Abstract
Purpose
We, herein, examined the role of the intraluminal contents and continuity of colonic intrinsic neurons in intracolonic capsaicin-induced enhancement of colonic motility and defecation.
Methods
Five beagle dogs were equipped with three strain gauge force transducers throughout the colon. The colonic contractile activity in response to intracolonic capsaicin was studied in intact dogs, dogs after colonic cleansing and dogs with transection/re-anastomosis (T/R) between the proximal and middle colon. The effects of intravenous yohimbine, a α2 adrenergic antagonist, on the colonic motility and defecation were also studied in the same models.
Results
In intact dogs, capsaicin (10 mg) and yohimbine (2 mg/kg) immediately induced contractions throughout the colon, with defecation occurring in all experiments. In dogs after colonic cleansing and T/R, the capsaicin (10 mg)-induced enhancement of colonic motility was decreased in the middle and distal colon, and capsaicin-induced defecation was observed in 0–20 % of experiments (p < 0.05 compared to intact dogs). The effect of yohimbine (2 mg/kg) in inducing colonic contractions was unaltered after colonic cleansing and T/R; in contrast, yohimbine-induced defecation was not observed after colonic cleansing, but was unchanged after T/R.
Conclusions
The continuity of the colonic intrinsic nerves as well as the intraluminal contents appear to play an important role in the colonic motor response to intracolonic capsaicin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We previously reported that the effects on gastrointestinal motility after intraluminal administration of capsaicin, a pungent ingredient of chili, differ according to the site of administration in conscious dogs. Intragastric administration of capsaicin stimulated colonic motility and induced defecation, but also induced vomiting [1]. When administered into the duodenum and ileum, capsaicin inhibited gastric and upper gastrointestinal contractility [2, 3]. The administration of capsaicin into the proximal colon enhanced the colonic motility and defecation, without inducing vomiting [4]. These motor responses were considered to be neural reflexes mediated via the receptor for capsaicin, the transient receptor potential cation channel subfamily V member 1 (TRPV1), on afferent neurons [5, 6].
Intracolonic capsaicin-induced enhancement of colonic motility and defecation may have a possible clinical application as an agent to induce defecation. The mechanism underlying the colonic motor response to intracolonic capsaicin has not been well described. We previously suggested that extrinsic nerves innervating the colon play an important role in the enhancement of colonic motility and defecation induced by intracolonic capsaicin [3], but the role of the intracolonic contents and continuity of intrinsic neurons within the colon in the intracolonic capsaicin-induced colonic motor response is still unclear.
The canine colon is usually filled with feces and is seldom empty [7]. We were interested in whether the enhancement of colonic motility and defecation after administration of intracolonic capsaicin would be preserved when the colon is completely empty, as after colonic lavage to “cleanse” the colon and thereby evacuate the intraluminal fecal content. Although intracolonic capsaicin may not evoke defecation in the empty colon, the colonic contractility might still be enhanced. We were also interested in the role of the continuity of the colonic intrinsic neurons in the intracolonic capsaicin-induced colonic motor response. Transected intrinsic neurons are functionally ‘reconnected’ 4–8 weeks after transection and re-anastomosis (T/R) of the intestinal wall [8]. Studying the effects of intracolonic capsaicin within 4 weeks after T/R between the proximal and middle colon would give us the answer to this question.
Two types of colonic contractions are observed in conscious dogs; one is colonic motor complexes (CMCs), which consist of a tonic increase of the baseline with superimposing phasic contractions [9–11]. Most CMCs occur in the proximal colon and propagate to the distal direction with a relatively slow velocity (4 cm/min). CMCs are thought to be associated with slow ‘to and fro’ movement of the intracolonic contents. Another type of colonic contractile activity is giant migrating contractions (GMCs) of high amplitude and fast propagating velocity (1 cm/sec) [9, 12]. Because defecation is observed when GMCs reach the distal colon, GMCs are thought to be related to the rapid transit of feces and defecation [9, 12, 13].
