Abstract
Aims
The aim of the study was to construct and validate a risk nomogram for clinically significant macular edema (CSME) prediction in diabetes mellitus (DM) patients using systemic variables.
Methods
In this retrospective study, DM inpatients who underwent routine diabetic retinopathy screening were recruited and divided into training and validation sets according to their admission date. Ninety-three demographic and systemic variables were collected. The least absolute shrinkage and selection operator was used to select the predictive variables from the training set. The selected variables were used to construct the CSME prediction nomogram. Internal and external validations were performed. The C-index, calibration curve and decision curve analysis (DCA) were reported.
Results
A total of 349 patients were divided into the training set (240, 68.77%) and the validation set (109, 31.23%). The presence of diabetic peripheral neuropathy (DPN) symptoms, uric acid, use of insulin only or not for treatment, insulin dosage, urinary protein grade and disease duration were chosen for the nomogram. The C-index of the prediction nomogram was 0.896, 0.878 and 0.837 in the training set, internal validation and external validation, respectively. The calibration curves of the nomogram showed good agreement between the predicted and actual outcomes. DCA demonstrated that the nomogram was clinically useful.
Conclusions
A nomogram with good performance for predicting CSME using systemic variables was developed. It suggested that DPN symptoms and renal function may be crucial risk factors for CSME. Moreover, this nomogram may be a convenient tool for non-ophthalmic specialists to rapidly recognize CSME in patients and to transfer them to ophthalmologists for early diagnosis and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of people with diabetes mellitus (DM) has dramatically increased worldwide in the past few decades, and it is estimated to exceed 693 million in 2045 [1]. Diabetic retinopathy (DR) is one of the severe complications of DM. Diabetic macular edema (DME), characterized by subretinal or intraretinal fluid accumulation in the macula, is the most common form of sight-threatening DR in the global working population. One-fifteenth of DM individuals, that is, more than 20 million people, have been affected by DME [2]. Clinically significant macular edema (CSME) is a term commonly applied to describe DME cases with retinal thickening and adjacent hard exudates. Frequent follow-ups and timely treatments should be adopted in patients with DME, especially in those with CSME [3], because a longer time of exudative fluid accumulation may further impair visual function; this requires physicians to recognize these CSME patients as early as possible during the DM treatment process. It is especially needed in local and rural hospitals without ophthalmologists or retina specialists. Nevertheless, the systemic risk factors for DME or CSME remain unclear and inconsistent in different studies. Vié and colleagues [4] found that obstructive sleep apnoea is associated with DME because of nocturnal hypoxemia. Glitazones, a well-known oral antidiabetic drug (OAD), was found to be closely related to DME risk [5]. Other studies [6, 7] reported dyslipidemia, smoking and high systolic blood pressure as DME or CSME risk factors. Liu [8] suggested that emerging genetic influences should be emphasized in DME. In addition, epigenetic mechanisms including histone modifications, DNA methylation and non-coding RNA regulation may also contribute to the modulation of pathways associated with oxidative stress, inflammation and other biological processes in DR or DME [9]. These studies tell us which parameters might be DME risk factors, but they lack quantitative standards to measure how much these factors may affect a DM patient.
Nomograms are a common tool used to predict the probability of a clinical event in oncology and chronic diseases. It is a pictorial and quantitative visualization of a complex mathematical model. It helps clinical decision-making and fulfils the drive of personalized medicine [10]. Recently, several predictive nomograms have been developed to assess diabetic complications [11,12,13], such as diabetes nephropathy (DN), DR and diabetes foot (DF). These graphic tools have contributed benefit and convenience to diabetic complication evaluations. However, we still lack a convenient and quantitative tool for internal medicine specialists to rapidly recognize CSME in patients and to transfer them to ophthalmologists for early diagnosis and treatment. The reason why we need to emphasize CSME in DR is that CSME can be noticed in different stages of DR. Moreover, patients are likely be vision-threatened when macular fovea is involved and the condition deteriorates.
Therefore, the purpose of this study was to construct and validate a risk nomogram for CSME prediction in DM patients using systemic variables.
