Abstract
Aims
Diabetic retinopathy is the leading cause of blindness in people with type 2 diabetes. To enable primary care physicians to identify high-risk type 2 diabetic patients with diabetic retinopathy at an early stage, we developed a nomogram model to predict the risk of developing diabetic retinopathy in the Xinjiang type 2 diabetic population.
Methods
In a retrospective study, we collected data on 834 patients with type 2 diabetes through an electronic medical record system. Stepwise regression was used to filter variables. Logistic regression was applied to build a nomogram prediction model and further validated in the training set. The c-index, forest plot, calibration plot, and clinical decision curve analysis were used to comprehensively validate the model and evaluate its accuracy and clinical validity.
Results
Four predictors were selected to establish the final model: hypertension, blood urea nitrogen, duration of diabetes, and diabetic peripheral neuropathy. The model displayed medium predictive power with a C-index of 0.781(95%CI:0.741–0.822) in the training set and 0.865(95%CI:0.807–0.923)in the validation set. The calibration curve of the DR probability shows that the predicted results of the nomogram are in good agreement with the actual results. Decision curve analysis demonstrated that the novel nomogram was clinically valuable.
Conclusions
The nomogram of the risk of developing diabetic nephropathy contains 4 characteristics.
that can help primary care physicians quickly identify individuals at high risk of developing DR in patients with type 2 diabetes, to intervene as soon as possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes has become a significant public health concern worldwide. Globally, as of 2021, there were 463 million T2DM patients with a projection of 700 million by 2045, among which China had an estimated 120.9 million cases [1]. Diabetes patients are at elevated risk of developing complications such as diabetic retinopathy, neuropathy, and kidney disease [2]. Complications of T2DM not only increase medical costs but also affect the quality of life of patients [3]. As the prevalence of diabetes increases, the number of people with diabetic retinopathy is also on the rise [4].
DR, the primary retinal vascular complication of diabetes mellitus, is a leading cause of visual impairment in patients with T2DM [5]. Several studies reported that the prevalence of DR in Chinese diabetic patients ranged from 11.9% to 43.1% [6, 7]. The World Health Organization estimates that the number of DR and vision threatening Dr will increase to 191 million and 56.3 million respectively by 2030 [8]. In the early stages, DR is usually asymptomatic, as the disease progresses DR can affect vision and eventually lead to blindness. The severe impact of DR on vision places a significant burden on individuals, families, communities, and society [9, 10].
DR screening is considered to be an important tool for the early identification of DR [11]. Unfortunately, in China, due to the lack of a convenient tool, DR screening for diabetes is not yet available in most areas.
China is the largest developing country with a mountain of diabetic patients, many of whom suffer from DR, and the current treatment methods have very limited relief of DR. Early detection and diagnosis become particularly important. Hence, in the present study, we aimed to develop a risk nomogram for the prediction of DR.
Materials and methods
Study population
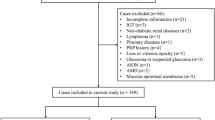
This study retrospectively analyzed T2D patients hospitalized in the First Affiliated Hospital of Xinjiang Medical University from March 2021 to December 2021. The diagnosis of type 2 diabetes was based on the diagnostic criteria for type 2 diabetes issued by the World Health Organization in 1999 [12]. The exclusion criteria were as follows: diabetic ketoacidosis; diabetic foot; Serious chronic diseases including advanced malignancy, liver cirrhosis, liver failure, heart failure, and incomplete information. Finally, 706 patients were included in the study. We divided patients into training and validation sets based on the date of data collection (Fig. 1).
Data collection
The clinicians collected clinical and laboratory data, including age, gender, duration of diabetes, body mass index (BMI), blood pressure, serum insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3), fasting blood glucose, glycosylated hemoglobin A1c (HbA1c), total cholesterol (TC), triglycerides (TG), Low-density lipoprotein (LDL-C) and high-density lipoprotein (HDL-C), BUN, serum creatinine (Scr), as determined by Beckman Coulter Fully Automatic Biochemistry Analyzer.