Our hypotheses were that (1) intracolonic capsaicin evokes CMCs, but not GMCs and defecation, in the empty “cleansed” colon, (2) the colonic motor response to intracolonic capsaicin does not differ between the normal colon and colon early after T/R. The aim of the present study was to investigate the role of the intracolonic contents and the continuity of the intrinsic neurons in the intracolonic capsaicin-induced colonic contractile activity and defecation. We also studied the effects of intravenous yohimbine, which stimulates colonic motility and induces defecation by blocking α2 adrenergic receptors on the enteric neurons [14], in the same models.
Materials and methods
Preparation of animals
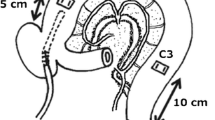
All procedures and animal care were performed according to the guidelines of the Animal Care and Use Committee of Tohoku University. All surgical preparations were performed using sterile techniques. Experiments were performed in 15 healthy adult beagle dogs (range of weight: 10–12 kg) divided into three groups: intact control dogs, dogs after colonic cleansing, and dogs after T/R. The dogs were anesthetized with intravenous sodium thiopental (Ravonal; Mitsubishi Tanabe Seiyaku Co., Osaka, Japan) at 20 mg/kg and were maintained by inhaled halothane (Fluothane; Takeda Chemicals Co., Osaka) with oxygen. Just prior to laparotomy, we placed a silicone catheter (SH No.2, Create Medic CO., Yokohama, Japan) into the superior vena cava via the right jugular vein to administer drugs. Via a midline incision, three strain gauge force transducers (F-12IS; Star Medical Inc., Tokyo, Japan) were sewn onto the seromuscular surface of the colon to measure the contractile activity of the circular muscle. The first transducer was placed on the proximal colon 5 cm distal to the ileocolic junction. The third transducer was placed on the distal colon 10 cm proximal to the peritoneal reflection, and the second transducer was implanted midway between the other two transducers (Fig. 1). A silicone catheter to administer test materials was placed in the proximal colon via the cecum, with its tip 5 cm distal to the ileocolic junction (Fig. 1). The transducers on the proximal, middle, and distal colon were termed C1, C2, and C3, respectively. The lead wires from the transducers and the silicone catheters were tunneled subcutaneously and exteriorized via a stab wound between the scapulae, and were covered with a canvas jacket for protection from self-inflicted trauma. These procedures were performed in intact dogs (N = 5), and in dogs after colonic cleansing (N = 5). In the other five dogs, T/R at the midpoint between C1 and C2 was also performed (Fig. 1). All dogs were allowed to recover for 14 days after the operation. Colonic motility was then monitored in the conscious state by connecting the lead wire from the transducers to an amplifier (MS-08; Star Medical Inc.), and then to a computer with a software program (Chart; AD Instruments, Victria, Australia) through Mac Lab (AD Instruments). The dogs were fed a solid meal (CD-55α; Clea Japan Inc., Tokyo) once a day in the evening. Water was given ad libitum except during the experiments.
A schematic diagram showing canine preparation. The first (C1) and the third (C3) transducers were placed on the proximal colon 5 cm distal to the ileocolic junction and on the distal colon 10 cm proximal to the peritoneal reflection, respectively. The second transducer (C2) was implanted midway between C1 and C3. A silicone catheter was placed in the proximal colon via the cecum. Transection and re-anastomosis (T/R) were performed at the midpoint between C1 and C2
Experimental protocol
Measurement of the colonic motility was started in the morning at 10 o’clock. In the intact dogs and dogs after T/R, the basal colonic motility was measured for the first 3 h and then intracolonic or intravenous administration of agents was performed. In dogs to be studied after colonic cleansing, we infused the colonic lumen with 154 mM NaCl solution at room temperature via the intracolonic silicone catheter at 25 mL/min for 30–90 min (infusion time 60 min and total volume of perfusion 1,500 mL) after starting the measurement of colonic motility. All dogs were defecated several times during this lavage, and when the colonic effluent contained no fecal matter, the colonic lavage was stopped. As a preliminary study, three other dogs undergoing this colonic cleansing were anesthetized, and we confirmed that the whole colon was empty by directly inspecting the colonic lumen. To study the effects of cleansing on the basal colonic contractile activity, we measured the colonic motility for 3 h after the colonic cleansing, and no further experiments were carried out on that day. Intracolonic or intravenous administration of agents was performed 5–10 min after colonic cleansing.