Methods
Study design and participants
This retrospective study was approved by the institutional review board of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (SYSEC-KY-KS-2021-263) and was performed in accordance with the tenets of the Declaration of Helsinki. In this study, DM inpatients from the endocrinology department who underwent routine DR screening in the ophthalmology department were recruited from November 2017 to October 2021. The inclusion criteria were a diagnosis of DM according to the 2015 American Diabetes Association (ADA) criteria [14] and a complete record of systemic and ocular variables. The exclusion criteria were as follows: (1) incomplete clinical information; (2) suspected DM individuals were finally diagnosed with impaired glucose tolerance instead of DM: as some individuals were diagnosed with suspected DM when referring to our routine DR screening, while the biochemical indexes of these patients did not reach the standards of DM after thorough examinations; (3) uncontrollable high blood pressure (HBP) (≥ 180/110 mmHg); (4) non-diabetic renal diseases; (5) malignant tumors, tumors that may affect visual function and other severe systemic diseases; (6) corneal or lens opacities affecting imaging: as blurred retinal images may make it difficult to determine whether an individual has DME; (7) high myopia (refractive error > -6 dioptres or axial length > 26 mm); (8) macular edema and retinal diseases caused by non-diabetic diseases; (9) glaucoma, optic neuropathy and other optic neuropathies; and (10) a history of retinal laser photocoagulation or intraocular surgery for glaucoma or retinal diseases.
Patients from November 2017 to December 2019 were assigned to the training set, which was used to construct the model, and patients from January 2020 to October 2021 were assigned to the validation set, which was used to evaluate the model performance.
Collection of clinical data
For the demographic and systemic data, 93 common variables which were routinely tested or recorded in the endocrinology department were collected. These variables included sex, age, height, weight, body mass index, body fat rate, waist circumference, hip circumference, waist-to-hip ratio, basal metabolic rate, disease duration, random blood glucose, glycosylated hemoglobin (HbAlc), fasting blood glucose (GLU0 h), DM type, diabetes treatment (OAD only, insulin only, OAD and insulin; and insulin dosage), presence of HBP, systolic pressure (SP); diastolic pressure, presence of coronary heart disease, phosphocreatine kinase, lactate dehydrogenase, creatine kinase-MB, percutaneous coronary intervention history, stroke history, total cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, apolipoprotein (Apo) A1, Apo B, Apo E, n-terminal pro-brain natriuretic peptide, smoking and alcohol consumption status, presence of diabetic peripheral neuropathy (DPN) symptoms, urea, creatinine (Cre), estimated glomerular filtration rate (eGFR), blood urea nitrogen to creatinine ratio (BUN/Cre), uric acid (UA), cystatin C (Cys C), urinary albumin excretion rate (UAER), the presence of diabetic foot, diabetic ketoacidosis, free triiodothyronine (FT3), free thyroxine, thyroid-stimulating hormone (TSH), anti-thyroid peroxidase, anti-thyroglobulin, alanine aminotransferase, aspartate transaminase, total bilirubin (TBIL), direct bilirubin, indirect bilirubin, γ-glutamyl transpeptidase, alkaline phosphatase, essential elements (K, Na, Cl, Ca, P, Mg), carbon dioxide combining power, β-hydroxybutyrate, prealbumin (PA), total protein, albumin (ALB), GLO (globulin), ALB-to-GLO ratio (ALB/GLO), total bile acid, glycocholic acid, high sensitive C reaction protein, cholinesterase, leucyl aminopeptidase, retinol binding protein (RBP), α- L-fucosidase, serum amylase, serum iron (SIR), unsaturated iron binding force, total iron binding capacity, serum iron saturation (SIS), serum ferritin (SF), transferrin, adenosine deaminase, superoxide dismutase (SOD) and free fatty acids (FFA). BP was measured twice when patients were in resting status, and an average value was calculated. The eGFR was calculated based on the Xiangya equation, which is more suitable for Chinese patients [15].
A rapid bedside neuropathy disability score (NDS) was applied to assess whether these inpatients have DPN symptoms. The NDS include scores were obtained from examinations of pin-prick, vibration and temperature sensations in the great toes and the presence or absence for ankle reflex. For each side, the sensory functions were scored as present (0) or reduced/absent (1), and ankle reflex was scored as normal (0), present with reinforcement (1) or absent (2). Patients with a score of > 2 was considered to have DPN symptoms. Besides, according to the Toronto Diabetic Neuropathy Expert Group [16], DPN symptoms refer to decreased sensation and positive neuropathic sensory dysfunctions (burning, aching, prickling, stabbing or asleep numbness) mainly in the legs, feet and toes. If patients report one or more of these symptoms in their medical records, they will also be considered to have DPN symptoms.