Serum IGFBP-3 and IGF-1 levels were measured by the chemiluminescence immunometric method (Siemens Healthcare Diagnostics Products Ltd, United Kingdom). Serum creatinine concentration was determined by the Jaffé method. high-density lipoprotein (HDL-C) and Low-density lipoprotein (LDL-C) were measured by the direct method, TG by GPO-POD method, TC by enzymatic method, and fasting blood glucose (FBG) by hexokinase method (Beckman Coulter Fully Automatic Biochemistry Analyzer, Beckman Coulter, Inc, USA). GFR is estimated using the simplified Modification of Diet in Renal Disease (MDRD) formula [13].
Definitions
We used the Optos Daytona Ultra-wide field fundus camera (Scanning Laser Inspection Ophthalmoscope, Optos, Daytona, UK) to image all subjects for assessment of retinopathy. DR was confirmed if the existence of the following retinal lesions: soft exudates, Microaneurysms, hard exudates, intraretinal hemorrhagic dots, intraretinal microvascular abnormality, venous beading, preretinal hemorrhage, neovascularization, or vitreous hemorrhage [14].
In this study, patients with a previous history of hypertension we defined as hypertensive. Diabetic nephropathy was defined according to Mogensen's (1986) criteria, with both microalbuminuria (20 ≤ UAER ≤ 200 μg/min) and clinical albuminuria (UAER > 200 μg/min) indicative of DN [9, 10].
DPN was screened by symptoms and physical examination. In all patients, other causes of neuropathy were excluded. The diagnosis was confirmed by electromyography and/or nerve conduction study.
Statistical analysis
Statistical analysis was performed using the R software (version 4.1.1; https://www.R-project.org). The 706 patients with type 2 diabetes in this study were divided into a training set of 521 participants and a validation set of 185 participants according to the date of data collection for validation, consistent with a theoretical 3:1 ratio [15].
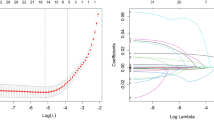
Comparisons of continuous and categorical variables between training and validation sets were performed using the t-test and chi-square test, respectively. Clinical variables were analyzed using univariate logistic regression analysis. According to the research results, the stepwise regression method was used to further screen variables, and select statistically significant indicators to construct a nomogram. The most ‘parsimonious’ was chosen by Akaike's Information Criterion (AIC) in a stepwise algorithm.
We evaluated the model by three aspects including discriminatory ability, accuracy, and clinical utility. To evaluate the predictive power of the model, we calculated the C-index. To reduce the bias, the calibration was conducted by using 1000 bootstrap samples [3]. AUC is the area under the ROC curve, also known as the C statistic, used to assess the discriminatory power of the model. A value of AUC between 0.7 and 0.9 indicates that the model has moderate predictive power and can be combined with clinical practice for predictive assessment [3]. The decision curve analysis takes into account the clinical benefit of the model unlike traditional diagnostic test metrics and assesses the clinical validity of the model by calculating the net benefit based on different threshold probabilities for patients with T2 DM [3].
Results
Participant characteristics.
A total of 706 patients with T2DM included 268 women and 438 men. The data were divided into training set and validation set according to the date of collection. Baseline characteristics such as demographics, physical examination findings, diabetic complication status, and biochemical indices of patients in both groups are shown in Table 1.
Feature selection
The clinical variables of T2DM patients with proteinuria were included in univariate logistic regression. The related factors of DR included age, diabetes duration, hypertension, DPN, DN, eGFR, Scr, BUN, TC, and LDL-C (Table 2). Based on the results of univariate logistics, the risk factors related to the risk of DR in T2DM patients were further screened by stepwise regression according to the principle of the Akaike Information Criterion.
Construction of the nomogram prediction model
The multivariate logistic regression analysis showed the correlation among DPN, hypertension, Diabetes duration, and BUN independent factors for DR in T2DM patients (Table 3). The results of the multivariate logistic regression analysis establish a nomogram model for predicting the risk of DR (Fig. 2). The binary logistic regression results are shown in the forest plot (Fig. 3).