Intracolonic administration of 10 mL 154 mM NaCl solution containing capsaicin (2 or 10 mg) as a bolus, or intravenous administration of 10 mL 154 mM NaCl solution containing yohimbine (0.5 or 2 mg/kg) as a bolus were performed when all colonic transducers were in the quiescent period. The colonic motor response induced by intracolonic administration of 10 mL 154 mM NaCl solution, which contained 1.0 mL of 95 % ethanol as the vehicle for capsaicin and by intravenous administration of 10 mL 154 mM NaCl solution, which contained 1.5 mL dimethyl sulfoxide as the vehicle for yohimbine, was used as a placebo control for experiments with capsaicin and yohimbine, respectively. Each experiment was done once a day in intact dogs and in dogs with T/R, and once every 2 days in dogs undergoing colonic cleansing in random order. All experiments were repeated twice, and the mean of the two studies was regarded as a representative value for that dog.
Data analysis
The duration of CMCs was measured by a visual inspection during the period of basal colonic motility in the three groups. The occurrence of GMCs was analyzed by a visual inspection. GMCs were defined as single event contractions with a mean amplitude greater than 2.8 times higher than that of CMCs and propagating with a mean velocity of 1.0 cm/s [9]. If defecation or GMCs were observed within 30 min after administration of agents, then these were regarded to be agent-induced. The area under the contractile waves measured for 30 min after administration of each solution was expressed as a motility index (MI). A commercial computer program (Chart; AD Instruments) was used to calculate the MI. The Mann–Whitney U test and the Chi-square test were used to compare the MI and the occurrence of defecation and GMCs, respectively. All data were expressed as the mean ± standard error of the mean (SEM), and values of p < 0.05 were regarded to be significant.
Drugs Capsaicin and yohimbine were purchased from Wako Pure Chemical Industries, Ltd., Osaka.
Results
Effects of colonic cleansing and T/R on the basal colonic motor activity
The duration of CMCs in intact dogs was 11.5 ± 2.5 min at C1, 12.6 ± 3.1 min at C2, and 15.3 ± 3.6 min at C3 (Table 1). In dogs after colonic cleansing, the phasic contractions without an increase in the baseline tone continued, and the duration of these contractions in dogs after colonic cleansing was prolonged compared with that in the intact dogs without colonic cleansing at all sites (p < 0.05, Table 1). The duration of CMCs in dogs after T/R was not significantly different from that in intact dogs (Table 1).
The effects of intracolonic capsaicin on colonic motility
In intact dogs, intracolonic capsaicin at doses of 2 and 10 mg caused CMCs in eight of 10 and 10 of 10 experiments (Fig. 2a), and induced GMCs with defecation in five of 10 and 10 of 10 experiments, respectively (Table 2). Intracolonic capsaicin increased the MI dose-dependently; the MI in the dogs given the 10 mg dose was enhanced compared with the placebo control at all colonic sites in intact dogs (p < 0.05, Fig. 3a).