In addition, all patients underwent thorough ophthalmic examinations, including best-corrected visual acuity (BCVA), intraocular pressure (IOP), anterior chamber depth (ACD), axial length (AL), fundus photographs (FP) (Canon, Inc., Tokyo, Japan), dilated-pupil fundus examination with slit-lamp biomicroscopy and indirect ophthalmoscopy, optical coherence tomography angiography (OCTA) and fluorescein fundus angiography (FFA), if necessary. IOP was measured using the Canon TX-20 non-contact tonometer (Canon Inc., Tokyo, Japan). AL and ACD were measured using IOLMaster (Carl Zeiss Meditec, Inc., Dublin, USA). OCT B-scan images, including a retina map image and a cross line image, were obtained using AngioVue software 2.0 of the RTVue XR Avanti device (Optovue, Inc., CA, USA). DR staging was confirmed mainly based on dilated-pupil fundus examination and FP, and FFA were performed if needed. The staging criteria were in accordance with the position statement released by the ADA in 2017 [16]. An eye with a more severe stage was chosen if the severity was different in both eyes, and a random eye was chosen if the severity was the same. The diagnosis of CSME was determined based on the OCT B-scan images and FP [3].
Statistical analysis
The clinical data, including the demographic, systemic and ocular variables, were compared between the training set and the validation set using SPSS 24.0 (SPSS Inc., IL, USA). Independent Student’s t tests were applied for normally distributed data, and chi-squared tests were applied for categorical variables. A p value of < 0.05 was considered to indicate statistical significance.
The model was constructed and validated using R statistical software (version 3.6.3, http://www.R-project.org/). The least absolute shrinkage and selection operator (LASSO) regression algorithm is an advanced machine-learning technique to screen predictive variables and to construct predictive models [17]. In LASSO regression algorithm, the values of data are shrunk toward a central point. A penalty equal to the absolute value of the magnitude of coefficients is added. When some coefficients become zero, they are finally eliminated from the model. The compression of the coefficients of variables helps to avoid the occurrence of model overfitting (including too many variables) using a penalty function. In our study, LASSO regression algorithm which is suitable for small sample sizes and high-dimensional data with collinearity, was used to choose the predictive variables from the training set. Significant and proper variables with nonzero coefficients identified by the LASSO regression model were chosen for binary multivariable logistic regression analysis. The regression coefficients (β), odds ratios (ORs), 95% confidence intervals (CIs) and p values for the chosen variables were reported. Meanwhile, a static nomogram and its corresponding dynamic nomogram were constructed. A calibration curve was plotted to evaluate the calibration of the nomogram, and a C-index was reported to assess the concordance between the predicted and actual outcomes. An internal validation for the nomogram was performed in the training set using 1,000 bootstrapping. An external validation was then performed in the validation set. The calibration curve and C-index for each validation were also provided. Finally, decision curve analysis (DCA) was performed to quantitatively determine the clinical use of the nomogram using net benefits in the training set and the validation set.
Results
Participant characteristics
Among 395 recruited patients, 46 patients were excluded, and a total of 349 patients were finally included in this study. The included patients were divided into the training set (240, 68.77%) and the validation set (109, 31.23%) according to the admission date. The detailed flowchart is shown in Fig. 1.
The clinical features were similar in these two sets. There was no significant difference between the two sets with regard to sex, age or most of the variables. However, some variables (disease duration, eGFR, BUN/Cre, TBIL, GLO, UIBC) in the training set had significantly higher values than those in the validation set, while others (GLU0 h, LDL-C, urea, Cre, FT3, TSH, P, Mg, Cys C, PA, ALB, RBP, SIS, SF, AD, SOD, FFA, AL) in the training set had significantly lower values than those in the validation set. There was a significantly higher proportion of mild or moderate non-proliferative diabetic retinopathy (NPDR) and a significantly lower proportion of severe NPDR in the training set than in the validation set.
