Abstract
Purpose
Post-hepatectomy liver failure (PHLF) is the major risk factor for mortality after hepatectomy. Preoperative planning of the future liver remnant volume reduces PHLF rates; however, future liver remnant function (FLR-F) might have an even stronger predictive value. In this preliminary study, we used a new method to calculate FLR-F by the LiMAx test and computer tomography-assisted volumetric-analysis to visualize liver function changes after portal vein embolization (PVE) before extended hepatectomy.
Methods
The subjects included patients undergoing extended right hepatectomy either directly (NO-PVE group) or after PVE (PVE group). Computed tomography (CT) scan and liver function tests (LiMAx) were done before PVE and preoperatively. FLR-F was calculated and correlated with the postoperative liver function.
Results
There were 12 patients in the NO-PVE group and 19 patients in the PVE group. FLR-F and postoperative liver function correlated significantly in both groups (p = 0.036, p = 0.011), although postoperative liver function was slightly overestimated, at 32 and 45 µg/kg/min, in the NO-PVE and PVE groups, respectively. LiMAx value did not change after PVE.
Conclusions
Volume–function analysis using LiMAx and CT scan enables us to reliably predict early postoperative liver function. Global enzymatic liver function measured by the LiMAx test did not change after PVE, confirming that liver function distribution in the liver stays constant after PVE. An overestimation of FLR-F is needed to compensate for the intraoperative liver injury that occurs in patients undergoing extended hepatectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The expanding resectability criteria for liver malignancies has made partial liver resection a standard procedure for an increasing number of patients [1, 2]. This procedure is offered even to elderly patients, including those over 80 years old, without increased liver related-morbidity and mortality [3].
Low future liver remnant volume (FLR-V) and underlying parenchymal disease have been identified as important risk factors for post-hepatectomy liver failure (PHLF) and impaired liver regeneration after partial liver resection [4–6]. An FLR-V > 25 % of the total functional liver volume is considered to indicate a safe and feasible partial hepatectomy [7–9]. Selective embolization of the right portal branch (PVE) has been shown to increase the FLR-V and thereby minimize the PHLF risk [6, 10]. However, to our knowledge, the process of liver function shift after PVE has not been described before.
If there is dysfunction of the liver parenchyma, such as that caused by steatosis, cirrhosis, or preoperative chemotherapy, the FLR-V alone is insufficient to assess the individual PHLF risk [11–14]. Thus, the future liver remnant function (FLR-F) should be used to predict the post-hepatectomy outcome [4, 15]. Different methods of investigating the FLR-F have been investigated, including the indocyanin green retention test [16], 99mTc-labelled galactosyl-human serum albumin scintigraphy [17–19], and 99mTc-labelled mebrofenin hepatobiliary scintigraphy [18, 20, 21]. Mizuguchi et al. [22] recently reviewed the literature on currently available methods of preoperative liver function assessment. We propose a new diagnostic approach to estimate FLR-F by using the LiMAx test and CT scan-assisted volume/function analysis.
The LiMAx is a 13C-based breath test that assesses actual liver function, the diagnostic power of which has been proven in several clinical settings. In particular, the LiMAx test has been shown to predict postoperative morbidity and mortality after partial liver resection when performed on postoperative day (POD) 1 [4]. Recently, our group proposed guidelines for using the LiMAx test with CT-assisted volumetric analysis to predict FLR-F [5]. The aim of this preliminary study was to analyze the changes in liver function after PVE and prove the feasibility to predict FLR-F by the LiMAx test and CT-scan assisted volume-function analysis in patients undergoing extended hepatectomy either after PVE, or without any preconditioning.