A representative tracing showing the effects of intracolonic capsaicin on the colonic motility in intact dogs (a), in dogs after colonic cleansing (b) and in dogs after T/R (c). a In intact dogs, the intracolonic administration of capsaicin at a dose of 10 mg evoked GMCs in the colon and induced defecation. b In dogs after colonic cleansing, GMCs propagating from the middle to the distal colon were observed at the dose of 10 mg. c In dogs after T/R, giant contractions occurred at C1 in three experiments, but they never propagated to the middle colon across the anastomosis. Arrows and arrowheads indicate CMCs and GMCs, respectively
The motility index for 30 min after intracolonic capsaicin in intact dogs (a), dogs after colonic cleansing (b) and in dogs after T/R (c). a Intracolonic capsaicin at a dose of 10 mg increased the motility index compared with the placebo control at all colonic sites examined in intact dogs. b In dogs after colonic cleansing, the motility index at the dose of 10 mg at C2 and C3 did not differ from that of the placebo control, and was decreased compared to that in intact dogs. c The motility index at the dose of 10 mg was greater than that of the placebo control at all colonic sites in dogs after T/R, but the motility index at the dose of 10 mg at C2 and C3 was decreased compared to that in intact dogs. *p < 0.05 compared to the placebo control, #p < 0.05 compared to intact dogs at the same site
In dogs after colonic cleansing, capsaicin induced CMCs in five of 10 and eight of 10 experiments at the doses of 2 mg and 10 mg, respectively (Fig. 2b). GMCs were not observed in any dog at the dose of 2 mg, and GMCs propagating from the proximal or middle colon to the distal colon were observed in five of 10 experiments at the dose of 10 mg; thus, the number of capsaicin-induced GMCs in dogs after colonic cleansing was decreased compared to that in intact dogs (Table 2, p < 0.05). These GMCs in dogs after colonic cleansing were not accompanied by defecation (Table 2). In dogs after colonic cleansing, intracolonic capsaicin increased the MI in a dose-dependent manner (Fig. 3b). Although the MI at C1 at the dose of 10 mg was significantly greater than that of the placebo control, the MI at C2 and C3 at the dose of 10 mg did not differ significantly from that of the placebo control (Fig. 3b). Moreover, the MI at C2 and C3 at the dose of 10 mg in dogs with cleansing was decreased compared to that in intact dogs (Fig. 3b, p < 0.05).
In dogs after T/R, the intracolonic administration of capsaicin at the doses of 2 mg and 10 mg evoked CMCs in two of 10 and 10 of 10 experiments, and GMCs associated with defecation occurred in one of 10 and two of 10 experiments, respectively (Fig. 2c, Table 2). All GMCs in dogs after T/R occurred in the middle colon distal to the T/R site and propagated to the distal colon. The number of capsaicin-induced GMCs in dogs after T/R was decreased compared to intact dogs (Table 2, p < 0.05 for capsaicin at 10 mg). Intracolonic capsaicin increased the MI in dose-dependent fashion at all colonic sites in dogs after T/R, and the MI at the dose of 10 mg was greater than that of the placebo control at all colonic sites (Fig. 3c, p < 0.05). However, the MI at C2 and C3 at the dose of 10 mg in dogs after T/R was still decreased compared to that in intact dogs (p < 0.05, Fig. 3c).
The effects of intravenous yohimbine on colonic motility
In intact dogs, yohimbine-induced CMCs at the dose of 2 mg/kg in all experiments, while yohimbine at 0.5 mg/kg induced CMCs in three of 10 experiments (Fig. 4a). GMCs associated with defecation were observed in three of 10 and 10 of 10 experiments at the doses of 0.5 and 2 mg/kg, respectively (Table 2).
Representative tracing showing the effects of intravenous yohimbine on the colonic motility in intact dogs (a), in dogs after colonic cleansing (b) and in dogs after T/R (c). a In intact dogs, intravenous yohimbine at a dose of 2 mg/kg evoked CMCs and GMCs in the colon and induced defecation. b In dogs after colonic cleansing, yohimbine-induced CMCs along the entire colon at the dose of 2 mg/kg. c In dogs after T/R, GMCs associated with defecation occurred at the middle colon and propagated to the distal colon. Arrows and arrowheads indicate CMCs and GMCs, respectively
In dogs after colonic cleansing, yohimbine at the doses of 0.5 and 2 mg/kg induced CMCs in four of 10 and eight of 10 experiments, respectively (Fig. 4b). GMCs were not observed at the dose of 0.5 mg/kg, and GMCs were not related to defecation propagated from the proximal to the distal colon in two of 10 experiments at the dose of 2 mg/kg (Table 2). The number of GMCs in dogs after colonic cleansing at the dose of 2 mg/kg was significantly decreased compared to that in intact dogs (Table 2, p < 0.05).