In both sets, a longer disease duration, a higher level of insulin dosage, SP, LDH, urea, Cre, UAER, urinary protein grade, Cys C and BCVA (logMAR), a higher proportion of “insulin only” users, the presence of DPN symptoms, HBP, severe NPDR and proliferative diabetic retinopathy (PDR), a lower level of eGFR, IBIL, TP, ALB, ALB/GLO, SIR and SOD and a lower proportion of “OAD only” users were observed in the CSME group when compared to the non-CSME group. Additional significant differences between the CSME and non-CSME groups could also be observed in a single set.
Details are shown in Supplemental Tables 1 (demographic and systemic variables) and 2 (ocular variables).
Clinical feature selection
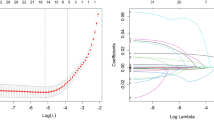
In the LASSO binary logistic regression model, 93 demographic and systemic variables were introduced, and their coefficients were calculated (Fig. 2A). In the cross-validated error plot (Fig. 2B), 9 variables with nonzero coefficients were included at minimum cross-validated error, while only 2 variables with nonzero coefficients were obtained at minimum cross-validated error within 1 standard error of the minimum. To avoid overfitting, 6 variables (presence of DPN symptoms, UA, insulin only or not, insulin dosage, urinary protein grade and HDL-C) were chosen when the λ value was 0.059. However, HDL-C was a controversial variable. Some studies emphasized its role in ameliorating DM, and others argued that the level of HDL-C did not refer to the level of functional HDL-C [18]. In addition, disease duration is usually recognized as a risk factor for DME [19,20,21]. Therefore, the presence of DPN symptoms, UA, insulin only or not, insulin dosage, urinary protein grade and disease duration were finally chosen to construct the prediction model.
Clinical feature selection using the least absolute shrinkage and selection operator (LASSO) binary logistic regression model. A LASSO coefficient profiles of 93 clinical variables. A coefficient profile plot was produced against log(lambda). B The optimal lambda in the LASSO model was validated using tenfold cross-validation. The partial likelihood deviance (binomial deviance) curve was plotted against log(lambda). The left vertical dotted line represents the minimum cross-validated error, and the right vertical dotted line represents the minimum error within 1 standard error of the minimum
Development and validation of the prediction nomogram
The results of the binary multivariable logistic regression analysis among the presence of DPN symptoms, UA, insulin only or not, insulin dosage, urinary protein grade and disease duration are shown in Table 1. A static nomogram for CSME risk prediction was formed and is presented in Fig. 3. Meanwhile, a corresponding dynamic nomogram online application was developed by using the shiny package in R statistical software (Fig. 4) (URL: https://csmeprediction.shinyapps.io/dynnomapp/).
Nomogram for predicting the risk of clinically significant macular edema in diabetes mellitus inpatients. DPN, diabetic peripheral neuropathy; UA, uric acid; CSME, clinically significant macular edema. Draw a straight line upward to the “points” axis from the corresponding value of each variable and obtain points. Locate the total number on the “total points” axis after adding up the points. Afterward, determine the corresponding CSME risk by drawing a straight line down to the “CSME” axis. Units: UA, μmol/L; insulin dosage, IU; disease duration, years
The C-index of the prediction nomogram was 0.896 in the training set and was 0.878 after 1,000 bootstrapping internal validation. The C-index still reached 0.837 in external validation. The calibration curves of the nomogram for CSME also presented good agreement between the predicted and actual outcomes (Fig. 5).
Calibration curves for the clinically significant macular edema (CSME) nomogram in the training set (A) and the validation set (B). The y-axis represents the actual risk of CSME, and the x-axis represents the predicted risk of CSME. The diagonal line (A & B “ideal” line) represents a perfect prediction by an ideal model. A The “apparent” line represents the apparent performance of the nomogram in the training set, while the “bias-corrected” line represents the performance of the nomogram after 1,000 bootstrapping iterations in the training set. B The “nonparametric” line represents the performance of the nomogram in the validation set. A closer fit to the diagonal line meant a better predictive effect
Clinical use of the prediction nomogram
The results of DCA for the nomogram are presented in Fig. 6. According to the DCA, the use of this nomogram for CSME prediction is more accurate than previous schemes when the CSME risk threshold probability was between 1 and 83% in the training set and was generally between 1 and 80% (except 67 70 and 71%) in the validation set.