A nomogram for predicting the incidence of DR in T2DM patients. Notes To use the nomogram it is necessary to substitute the variables into the nomogram and draw a vertical line upward for each variable to give the score of each variable, and finally add the scores of the 4 variables to obtain the total score. The probability of DR in type 2 diabetes is obtained by drawing a vertical line down the total score.
Performance of the DR risk nomogram
For the 706 T2DM patients in this study, the calibration curves of both the training set (Fig. 4A) and validation set (Fig. 4A) showed good agreement in predicting DR risk. Based on the nomogram prediction model, the AUC value of the training set (Fig. 5A) was 0.781 (95% CI; 0.741–0.822), and the AUC value of the validation set (Fig. 5B) was 0.865 (95% CI; 0.807–0.923).
Calibration curves for the nomogram. Notes A represents the training set. B represents a validation set. The x-axis represents the predicted probability of DR. The y-axis represents the actual diagnosed DR. The diagonal dotted line represents a perfect prediction by an ideal model. The solid line represents the performance of the nomogram, of which a closer fit to the diagonal dotted line represents a better prediction.
ROC curves for the nomogram. A represents the training set and B represents the validation set. Notes The x-axis represents the false positive rate of the risk prediction, while the y-axis represents the true-positive rate of the risk prediction. The red line displays the performance of the nomogram.
These results suggest that the nomogram can accurately predict the incidence of DR in T2DM patients.
Clinical use
The decision curve of the DR risk nomogram is shown in Fig. 6. According to decision curve analysis, nomograms are clinically beneficial in predicting the risk of DR incidence between a considerable range of threshold probabilities. One patient was randomly selected from the population based on the characteristic indicators selected by the model. Patient indicators were as follows: DPN = yes, BUN = 6.7 mmol/L, Diabetes duration = 5 years, and hypertension = yes. A dynamic nomogram was created to predict the incidence of DR (Fig. 7).
Decision curve analysis for the DR incidence risk nomogram. A represents the training set and B represents the validation set. Notes The y-axis represents the net benefit. The gray slash indicates the hypothesis that all patients have DR, while the black solid line indicates the hypothesis that all patients do not have DR. The blue line represents the risk nomogram.
Dynamic Nomogram. Notes To use the nomogram it is necessary to substitute the variables into the nomogram and draw a vertical line upward for each variable to give the score of each variable, and finally add the scores of the 4 variables to obtain the total score. The probability of DR in type 2 diabetes is obtained by drawing a vertical line down the total score. *** p < 0.001, ** p < 0.01, * p < 0.05
Discussion
DR is one of the most common and serious microvascular complications of diabetes and is the ocular manifestation of end-organ damage in diabetes. Data from 35 diabetes-based studies worldwide show that the overall prevalence of DR is approximately 34.6% worldwide. Blindness due to DR accounts for 2.6% of all blindness cases and is increasing at a rate of 8,000 new cases per year[16]. Despite the great progress in the recognition and treatment of DR, the rising prevalence of diabetes and DR still shows that the task of preventing DR, preventing DR progression, and delaying DR blindness is daunting. Therefore, we developed a diagnostic model based on the risk factors for the occurrence of DR to facilitate early detection of DR.
In the present study, we found that hypertension, BUN, duration of diabetes, and DPN were independently associated with the risk of DR in patients with T2DM. Our findings are consistent with the risk factors for DR that have been reported in the literature. Longer duration of diabetes, higher systolic blood pressure, and elevated BUN levels were found to be independent risk factors for DR in a multicenter study conducted in eight hospitals in China [16]. In a study of risk factors for DR in community-based diabetic patients, Li et al. found that hypertension, duration of diabetes, and DPN were independent risk factors [16]. We can assume that patients with long-term diabetes, higher BUN, DPN, and hypertension were more susceptible to DR.