In dogs after T/R, yohimbine at the doses of 0.5 and 2 mg/kg induced CMCs in four of 10 and 10 of 10 experiments, and induced GMCs associated with defecation in two of 10 and eight of 10 experiments (Fig. 4c, Table 2). The number of yohimbine-induced GMCs in dogs with T/R did not differ from that in intact dogs (Table 2).
In intact dogs, intravenous yohimbine increased the MI in a dose-dependent manner with significant differences observed between the placebo control and 2 mg/kg at all colonic sites (Fig. 5a). The same phenomenon was observed in dogs after colonic cleansing and T/R, and the MI at the dose of 2 mg/kg did not differ across the three groups (Figs. 5b, c).
The motility index for 30 min after intravenous yohimbine in intact dogs (a), dogs after colonic cleansing (b) and dogs after T/R (c). Intravenous yohimbine at a dose of 2 mg/kg increased the motility index, with significant differences observed at all colonic sites compared to the placebo control. This phenomenon was observed in all three groups. *p < 0.05 compared to placebo control
Discussion
In the present study, we chose capsaicin and yohimbine based on our previous reports. Intracolonic capsaicin and intravenous injection of the α2 adrenergic antagonist, yohimbine, enhanced the colonic motility and induced defecation, and we found an important role of extrinsic nerves innervating the colon in these responses [4, 14]. However, the role of the intracolonic contents and continuity of the colonic intrinsic nerves is still controversial in these motor responses. Because the route of administration differs between capsaicin (intracolonic administration) and yohimbine (intravenous administration), different roles of the intracolonic contents and continuity of colonic intrinsic nerves may exist between these drugs.
We showed that there was a decrease in the number of defecation-related GMCs and the enhancement of MI in the middle and distal colon after the administration of intracolonic capsaicin in dogs after colonic cleansing and after T/R. These results suggest that the continuity of the intrinsic nerves, as well as the colonic intraluminal contents, play an important role in the enhancement of colonic motility and defecation in response to intracolonic capsaicin. Considering the results of the present study and our previous report, which demonstrated that the enhancement of colonic motility and defecation induced by intracolonic capsaicin were decreased after extrinsic denervation of the colon [4], intracolonic capsaicin-induced enhancement of colonic motility and defecation are mediated by some combination of all these three elements: the colonic intraluminal contents, continuity of colonic intrinsic neurons, and the intact extrinsic nerves.
Stimulation of the TRPV1 receptor with intracolonic capsaicin, thus, appears to mediate the stimulation of proximal colonic contractility, either directly or indirectly. The increase in proximal colonic contractile patterns migrates caudally along the intrinsic nerves, and is associated with the rapid transit of intraluminal contents distally in the colon. The rapid transit of feces is associated with GMCs, and when GMCs reach the distal colon, defecation occurs. The extrinsic nerves are necessary for the occurrence and organized migration of GMCs.
We also studied whether intravenous yohimbine exerts its effects on colonic motility via the same or a related mechanism as intracolonic capsaicin. Although the number of defecation-related GMCs induced by intravenous yohimbine in intact dogs was decreased after colonic cleansing, the yohimbine–induced enhancement of the MI did not differ between these two groups. Moreover, the frequency of defecation-related GMCs and enhancement of the MI induced by yohimbine did not differ between intact dogs and dogs after T/R. These results indicate that the continuity of intrinsic nerves appears to have little or no role in mediating the enhancement of colonic motility and defecation in response to intravenous yohimbine.
This observation suggests that the continuity of the intrinsic colonic nerves is not necessary either for the induction of these responses or for the propagation/migration of the motor patterns. The intraluminal contents appear to have a role in inducing defecation-related GMCs, but not in the enhancement of the colonic MI. It is well known that the α2-antagonist yohimbine enhances colonic motility and defecation [14–16]. We previously studied the effect of intravenous yohimbine on an enterically isolated “empty” colon constructed by transection of the terminal ileum and the distal colon. In this model, we showed that intravenous yohimbine-induced CMCs, but not GMCs [14]. These observations are not in conflict with our current study showing that T/R did not alter the yohimbine-induced colonic motor response, and that the number of GMCs decreased after colonic cleansing.