Decision curve analyses for the clinically significant macular edema (CSME) nomogram in the training set (A) and the validation set (B). The y-axis represents the net benefit, and the x-axis represents the threshold probability. The green line represents the assumption that all patients have CSME, while the black line represents the assumption that all patients have non-CSME. The blue and red lines represent the performance of the nomogram in the training set (A) and the validation set (B), respectively. For example, if the CSME threshold probability of a patient is 50%, the net benefit is 0.111 in the validation set, which means that 11 out of 100 patients may benefit from using this nomogram
Discussion
Most previous studies on DME or CSME simply focus on analyzing the risk factors, while few of them developed a nomogram or validated it [20, 21]. Therefore, the reliability of these risk factors may be uncertain. In our nomogram, the six variables included the presence of DPN symptoms, UA, use of insulin only or not, insulin dosage, urinary protein grade and disease duration. The C-index in the internal validation and in the external validation exceeded 0.85 and 0.8, respectively. The calibration curves of the nomogram yielded good agreement, and the DCA results were also acceptable in the two sets. The model was deemed sufficiently accurate and suitable. To our knowledge, this was the first study to construct and validate a nomogram for CSME risk prediction in DM patients. Although CSME or DME risk nomograms have not been developed before, a handful of DR risk nomograms have been presented in recent studies. In Mo’s research [22], HbA1c contributed the most to DR prediction. Good agreement between the predicted and actual outcomes was achieved, but the C-index and DCA results did not seem as satisfactory as ours. Chen’s study [23] showed that age and HbA1c may be key factors for DR development in a type 2 DM cohort. However, calibration curves and DCA were not further performed. Moreover, an increased arterial BP and the onset of diabetes at approximately age 30 was found to be more related to DME than to PDR in another study [24]. Therefore, risk factors might be different between DR and DME.
The application of LASSO regression in the clinical feature selection helped to avoid overfitting (too many variables selected) and reduce collinearity. In addition, controversial variables were removed, and generally admitted variables were introduced, which made the model more reasonable. Moreover, common variables were used during model construction, making the use of the nomogram widely and easily applicable. Therefore, our study not only provided a clinical-based CSME risk factor analysis but also developed a convenient and quantitative tool for non-ophthalmic specialists to rapidly recognize high-CSME-risk patients so as to assist them in receiving ophthalmic interventions.
It is of particular interest that the presence of DPN symptoms was rather crucial in this model (if the answer was “yes,” approximately 90 points were obtained, while the total point was 400. Zander [19, 25] the relationship between DPN and DME has been relatively lacking in previous studies. Only two studies showed similar results to our study. In an Indian study including 911 participants [25], DPN was found to be significantly associated with center-involved CSME (CI-CSME) and non-center-involved CSME (NCI-CSME). Moreover, the risk of DPN was greater in CI-CSME than in NCI-CSME. More interestingly, Zander and his colleagues [19] found that cardiovascular autonomic and peripheral neuropathy may contribute to DME. These facts suggested that a common and mutual process affecting the autonomic nervous system, the peripheral nervous system and the retinal microvasculature might exist. In the past few years, the concept of a “neurovascular unit” in the retina has been raised [26]. The unit, including neurons, microvasculature, glia and microglia, plays a pivotal role in the process of neurovascular coupling between neuronal activity and blood flow adjustment. Correspondingly, previous OCTA studies [27] showed that the ganglion cell complex (GCC) thickness and vessel density were simultaneously reduced in DR. Moreover, significantly decreased retinal nerve fiber layer and GCC thickness were noticed in DPN patients [28]. Therefore, the “neurovascular unit” in the retina and the relationship between retinal neuropathy and peripheral neuropathy may provide some related evidence to understand the link between DME and DPN. Nevertheless, the relationship between DPN and DME has not been well elucidated, and large-scale prospective clinical trials and animal experiments should be performed in the future.
