for NAC preservation in 164 of the 193 patients, after the exclusion of 24 patients with bloody nipple discharge that was positive for malignant cells and 5 with Paget’s disease. Table 2 summarizes the patient and tumor characteristics. We concluded that NSM would have been an option (convertible group) for 70 of the 164 patients treated with SSM and classified the other 94 for whom we felt NAC preservation was not an option (unconvertible group) according to the location of the malignant lesion/s as follows: in the nipple (n = 15), within a 1-cm radius from the base of the nipple in the section through the nipple (n = 58), and within 1 cm from the NAC skin surface (n = 21).
The mean age of the patients was 48.3 years in the convertible group and 47.8 years in the unconvertible group. The two groups did not differ significantly with regard to staging classification, histologic type, tumor size, lymphovascular invasion (LVI), number of patients with positive nodes, and number of patients with a positive extended intraductal component (EIC; referring to invasive breast cancer in which at least 25% of the tumor is composed of an intraductal component that extends beyond the invasive tumor [8]).
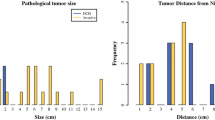
Magnetic resonance imaging (MRI) was done for 157 of the 164 patients. Based on the MR images, 72 (83.7%) of the 94 unconvertible group procedures were judged as unconvertible, and 43 (64.2%) of the 70 convertible group procedures were judged as convertible (sensitivity 64.2%, specificity 83.7%, accuracy 75.2%). We observed significant differences between the convertible vs. unconvertible groups in the distance between the nipple and the tumor, being 21.0 vs. 12.1 mm, respectively (P = 0.0001); between the areola and tumor, being 2.5 vs. − 6.1 mm, respectively (P = 0.0004); in the histological extent of intraductal spread, being 46.7 vs. 32.6 mm, respectively (P = 0.0001); and in the absence of intraductal spread on MRI, seen in 25 vs. 14 patients, respectively (P = 0.003).
Discussion
Modern radical surgery for breast cancer was established when William Stewart Halsted performed a standard radical mastectomy in 1882 [9]. At the end of the nineteenth century, most of these operations were performed for locally advanced disease, and widespread belief in Sappey’s theory (1885) of centripetal lymphatic drainage of the breast toward the subareolar lymphatic plexus [7, 10] precluded consideration of NAC preservation. However, demonstration by Turner-Warwick (1959) and Handley (1964) of the downward lymphatic drainage of the breast, not toward the subareolar lymphatic plexus, but toward the deep pectoral lymphatic plexus, implied that preservation of the NAC might be safe [7, 10]. In 1962, Freeman reported sparing the NAC in subcutaneous mastectomy, but advocated the procedure’s use only for benign lesions and not in therapeutic mastectomy or risk-reducing prophylactic mastectomy [11, 12]. It was not until the 1980s that breast-conserving surgery became common practice for breast cancer [13] and NAC preservation was accepted, although George Crile [14], a pioneer in breast cancer surgery, had performed a partial mastectomy as early as 1957.
A study reported by De La Cruz et al. in 2015, utilizing the Surveillance, Epidemiology, and End Results of the National Cancer Institute, demonstrated almost doubling of the number of NSM procedures performed between 2005 and 2009 in the United States [15]. However, many of these NSMs were performed for risk-reducing prophylactic mastectomy [16], and the numbers differed greatly by country, ranging from 0% in Norway to 49% in the United States [17]. As noted, risk-reducing prophylactic NSM is not covered by Japan’s national health insurance. In their meta-analysis of 20 studies involving 5594 patients, the De La Cruz team detected no adverse oncologic outcomes of NSM in carefully selected women with early stage breast cancer [15].
Although preservation of the nipple–areola complex in the treatment of breast cancer may significantly impact a woman’s body image, its value remains controversial because of concerns about possible residual cancer in the nipple [18]. Nipple involvement is defined as ductal carcinoma in situ (DCIS), invasive carcinoma, or Paget’s cells within 1 cm of the NAC [7]. Anatomically, 15–20 mammary ducts branch from the terminal ductal lobular units to form the lactiferous sinus under the NAC, then branch again and rise toward the nipple surrounded by a network of vessels. When mastectomy is performed, a skin flap is raised in the plane of Cooper’s ligament. However, there are neither Cooper’s ligaments nor subcutaneous fat between the skin and underlying glandular tissue of the breast at the NAC. The creation of a retro-nipple–areolar flap that is too thin can compromise the cosmetic results, and if the flap is too thick, oncologic safety is at risk because of the possibility of residual tumor cells. Torresan’s group reported an association between a high prevalence of glandular breast tissue and residual disease in the skin flap with flap thickness greater than 5 mm [19]. It can be technically difficult for the breast surgeon to create a retro-nipple–areolar skin flap that is thinner than 5 mm. This is why we consider NAC preservation for within a 1 cm radius from the base of the nipple and the NAC skin surface.