Methods
This prospective observational study was performed in the Department of General, Visceral and Transplantation Surgery at the Charité Berlin, Germany, between January 2005 and December 2009. The study was performed in cooperation with the Department of Diagnostic and Interventional Radiology at the Charité Berlin. The study protocol received prior approval from the local ethics committee and informed consent was obtained from all subjects before their inclusion. The trial was performed in accordance with the precepts established by the Helsinki Declaration. All patients evaluated to undergo extended right hepatectomy for primary or metastatic liver malignancy were screened for the exclusion criteria for this study, as follows: a history of liver surgery, apart from cholecystectomy; severe infectious disease such as HIV; and mental health disorders. Thereafter, the decision was made as to whether the patient would undergo portal vein embolization (PVE) to improve the FLR-V or whether they would undergo the hepatectomy directly (NO-PVE). This decision was made by the surgeon in charge according to the initial FLR-V volume. Postoperative outcome was assessed by 30-day mortality and complications were evaluated according to the Clavien/Dindo classification [23]. Liver function was measured using the LiMAx test consecutively in all patients until discharge and on POD 85. Liver histology was evaluated semi quantitatively according to the Batts and Ludwig scoring system [24]. Fibrosis was staged on a 0–4 scale as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and a few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis.
Portal vein embolization and hepatectomy
PVE was performed in an analogous manner by two experienced interventional radiologists, via a percutaneous transhepatic ipsilateral approach. A catheter was inserted into the right portal vein by a trans-hepatic CT-guided puncture of the right portal branch. Briefly, after establishing percutaneous ipsilateral transhepatic access, direct portography was performed to visualize the portal vein anatomy. Branches of the right portal vein were then catheterized selectively, followed by embolization with PVA particles (500–710 μm Contour, Boston Scientific, Natick, MA, USA) until complete stasis was achieved. If segment IV portal vein branches arose from the left portal vein, these branches would not have been embolized selectively. The success of portal vein embolization was controlled by direct portography. Patients were discharged 1 day after PVE and then readmitted after an interval of 4 weeks.
On readmission, they underwent standard preoperative work-up, including contrast-enhanced four-phase CT scan. Every patient underwent extended right hepatectomy, including segments V–VIII of the liver and partial or complete resection of segment IV and segment I resection (en bloc). Portal vein resection and biliary anastomosis was performed for all Klatskin tumors. Liver pedicle clamping (Pringle maneuver) was performed if there was profuse parenchymal bleeding, and this decision was made by the surgeon intraoperatively.
Dynamic liver function test
Liver function was assessed with the LiMAx (MAximum Liver function capacity) test, as described previously (9, 20). Based on the hepatocyte-specific metabolism of the 13C-labelled substrate Methacetin (Euriso-top, Saint-Aubin Cedex, France) by the cytochrome P450 1A2 enzyme, which is ubiquitously active throughout the liver, 13C-methacetin is instantly metabolized into acetaminophen and the demethylated 13C-group after its intravenous injection at 2 mg/kg. This is converted into 13CO2 and is exhaled, leading to a significant alteration of the regular 13CO2/12CO2 ratio in the expired breath. A suitable device connected to the patient measures this change (FLIP®, Humedics company, Berlin, Germany) and analyzes the breath automatically. Liver function capacity is calculated from the kinetic analysis of the 13CO2/12CO2 ratio over a maximum of 60 min.
Volumetric analysis
Patients in the PVE group had a multiphase contrast-enhanced CT scan done directly before PVE and before hepatectomy. Patients in the NO-PVE group were assessed directly before resection, at which time a volumetric analysis was performed using Amira® software (Visage Imaging GmbH, Richmond, Australia, Fig. 1). This software was validated previously in an experimental study [25] and is now used routinely by radiologists in our hospital. Total liver volume and tumor volume measurements were done manually by the delineation of margins in every CT slide by an experienced radiologist in collaboration with the attending liver surgeon. Since tumor volume is regarded as a non-functional tissue, total functional liver volume was calculated as follows:
Resected liver volume was measured by an intraoperative water displacement method. The measured compound volume was multiplied by a factor of 1.15 to compensate the blood volume [25]. The future liver remnant volume (FLR-V) was thus calculated as follows:
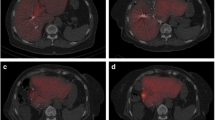
Computed tomography (CT) scans before (a, c) and after (b, d) portal vein embolization (PVE). Volumetric analysis was performed manually by a trained physician (supervised by a second physician), who delineated the margins in every CT slide with a handheld cursor. The volumes were calculated automatically according to the delineations and slice thickness. Arrows show the right portal branches before PVE (a) and the non-perfused areas after PVE (b). Grown liver segments II and III as well as a hyper-perfused left portal branch can be seen in picture ‘d’
Calculation of FLR-F
Future liver remnant function (FLR-F) was calculated based on a FLR-V as:
Additionally, the function of liver segments II and III was calculated according to the volume of those segments as:
Statistical analyses
Continuous quantitative variables are expressed as means ± 95 % confidence interval if not noted otherwise. Group comparisons were performed with the Chi-quadrant, unpaired student t test, the Mann–Whitney U test, or the Wilcoxon test, in accordance to date scale and distribution. Moreover, an ROC analysis was performed to assess the predictive value of preoperative parameters (area under the curve) for liver-related death. Statistical significance was accepted at a p value of <0.05. Calculations were performed with SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Thirty-one patients underwent extended right hepatectomy for primary or secondary liver malignancies. Table 1 provides detailed demographical and clinical data about the cohort. A group of 19 patients (59 %) received PVE prior to the operation, whereas 12 patients (31 %) underwent surgery without prior embolization (NO-PVE). There were 12 male patients in the PVE group (63 %) and 7 (58 %) in the NO-PVE group (male vs. female p = 0.043), All of the PVE patients and five of the NO-PVE patients had a Klatskin tumor, while two in the NO-PVE group had cholangiocarcinoma (17 %) and three (25 %) had metastases of colon adenocarcinoma (p = 0.019). Liver cirrhosis (F4) was found in the pathological workup of specimens from the two of the NO-PVE group patients, both of whom had a positive alcohol anamnesis (Table 1). None of the patients in our cohort had viral hepatitis positivity. There were no other significant differences in demographical, clinical or laboratory data between the study groups. Data regarding portal resection and the duration of hepatectomy did not differ between the groups (Table 1). Although liver-related postoperative complications such as bile leakage and bilioma tended to be more common in the PVE group, the difference was not significant. The mortality rates were 17 % in the NO-PVE and 12 % in the PVE group (p = 0.630). In the NO-PVE group, two patients died of portal vein thrombosis followed by liver failure. In the PVE group one patient died of intestinal perforation with peritonitis. Another patient in the PVE group had leakage of the biliary-enteric anastomosis, resulting in peritonitis and associated intestinal perforations. Overall, the three deaths were classified as “liver related”. Table 4 summarizes the clinical characteristic of the patients who died. An ROC analysis of the liver-related deaths and the associated parameters; namely, FLR-V, FLR-F, and LiMAx on POD 1 was also carried out (Fig. 2).
Predictive values for liver related death. Four patients died: three, of liver failure (liver-related death (Table 4). The area under the curve (AUC) from the receiver operating curve was calculated for the FLR-V (% of total functional liver volume) and FLR-F (µg/KG/h) before resection, as well as for the LiMAx on POD 1 (µg/KG/h) for liver-related death
The volume of liver segments II and III increased from 17.3 to 23.6 % after PVE. The measured liver-remnant volume after surgery was 34.7 % in the NO-PVE group and 36.6 % in the PVE group, without a significant difference. Table 2 summarizes further data on the liver volume measurements.
Table 3 lists the liver function parameters. The initial LiMAx value was within the normal range [>315 (µg/kg/h)] and did not differ between the groups [344 vs. 401 (µg/kg/h), p = 0.326]. The initial FLR-F before PVE also did not differ between the groups. However, four weeks after PVE, the FLR-F increased to 141 µg/kg/h, with subsequent outbalance of the PVE group over the NO-PVE group (141 vs. 114 µg/kg/h, respectively; p = 0.023). Liver function measured on POD 1 did not differ significantly between the groups [NO-PVE 86 vs. PVE 95 (µg/kg/min), p = 0.215]. An overestimation of the planned FLR-F was 32 and 45 (µg/kg/min) for the NO-PVE and PVE groups, respectively (p = 0.391). The FLR-F and LiMAx values on POD 1 correlated significantly (Fig. 3) in the PVE (r = 0.585, p = 0.011) and NO-PVE groups (r = 0.608, p = 0.036). The postoperative regeneration of liver function after resection, quantified by the LiMAx test in both groups was similar. The LiMAx value was higher in the NO-PVE group only on POD 10 (p = 0.05, Fig. 4).