The duration of diabetes mellitus is an unmodifiable risk factor for DR in many studies [17,18,19]. LALES et al. found that the risk of DR would increase by 8% for each additional year of diabetes duration [20]. A cross-sectional study of risk factors for DR conducted at Nanjing Drum Tower Hospital confirmed that the duration of diabetes was strongly associated with the risk of DR [21]. Similarly, in another study on risk factors for DR in southern China, the authors concluded that the duration of diabetes was one of the most important risk factors for DR [22]. Singh et al. showed that in type 2 diabetes, the prevalence of DR was five times higher in patients with a disease duration of > 15 years compared to those with a disease duration of fewer than 5 years [23]. In the present investigation, the duration of diabetes was significantly correlated with the occurrence of DR in the multiple regression model (P < 0.001), so we also included the duration of diabetes in the prediction model.
Hypertension was found to be a contributing factor to DR in a Chinese community-based study of risk factor screening for microvascular disease [24]. In the present study, we found that hypertension was closely associated with the progression of DR. In a population-based study, it was found that patients with poorly controlled blood pressure had a higher risk of developing DR compared to diabetic patients with well-controlled blood pressure [25]. Additionally, in a long-term follow-up study conducted in the United States, it was found that elevated blood pressure can have long-term damage to small retinal arteries, and this damage accumulates gradually. The authors concluded that hypertension may serve as a long-term marker in response to retinal microangiopathy [26]. In this study, we found a positive association between hypertension and the risk of DR, which is consistent with the findings reported above.
Hypertension, BUN, and course of the disease are important risk factors for T2DM patients, among which the course of diabetes is an unmodifiable risk factor. The results showed that the long course of the disease, the higher BUN, and hypertension was closely related to the incidence rate of DR. We should look at them as a whole when we consider the association in it. Here, these risk factors may be about the element of social and era development. With the continuous development of the social economy, People's lifestyle has changed a lot, so that they may spend more time in the office rather than in the gym [27, 28]. In addition, the pursuit of all kinds of delicious food has reached a crazy degree, which to a certain extent increases the risk of hypertension, but also increases the burden on the kidney. Patients with a longer course of the disease have greater psychological and mental stress. These common factors increase the probability of DR.
A retrospective study in Taiwan found that DPN increased the risk of DR onset and progression [29]. Interestingly, Rasheed et al. found that retinal neurodegenerative changes parallel DPN during diabetes treatment. The authors found a significant association between DR and DPN from a novel perspective assessment [30]. In the present study, we found that DPN was also a risk factor for the development of DR.
It is well known that urea nitrogen is an important component of urea, which is the culprit of chronic kidney disease [31]. Therefore, metabolic indicators of abnormal renal function can also indicate the risk of indirectly related diseases such as eye disease. Elevated levels of circulating urea can directly impair pancreatic beta-cell function, which can lead to disruption of glucose homeostasis and ultimately bring about the development of T2DM and decreased renal function [32]. In addition, Shi et al. noted BUN as a predictor of DR in a columnar line graph study of DR risk, OR: 1.053, 95% CI (1.017–1.091) [33]. Another showed that BUN was a characteristic variable in the presence of DR [16]. Our study showed that high levels of BUN were positively associated with the risk of DR, which is consistent with the results of previous studies.
Our study has many strengths, the parameters used for nomogram construction were derived from clinical practice and could be collected by simple biochemical tests and questionnaires during follow-up. Furthermore, the nomogram does not require the results of an ophthalmic examination, reducing the burden on the patient.
Limitation of this study
This was a cross-sectional study, the patients with DR were observed in the static state, without the monitoring of the dynamic change process. If the patients’ indicators were tested dynamically, the accuracy of the prediction model would be improved to a certain extent. In a Cross-Sectional study, we can predict this. However, this is one of the limitations of our article. Secondly, the research design of this study is a cross-sectional study, which cannot accurately determine the causal relationship between exposure and results, and further prospective studies are needed to confirm the results. Thirdly, since all data in this study were derived from fundus color photography, rather than fundus fluorescein angiography, the DR diagnosis may lack rigor. Furthermore, the patients included in this study are all Chinese, so enlarging the range of the population would be better to increase the representation. Finally, all medical history data and laboratory indicators are obtained from the electronic medical record system, which cannot cover all risk factors for the occurrence of DR, and the accuracy may be biased.