The incidence of nipple involvement has been reported to range from 0 to 58% among patients with breast cancer [7, 10] and from 0 to 14% among patients selected to undergo therapeutic NSM according to strict criteria [20]. Moreover, most studies reported tumor recurrence in the NAC in fewer than 1% of cases [20]. Patient selection is most important for the safe outcome of NSM, and various methods have been proposed to assess nipple involvement before, during, and after surgery. Studies reporting the usefulness of MRI in predicting nipple involvement prior to surgery [21,22,23,24,25] have suggested a correlation between nipple involvement and distance between the nipple and tumor of no greater than 2 cm or distance between the nipple–areola complex and tumor of no greater than 0.5 cm. Fortunato et al. reported the usefulness of intraoperative histological examination and concluded that NSM can be performed safely if the margin of the retro-areolar resection is clear and the maximal surgical margin clearance is performed [26]. Petit’s group delivered a single intraoperative dose of radiation (ELIOT) to the NAC and observed no recurrence in the NAC after 19 months of follow-up [27]. However, intraoperative radiation is not widely available, and therefore, not an option for most centers.
According to Murthy et al., the best available evidence suggests that NSM can be considered for patients whose tumor is smaller than 2.5 cm and located more than 4 cm from the nipple with negative axillary status, LVI, or EIC [10]. However, only one of our 164 patients treated with SSM satisfied all these criteria.
We judged that only 70 of our 193 patients (36.3%) would have been suitable candidates for NSM. Among the total 193 patients who underwent SSM, including the 24 with positive bloody nipple discharge and 5 with Paget’s disease, none were considered candidates for sparing NAC. However, SSM was safely completed in all these patients, with 88.7% of them and 95.3% of their breast surgeons reporting levels of satisfaction with the reconstructed breast that were excellent, very good, or good. Furthermore, local control failed in only two patients (1.0%) during a median follow-up of 65 months. Based on the results of our small retrospective study, excluding cases of risk-reducing prophylactic NSM for gene mutation, we identified few candidates for therapeutic NSM convertible from SSM for early breast cancer.
Conclusion
We achieved excellent oncological safety and cosmetic outcomes in 193 patients treated with SSM and judged that nipple preservation might have been an option for 70, representing about one-third of our study patients. We believe that NSM can be an option in SSM, but should be considered only for carefully selected women with early stage breast cancer to avoid the risk of residual cancer in and around the nipple–areola complex.
References
Fisher B. From Halsted to prevention and beyond: advances in the management of breast cancer during the twentieth century. Eur J Cancer. 1999;35(14):1963–73.
Patani N, Devalia H, Anderson A, Mokbel K. Oncological safety and patient satisfaction with skin-sparing mastectomy and immediate breast reconstruction. Surg Oncol. 2008;17(2):97–105.
Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87(6):1048–53.
Kinoshita S, Nojima K, Takeishi M, Imawari Y, Kyoda S, Hirano A, et al. Retrospective comparison of non-skin-sparing mastectomy and skin-sparing mastectomy with immediate breast reconstruction. Int J Surg Oncol. 2011;2011:876520. https://doi.org/10.1155/2011/876520.
Kinoshita S, Kyoda S, Hirano A, Akiba T, Nojima K, Uchida K, et al. Clinical comparison of four types of skin incisions for skin-sparing mastectomy and immediate breast reconstruction. Surg Today. 2014;44(8):1470–5.
Kinoshita S, Nojima K, Miyake R, Shimada N, Hirano A, Uchida K, et al. Clinical assessment of skin incisions used for skin-sparing mastectomy. Intern Med Rev. 2016. https://doi.org/10.18103/imr.v2i8.197
Cense HA, Rutgers EJ, Lopes Cardozo M, Van Lanschot JJ. Nipple-sparing mastectomy in breast cancer: a viable option? Eur J Surg Oncol. 2001;27(6):521–6.