Correlation between predicted and measured liver function on POD 1. Future liver remnant function (FLR-F) can be predicted before resection by using CT-scan volumetric analysis and liver function assessment with the LiMAx test. FLR-F and LiMAx on POD 1 correlated significantly (r = 629, p < 0.001). The FLR-F was slightly overestimated in this clinical trial (adjusted line). This might be based on an additional intraoperative liver function loss caused by vascular clamping
Liver regeneration after PVE and extended right hepatectomy. Liver regeneration was assessed by the LiMAx test. Liver function developed similarly after partial liver resection in both groups. On postoperative day 10, liver function in the NO-PVE group appeared to be higher, although this difference had diminished by 3 months postoperatively
Discussion
Insufficient remnant liver function after hepatectomy leads to a posthepatectomy liver failure (PHLF), which increases the risk of postoperative mortality. We conducted the present study to predict FLR-F, using volume-function analysis by combining the LiMAx liver function test with CT/MRT liver volumetric analysis.
FLR-F is a crucial predictor of post-hepatectomy complication and mortality rates [26]. Different approaches, such as 99mTc-GSA scintigraphy, have been used to assess FLR-F in patients undergoing extended hepatectomy with or without prior PVE, but none has proven effective and limited data have been published [20, 27, 28]. One of the reasons for this might be that nuclear imaging techniques are cost- and facility-intensive. A volume function analysis using the LiMAx test and CT/MRT volumetric analysis was published recently by our group. We found an excellent correlation between planed FLR-F and morbidity and mortality (Table 2, page 142 [5]). Moreover, a high predictive power of the LiMAx test on POD 1 for liver failure and liver-related death was shown (AUC 0.99 and 0.99 respectively; Table 4, page 125 [4]). The findings of the present study are consistent with those of the previous reports and they also present the feasibility of volume function analysis to predict FLR-F in patients undergoing preoperative PVE.
An important parameter influencing the FLR-F calculation is intraoperative liver injury. After intraoperative hepatic pedicle clamping with ensuing liver ischemia, an unknown liver function loss may occur [29]. Another disturbing factor is the presence of non- or hypo-perfused liver areas after hepatectomy. In the present series, all Klatskin tumors were resected according to the no-touch technique by Neuhaus et al. [30], which involves portal vein resection, accompanied by some liver ischemia. Assessing the hypo-perfused areas is difficult and requires a CT\MRT scan directly after hepatectomy, which was not performed in our study. Thus, the feasibility of volume function planning to predict the FLR-F is limited in patients undergoing complex hepatectomy. An over-calculation of FLR-F with a respective safety range is needed for those patients.
Another interesting finding of this study relates to the liver function distribution after PVE. Since blood supply to the right liver lobe is depleted after PVE, a loss of function may result; however, our data showed the opposite. First, we did not see any loss of function directly after PVE (Table 3; Fig. 4). Thus, we believe that loss of portal vein blood does not lead to an acute function alteration, as long as the arterial perfusion is preserved. Through a hepatic artery buffer response an arterial hyper-perfusion takes place in those portal hypo-perfused areas [31]. This hypothesis is supported by the fact that PVE, in contrast to arterial embolization, does not lead to necrosis or to a loss of hepatocyte mass. Second, the underestimation of the FLR-F did not differ between the PVE and NO-PVE groups in this study. This finding also suggested similar distribution of liver function in patients with and those without PVE. We assume that the shift in liver function from the right to the left lobe is a slow remodeling process, which includes proliferation and atrophy of the right liver lobe. Thus, the liver function distribution does not change at each time point after PVE and the data presented here support this hypothesis.
We observed an insignificant increase in the LiMAx value, from 360 to 401 µg/kg/h, between PVE and hepatectomy. This slight increase was supposedly due to improved therapy for cholestasis and cholangitis, which are known to impair liver function [32, 33]. In our department, biliary stenting is usually performed directly before PVE in patients with central cholangiocarcinoma. In the present series we noted a decrease in the total bilirubin serum level from 5.3 (2:7) (µg/dl) before PVE to 2.1 (1:3) (µg/dl) before hepatectomy (p < 0.001).