These different roles of the intraluminal contents and continuity of intrinsic nerves between intracolonic capsaicin and intravenous yohimbine are likely related to the route of administration, as well as kind of stimulus. Yohimbine, which is administered intravenously, binds to the α2-adrenoreceptors on the enteric nervous system along the entire colon, and thus, may have a more “diffuse” effect on colonic motility. In contrast, we assume that the capsaicin administered into the proximal colon stimulates only the afferent nerves in the proximal colon, and thereby, has a “local” effect on stimulating the colonic motility. It is also likely that this local effect then affects the entire colon via the movement of the intraluminal contents, the continuity of the intrinsic neural system, and possibly via extrinsic neural reflexes.
GMCs are thought to be associated with the rapid transit of feces and ultimately defecation, which might suggest that GMCs do not occur in the “cleansed” colon without intraluminal contents. Sarna et al. [7] studied the effects of cleansing the colon on the colonic motor activity and found that the frequency of GMCs increased after colonic cleansing. We previously reported two motor patterns of GMCs in the canine colon [9]. One pattern is GMCs migrating from the proximal to the middle colon, while another pattern involves GMCs migrating from the proximal or middle colon all the way to the distal colon. The former pattern is not associated with defecation, while the later pattern is strongly associated with defecation. In our current study, GMCs propagating to the distal colon, but not associated with defecation, still occurred in the cleansed empty colon. Therefore, we concluded that the intraluminal contents play an important, but not mandatory, role in the occurrence of GMCs.
We studied the effects of intracolonic capsaicin and intravenous yohimbine within 4 weeks after transecting and re-anastomosing the colon at the midpoint between C1 and C2, as a model for disrupted continuity of the colonic intrinsic neurons. This study was designed based on the fact that intrinsic neurons are reconnected in 4–8 weeks after T/R in the guinea pig small intestine [8]. We have no data indicating whether the same phenomenon occurs in the canine colon. However, we believe that the decreased colonic motor responses and defecation in response to intracolonic capsaicin is due to the disrupted colonic intrinsic nerves.
We showed that T/R did not affect the duration of CMCs with regard to the basal motor activity. This result does not conflict with our previous report that described no change in the duration or interval of CMCs in enterically isolated colonic loops compared with neurally intact dogs [10]. In the present study, cleansing the colon induced colonic phasic contractions without a basal tone increase that continued for nearly 60 min. These phasic contractions differed from spontaneous CMCs in terms of their longer duration and lack of a basal tone increase. These results suggest a possible association between the presence of intracolonic contents and the duration or basal tone increase of CMCs, although we cannot currently explain the mechanisms connecting these elements.
A possibility remains that capsaicin or yohimbine could be clinically effective as a prokinetic for the colon. Some Japanese herbal medicines, including Dai-Kenchu-To (DKT), are effective for relieving chronic constipation [17, 18]. We found that the intra gastric administration of DKT increased the colonic motility, but did not induce defecation (Kikuchi et al., unpublished data). Compared to DKT and commercially available laxatives, intracolonic capsaicin and intravenous yohimbine are characteristic in terms of their prompt and marked stimulation of the colonic motility and their induction of defecation. Considering the fact that yohimbine might cause systemic adverse effects following intravenous injection, capsaicin has a greater possibility of being useful as a drug to induce defecation than yohimbine. The development of an oral form of capsaicin that would dissolve in the colon would be necessary for this purpose.
In conclusion, the continuity of the colonic intrinsic nerves, as well as the intraluminal contents, plays an important role in the enhancement of the colonic motor response and defecation in response to intracolonic capsaicin. In the colonic motor response and defecation resulting after the intravenous administration of yohimbine, the continuity of the intrinsic nerves seems to have little or no role, while the intraluminal contents play an important role, especially in the induction of defecation-related GMCs.
References
Shibata C, Sasaki I, Naito H, Tsuchiya T, Takahashi M, Ohtani N, et al. Intragastric capsaicin stimulates colonic motility via a neural reflex in conscious dogs. Gastroenterology. 1995;109:1197–205.