Conclusion
In this study, we developed a nomogram model for patients at high risk of developing DR in type 2 diabetes based on Xinjiang patients with type 2 diabetes. It helps primary care physicians to provide a reference for the screening and early diagnosis of DR.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- DR:
-
Diabetic Retinopathy
- DN:
-
Diabetic nephropathy
- DPN:
-
Diabetic peripheral neuropathy
- ROC:
-
Receiver operating characteristic
- DCA:
-
Decision curve analysis
- BMI:
-
Body mass index
- HbA1c:
-
Glycosylated hemoglobin A1c
- Scr:
-
Serum creatinine
- BUN:
-
Blood urea nitrogen
- LDL-C:
-
Low-density lipoprotein
- HDL-C:
-
High-density lipoprotein
- TC:
-
Total cholesterol; TG: triglycerides
- IGF-1:
-
Insulin-like growth factor-1
- IGFBP-3:
-
Insulin-like growth factor binding protein-3
- FBG:
-
Fasting blood glucose
- OR:
-
Odds ratio
- MDRD:
-
Modification of diet in renal disease
References
Guo Z, Priefer R (2021) Current progress in pharmacogenomics of Type 2 diabetes: a systemic overview[J]. Diabetes Metab Syndr 15(5):102239
Joshi RD, Dhakal CK (2021) Predicting Type 2 Diabetes Using Logistic Regression and Machine Learning Approaches[J]. Int J Environ Res Public Health 18(14):7346
Yoon J, Oh IH, Seo H et al (2016) Disability-adjusted life years for 313 diseases and injuries: the 2012 Korean burden of disease study[J]. J Korean Med Sci 31(Suppl 2):S146–S157
Yau JWY, Rogers SL, Kawasaki R et al (2012) Global prevalence and major risk factors of diabetic retinopathy.[J]. Diabetes care 35(3):556–564
The EDPR (2004) The Prevalence of diabetic retinopathy among adults in the united states[J]. Arch Ophthalmol 122(4):552–563
Pang C, Jia L, Jiang S et al (2012) Determination of diabetic retinopathy prevalence and associated risk factors in Chinese diabetic and pre-diabetic subjects: shanghai diabetic complications study.[J]. Diabetes/metabm Res Rev 28(3):276–283
Xu J, Wei WB, Yuan MX et al (2012) Prevalence and risk factors for diabetic retinopathy: the Beijing communities diabetes study 6.[J]. Retina (Philadelphia, Pa.) 32(2):322–329
Koziol M, Nowak MS, Udziela M et al (2020) First nation-wide study of diabetic retinopathy in Poland in the years 2013–2017[J]. Acta Diabetol 57(10):1255–1264
Jinan BS, Amanda AH, VenkatZhang KM et al (2008) Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: united states, 2005–2050[J]. Archives of Ophthalmology 126(12):1740–1747
Kashif M, Rohit V, Farzana C et al (2011) Severity of diabetic retinopathy and health-related quality of life[J]. Ophthalmology 118(4):649–655
Zhang G, Chen H, Chen W et al (2017) Prevalence and risk factors for diabetic retinopathy in China: a multi-hospital-based cross-sectional study.[J]. British j ophthalmol 101(12):1591–1595
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications Part.1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation[J]. Diabet Med 15(7):539–553
Jalalonmuhali M, Lim SK, Md SMN et al (2017) MDRD vs. CKD-EPI in comparison to 51Chromium EDTA: a cross sectional study of Malaysian CKD cohort.[J]. BMC nephrol 18(1):1–6
Tien Y W, Chui M G C, Michael L, et al. (2016) Diabetic retinopathy.[J]. Nat Rev Disease prim 2(1).