We presented data from a single-center prospective observational study of patients undergoing standard treatment for liver malignancy in our department. Every patient who fulfilled inclusion criteria was evaluated so there was no bias of patient selection criteria in relation to initial liver function. Portal vein embolization (PVE) was performed by the same method in all patients, thereby eliminating bias related to this issue in our trial [7]. However, this study has several limitations which need to be addressed in further research studies. First, the limited number of patients and too few “hard” end points such as liver failure-related death make it impossible to perform logistic regression in this selected population. Furthermore, none of the patients in this clinical trial had a history of liver diseases, such as hepatitis or cirrhosis, or any marked comorbidity impairing global liver function, as well as FLR-F. On the other hand, the LiMAx could be carried out easily in a common clinical situation, by using a mobile device. In our experience, the LiMAx test might even be used in patients with acute cholestasis, which is known to affect the results of other liver function tests, such as the ICG-PDR [34].
Conclusions
The LiMAx test combined with CT-assisted volume/function analysis allows for a reliable calculation of the future liver remnant function (FLR-F) directly before extended right hepatectomy, or after portal vein embolization (PVE). An overestimation of the FLR-F with a respective safety range is needed for complex hepatectomy to compensate for the intraoperative liver injury caused by vascular clamping or the presence of non-functional (hypoperfused) liver areas. The prediction of early postoperative liver function by the preoperative calculation of FLR-F could be used to stratify the risk of postoperative liver failure. Liver function distribution does not change after PVE showing a slow and homogeneous remodeling process.
Abbreviations
- ICG:
-
Indocyanine green
- POD:
-
Postoperative day
- PVE:
-
Portal vein embolization
- FLR-F:
-
Future liver remnant function
- FLR-V:
-
Future liver remnant volume
- PHLF:
-
Postoperative liver failure
References
Ribero D, Curley SA, Imamura H, Madoff DC, Nagorney DM, Ng KK, et al. Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection. Ann Surg Oncol. 2008;15:986–92.
Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–80.
Nozawa A, Kubo S, Takemura S, Sakata C, Urata Y, Nishioka T, et al. Hepatic resection for hepatocellular carcinoma in super-elderly patients aged 80 years and older in the first decade of the 21st century. Surg Today. 2015;45:851–7.
Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke M, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. 2009;250:119–25.
Stockmann M, Lock JF, Malinowski M, Niehues SM, Seehofer D, Neuhaus P. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB (Oxford). 2010;12:139–46.
Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg. 2011;98:1188–200.
Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57.
Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–94.
May BJ, Talenfeld AD, Madoff DC. Update on portal vein embolization: evidence-based outcomes, controversies, and novel strategies. J Vasc Interv Radiol. 2013;24:241–54.
Yigitler C, Farges O, Kianmanesh R, Regimbeau JM, Abdalla EK, Belghiti J. The small remnant liver after major liver resection: how common and how relevant? Liver Transpl. 2003;9:S18–25.
Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–59.
Seehofer D, Stockmann M, Schirmeier A, Nussler AK, Cho SY, Rayes N, et al. Intraabdominal bacterial infections significantly alter regeneration and function of the liver in a rat model of major hepatectomy. Langenbecks Arch Surg. 2007;392:273–84.
Yokoyama Y, Nagino M, Nimura Y. Mechanism of impaired hepatic regeneration in cholestatic liver. J Hepatobiliary Pancreat Surg. 2007;14:159–66.
Field KM, Dow C, Michael M. Part I: liver function in oncology: biochemistry and beyond. Lancet Oncol. 2008;9:1092–101.
Lock JF, Malinowski M, Seehofer D, Hoppe S, Rohl RI, Niehues SM, et al. Function and volume recovery after partial hepatectomy: influence of preoperative liver function, residual liver volume, and obesity. Langenbecks Arch Surg. 2012;397:1297–304.
Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–72.
Hirai I, Kimura W, Fuse A, Suto K, Urayama M. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99 mTc-GSA SPECT scintigraphy. Surgery. 2003;133:495–506.