Dzierzanowski M, Melville KA, Barnes PJ, MacIntosh RF, Caines JS, Porter GA. Ductal carcinoma in situ in core biopsies containing invasive breast cancer: correlation with extensive intraductal component and lumpectomy margins. J Surg Oncol. 2005;90(2):71–6.
Halsted WS. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;56:1–19.
Murphy V, Chamberlain RS. Nipple-sparing mastectomy in modern breast practice. Clin Anat. 2013;26(1):56–65.
Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transpl Bull. 1962;30:676–82.
Garcia-Etienne CA, Borgen PI. Update on the indications for nipple-sparing mastectomy. J Support Oncol. 2006;4(5):225–30.
Fisher B, Bauer M, Margolese R, Poisson R, Pilch Y, Redmond C, et al. Five-year results of randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665–73.
Hermann RE, Esselstyn CB Jr, Cooperman AM, Crile G Jr. Partial mastectomy without radiation therapy. Surg Clin North Am. 1984;64(6):1103–13.
De La Cruz L, Moody AM, Tappy EE, Blankenship SA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple–areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22(10):3241–9.
Manning AT, Sacchini VS. Conservative mastectomies for breast cancer and risk-reducing surgery: the Memorial Sloan Kettering Cancer Center experience. Gland Surg. 2016;5(1):55–62.
Metcalfe KA, Lubinski J, Ghadirian P, Lynch H, Kim-Sing C, Friedman E, et al. Predictors of contralateral prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation: the Hereditary Breast Cancer Clinical Study Group. J Clin Oncol. 2008;26(7):1093–7.
Sisco M, Yao KA. Nipple-sparing mastectomy: a contemporary perspective. J Surg Oncol. 2016;113(8):883–90.
Torresan RZ, dos Santos CC, Okamura H, Alvarenga M. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol. 2005;12(12):1037–44.
Tang R, Coopey SB, Merrill AL, Rai U, Specht MC, Gadd MA, et al. Positive nipple margins in nipple-sparing mastectomies: rates, management, and oncologic safety. J Am Coll Surg. 2016;222(6):1149–55.
D’Alonzo M, Martincich L, Biglia N, Pisacane A, Maggiorotto F, Rosa GD, et al. Clinical and radiological predictors of nipple–areola complex involvement in breast cancer patients. Eur J Cancer. 2012;48(15):2311–8.
Steen ST, Chung AP, Han SH, Vinstein AL, Yoon JL, Giuliano AE. Predicting nipple–areolar involvement using preoperative breast MRI and primary tumor characteristics. Ann Surg Oncol. 2013;20(2):633–9.
Byon W, Kim E, Kwon J, Park YL, Park C. Magnetic resonance imaging and clinicopathological factors for the detection of occult nipple involvement in breast cancer patients. J Breast Cancer. 2014;17(4):386–92.
Karamchandani DM, Chetlen AL, Riley MP, Schetter S, Hollenbeak CS, Mack J. Pathologic–radiologic correlation in evaluation of retroareolar margin in nipple-sparing mastectomy. Virchows Arch. 2015;466(3):279–87.
Ponzone R, Maggiorotto F, Carabalona S, Rivolin A, Pisacane A, Kubatzki F, et al. MRI and intraoperative pathology to predict nipple–areola complex (NAC) involvement in patients undergoing NAC-sparing mastectomy. Eur J Cancer. 2015;51(14):1882–9.
Fortunato L, Loreti A, Andrich R, Costarelli L, Amini M, Farina M, et al. When mastectomy is needed: is the nipple-sparing procedure a new standard with very few contraindications? J Surg Oncol. 2013;108(4):207–12.
Petit JY, Veronesi U, Rey P, Rotmensz N, Botteri E, Rietjens M, et al. Nipple-sparing mastectomy: risk of nipple–areolar recurrences in a series of 579 cases. Breast Cancer Res Treat. 2009;114(1):97–101.
Acknowledgements
We thank Rosalyn A. Uhrig for English-language editorial assistance in the preparation of the initial version of our manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Miyake, R., Kinoshita, S., Shimada, N. et al. Preservation of the nipple–areola complex in skin-sparing mastectomy for early breast cancer. Surg Today 48, 591–597 (2018). https://doi.org/10.1007/s00595-018-1633-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-018-1633-z