Wei L, Bo X, Shanhu Q et al (2018) Non-lab and semi-lab algorithms for screening undiagnosed diabetes: a cross-sectional study[J]. EBioMedicine 35:307–316
Yin L, Zhang D, Ren Q et al (2020) Prevalence and risk factors of diabetic retinopathy in diabetic patients: a community based cross-sectional study[J]. Med (Baltimore) 99(9):e19236
Tien YW, Ronald K, Amirul FM et al (2005) Diabetic retinopathy in a multi-ethnic cohort in the united states[J]. Am J Ophthalmol 141(3):446–455
Xie XW, Xu L, Wang YX et al (2008) Prevalence and associated factors of diabetic retinopathy The Beijing eye study 2006 [J]. Gra Arch clin exp ophthalmol 246(11):1526
Rajalakshmi R, Amutha A, Ranjani H et al (2014) Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes[J]. J Diabet Complicat 28(3):291–297
Varma R, Macias GL, Torres M et al (2007) Biologic risk factors associated with diabetic retinopathy: the los angeles latino eye study[J]. Ophthalmology 114(7):1332–1340
Sun Q, Jing Y, Zhang B, et al. (2021) The Risk Factors for Diabetic Retinopathy in a Chinese Population: A Cross-Sectional Study[J]. Journal of Diabetes Research 2021.
Cui Y, Zhang M, Zhang L et al (2019) Prevalence and risk factors for diabetic retinopathy in a cross-sectional population-based study from rural southern China: dongguan eye study.[J]. BMJ Open 9(9):e023586
Singh HV, Das S, Deka DC et al (2021) Prevalence of diabetic retinopathy in self-reported diabetics among various ethnic groups and associated risk factors in north-east India: a hospital-based study [J]. Indian J Ophthalmol 69(11):3132
Pang C, Jia L, Hou X et al (2014) The significance of screening for microvascular diseases in Chinese community-based subjects with various metabolic abnormalities.[J]. PloS one 9(5):e97928
Ramachandran R, Anandakumar A, Harish R et al (2014) Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset Type 1 and Type 2 Diabetes[J]. J Diabetes Its Complicati 28(3):291–297
Christy LA, Anna K, Keri LM et al (2012) Impact of long-term measures of glucose and blood pressure on the retinal microvasculature[J]. Atherosclerosis 225(2):412–417
Sergi D, Boulestin H, Campbell FM et al (2021) The role of dietary advanced glycation end products in metabolic dysfunction[J]. Mol Nutr Food Res 65(1):e1900934
Pace LA, Crowe SE (2016) Complex relationships between food, diet, and the microbiome[J]. Gastroenterol Clin North Am 45(2):253–265
Tseng S, Chou S, Low B et al (2015) Risk factors associated with diabetic retinopathy onset and progression in diabetes patients: a Taiwanese cohort study[J]. Int j clin exp med 8(11):21507
Rasheed R, Pillai GS, Kumar H et al (2021) Relationship between diabetic retinopathy and diabetic peripheral neuropathy - neurodegenerative and microvascular changes [J]. Indian j ophthalmol 69(11):3370
Li S, Cui Y, Luo Z et al (2021) Association between blood urea nitrogen and incidence of type 2 diabetes mellitus in a Chinese population: a cohort study.[J]. Endocr j 68(9):1057–1065
Koppe L, Nyam E, Vivot K et al (2016) Urea impairs β cell glycolysis and insulin secretion in chronic kidney disease [J]. J clin invest 126(9):3598–3612
Shi R, Niu Z, Wu B et al (2020) Nomogram for the risk of diabetic nephropathy or diabetic retinopathy among patients with type 2 diabetes mellitus based on questionnaire and biochemical indicators: a cross-sectional study [J]. Diabetes metab syndr obes Targ Ther 13:1215
Funding
This work was supported by the fund project in the state key laboratory of Pathogenesis, Prevention, and Treatment of high incidence diseases in Central Asia. (Name of the fund: The role of TCF7L2/Wnt/GLP-1 signaling pathway and environmental factors in the pathogenesis of type-2 diabetes in Kazakhs (No.SKL-HIDCA-2019–15).
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be. accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this work.
Ethics approval
The study was performed by the ethical guidelines of the 1975 Declaration of Helsinki and was reviewed and approved by the human research ethics committee of the Affiliated Hospital of Xinjiang Medical University.
Informed consent
Written informed consent was waived due to the retrospective nature and low risk of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Jiang, S. Development and validation of a model that predicts the risk of diabetic retinopathy in type 2 diabetes mellitus patients. Acta Diabetol 60, 43–51 (2023). https://doi.org/10.1007/s00592-022-01973-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-022-01973-1