Nishiyama Y, Yamamoto Y, Hino I, Satoh K, Wakabayashi H, Ohkawa M. 99 mTc galactosyl human serum albumin liver dynamic SPET for pre-operative assessment of hepatectomy in relation to percutaneous transhepatic portal embolization. Nucl Med Commun. 2003;24:809–17.
Yumoto Y, Yagi T, Sato S, Nouso K, Kobayashi Y, Ohmoto M, et al. Preoperative estimation of remnant hepatic function using fusion images obtained by (99m)Tc-labelled galactosyl-human serum albumin liver scintigraphy and computed tomography. Br J Surg. 2010;97:934–44.
de Graaf W, van Lienden KP, van den Esschert JW, Bennink RJ, van Gulik TM. Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg. 2011;98:825–34.
Nanashima A, Tobinaga S, Abo T, Sumida Y, Araki M, Hayashi H, et al. Relationship of hepatic functional parameters with changes of functional liver volume using technetium-99m galactosyl serum albumin scintigraphy in patients undergoing preoperative portal vein embolization: a follow-up report. J Surg Res. 2010;164:e235–42.
Mizuguchi T, Kawamoto M, Meguro M, Hui TT, Hirata K. Preoperative liver function assessments to estimate the prognosis and safety of liver resections. Surg Today. 2014;44:1–10.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–17.
Niehues SM, Unger JK, Malinowski M, Neymeyer J, Hamm B, Stockmann M. Liver volume measurement: reason of the difference between in vivo CT-volumetry and intraoperative ex vivo determination and how to cope it. Eur J Med Res. 2010;15:345–50.
Dimitroulis D, Tsaparas P, Valsami S, Mantas D, Spartalis E, Markakis C, et al. Indications, limitations and maneuvers to enable extended hepatectomy: current trends. World J Gastroenterol. 2014;20:7887–93.
Wigmore SJ. Increase in future remnant liver function after preoperative portal vein embolization (Br J Surg 2011; 98: 825–834). Br J Surg. 2011;98:835.
Kim SH, Kim IK, Hong YK, Chol SB, Lee KH, Park SW, et al. The effect of preoperative portal vein embolization on liver regeneration after extended hepatic resection. Hepatogastroenterology. 2011;58:516–21.
Nuzzo G, Giuliante F, Giovannini I, Vellone M, De Cosmo G, Capelli G. Liver resections with or without pedicle clamping. Am J Surg. 2001;181:238–46.
Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–18; discussion 19.
Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–339.
Suda K, Ohtsuka M, Ambiru S, Kimura F, Shimizu H, Yoshidome H, et al. Risk factors of liver dysfunction after extended hepatic resection in biliary tract malignancies. Am J Surg. 2009;197:752–8.
Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, Nagino M. The adverse effects of preoperative cholangitis on the outcome of portal vein embolization and subsequent major hepatectomies. Surgery. 2014;156:1190–6.
Stockmann M, Malinowski M, Lock JF, Seehofer D, Neuhaus P. Factors influencing the indocyanine green (ICG) test: additional impact of acute cholestasis. Hepatogastroenterology. 2009;56:734–8.
Acknowledgments
We thank Rhea Roehl for her assistance in performing the study measurements.
Authors’ contributions
All authors contributed essentially to this paper, then revised and finally approved the manuscript. MM designed and organized the study, analyzed and interpreted data, and wrote the manuscript; JFL contributed to conception and design of the study; DS and BG contributed to the conception and design of the study, and performed the measurements. AS contributed to the conception, design and organization of the study. LD participated in organizing the study and collected data. JB analyzed and interpreted data. VS analyzed and interpreted data. PN contributed to the conception and design of the study. MS contributed to the conception and design of the study, analyzed and interpreted data, and organized the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
Martin Stockmann is the inventor of the LiMAx-Test and has capital interest in its sales (Humedics GmbH, Berlin, Germany). The other authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Malinowski, M., Lock, J.F., Seehofer, D. et al. Preliminary study on liver function changes after trisectionectomy with versus without prior portal vein embolization. Surg Today 46, 1053–1061 (2016). https://doi.org/10.1007/s00595-015-1293-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-015-1293-1