Shibata C, Naito H, Ueno T, Jin XL, Funayama Y, Fukushima K, et al. Intraduodenal capsaicin inhibits gastric migrating motor complex via an extrinsic neural reflex in conscious dogs. Neurogastroenterol Motil. 2002;14:543–51.
Shibata C, Jin XL, Naito H, Matsuno S, Sasaki I. Intraileal capsaicin inhibits gastrointestinal contractions via a neural reflex in conscious dogs. Gastroenterology. 2002;123:1904–11.
Hayashi K, Shibata C, Nagao M, Sato M, Kakyo M, Kinouchi M, et al. Intracolonic capsaicin stimulates colonic motility and defecation in conscious dogs. Surgery. 2010;147:789–97.
Holzer P. Capsaicin: cellular targets, mechanism of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201.
Matsumoto K, Hosoya T, Tashima K, Namiki T, Murayama T, Horie S. Distribution of transient receptor potential vanilloid 1 channel-expressing nerve fibers in mouse rectal and colonic enteric nervous system: relationship to peptidergic and nitrergic neurons. Neuroscience. 2011;172:518–34.
Sarna SK. Effect of fluid perfusion and cleansing on canine colonic motor activity. Am J Physiol. 1992;262:G62–8.
Galligan JJ, Furness JB, Costa M. Migration of the myoelectric complex after interruption of the myenteric plexus: intestinal transection and regeneration of enteric nerves in the guinea pig. Gastroenterology. 1989;97:1135–46.
Shibata C, Sasaki I, Matsuno S, Mizumoto A, Iwanaga Y, Itoh Z. Characterization of colonic motor activity in conscious dogs. J Gastrointest Motil. 1993;5:9–16.
Shibata C, Sasaki I, Matsuno S, Mizumoto A, Itoh Z. Colonic motility in innervated and extrinsically denervated loops in dogs. Gastroenterology. 1991;101:1571–8.
Sarna SK, Condon R, Cowles V. Colonic migrating and nonmigrating motor complexes in dogs. Am J Physiol. 1984;246:G355–60.
Karaus M, Sarna SK. Giant migrating contractions during defecations in the dog colon. Gastroenterology. 1987;92:925–33.
Matsushima Y, Okamoto E, Toyosaka A. Studies on colonic motor correlates of spontaneous defecation in conscious dogs. Jpn J Smooth Muscle Res. 1989;25:137–46.
Nagao M, Shibata C, Funayama Y, Fukushima K, Takahashi K, Jin XL, et al. Role of alpha-2 adrenoceptors in regulation of giant migrating contractions and defecation in conscious dogs. Dig Dis Sci. 2007;52:2204–10.
Doherty NS, Hancock AA. Role of alpha-2 adrenergic receptors in the control of diarrhea and intestinal motility. J Pharmacol Exp Ther. 1983;225:269–74.
Malcom A, Camilleri M. Coloanal motor coordination in association with high-amplitude colonic contractions after pharmacological stimulation. Am J Gastroenterol. 2000;95:715–9.
Iwai N, Kume Y, Kimura O, Ono S, Aoi S, Tsuda T. Effects of herbal medicine Dai-Kenchu-to on anorectal function in children with severe constipation. Eur J Pediatr Surg. 2007;17:115–8.
Sakakibara R, Odaka T, Lui Z, Uchiyama T, Yamaguchi K, Yamaguchi T, et al. Dietary herb extract dai-kenchu-to ameliorates constipation in parkinsonian patients (Parkinson’s disease and multiple system atrophy). Mov Disord. 2005;20:261–2.
Acknowledgments
The authors thank Michael G. Sarr, Department of Surgery, Mayo Clinic, MN, USA, for reviewing this manuscript.
Conflict of interest
Daisuke Kikuchi and co-authors have no potential conflicts of interest or financial interests to be disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kikuchi, D., Shibata, C., Imoto, H. et al. Role of the intraluminal contents and the continuity of intrinsic neurons in intracolonic capsaicin-induced contraction and defecation in dogs. Surg Today 44, 152–159 (2014). https://doi.org/10.1007/s00595-013-0493-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-013-0493-9