Another important risk factor for CSME in this model was the level of urinary protein. Similarly, the positive correlation between proteinuria level and DME severity has been illustrated in some studies [29, 30]. Lai’s study revealed that a thicker central subfoveal retinal thickness and a more frequent presence of intraretinal cysts were observed in patients with proteinuria than in those without proteinuria [31]. DME is a result of fluid imbalance. It is generally admitted that increased levels of vascular endothelial growth factor (VEGF) contribute to barrier disruption in the retina and are the main reason for DME [32]. Protein loss and overhydration might also contribute to DME. Obvious protein loss caused by proteinuria induces low oncotic pressure, which may subsequently drive fluid from the intervascular space to the interstitial tissue [33]. Overhydration was common in DN individuals, and furosemide helped patients partially recover from DME in some cases [34]. Therefore, proteinuria and DME may be the consequences of a similar process, and they were mutually related.
For the relationship between UA and DME, a positive correlation between serum UA and CSME risk levels existed in our study. This relationship has been explored in only a handful of previous studies. Our result was supported by Krizova’s and Zhu’s studies Krizova [35, 36]. Krizova et al. [35] found that the levels of serum and vitreous UA in DME patients with type 2 DM were higher than those in the controls. Zhu’s study [36] revealed that a high UA level based on high glucose status accelerated retinal inflammation by regulating the Notch signaling pathway. However, a prospective cohort study performed in Asia showed that a higher level of serum UA was correlated with increased DR risk in males with type 2 DM but not in females [37]. Notably, in another study in Denmark [38], a higher plasma UA level and DR severity were not significantly related after adjustment for diabetes duration. The varied results of these studies may be partially due to the variation in DM type, race, the collection method of UA (plasma or serum) and other factors. Although serum UA was chosen as a predictor in this nomogram, it did not mean that other factors were not important. The levels of urea, Cre, UAER, Cys C and eGFR were also vital renal function indicators. Interestingly, a significantly higher level of urea, Cre, UAER and Cys C and a significantly lower level of eGFR were found in the CSME group in both sets, which jointly suggested that DM patients with reduced renal function may be more vulnerable to CSME.
Insulin use only in DM treatment and a higher insulin dosage were correlated with CSME risk in our study, which was consistent with Wang’s and Acan’s results [21, 39]. It was quite easy to understand the reasons. Individuals taking insulin may have poorer glycemic control, worse islet function and consequently more severe complications. Previous studies Stratton [40, 41] have shown that early and intensive glycemic control decreased DR onset and progression. However, a rapid drop of blood glucose induced by intensive glycemic control has been reported to be related to a worsened DR in a growing number of studies. According to a meta-analysis [42], the DR progression risk increased after 6 to 12 months of intensive insulin therapy (odds ratio: 2.11) when compared to conventional insulin treatments. Nevertheless, the increased DR risk was reversed after 24 months of intensive insulin treatments. Similar results were also shown in various studies Zhao [43, 44]. The phenomenon may be explained by a synergistic hypothesis that high level of exogenous insulin and VEGF induced by ischemic retina may synergistically trigger proliferation of the vessels and the development of DR [45]. The mechanisms deserve further exploration. Regarding disease duration, a generally admitted risk factor for DME was not as pivotal as other factors in the nomogram. Most of the disease durations were self-reported, and many patients may have DM long before they were diagnosed clinically.
In addition to the variables included in the nomogram, a lower level of SOD was noticed in the CSME group in both sets. Oxidative stress is crucial in the presence of diabetic complications, and SOD, as an antioxidant enzyme, plays a pivotal role in oxidative stress. Decreased serum SOD activity was suggested to be related to more severe DR, DN and DPN in Chinese type 2 DM patients [46, 47]. However, there have been no SOD-related studies specific to CSME or DME in the past few years.
There were some limitations in our study. First, the validation set was retrospective and was from the same hospital. Therefore, to realize widespread use of the model, it should also be prospectively validated in different hospitals, different populations of different areas and be adjusted according to practical use in the future work. Second, type 1 and type 2 DM patients were combined for analyses in this study. In our future investigations, to establish CSME nomograms for type 1 and type 2 DM individuals, respectively, will be more precise and help to improve the quality and reliability of the models in different population. Moreover, this was a retrospective hospital-based study, and some bias may have occurred during the process. For instance, DPN symptom assessments were mainly based on daily clinical processes and medical records. A standardized/previously validated questionnaire or quantitative sensory tests were not applied in the assessments. A large-sample well-designed prospective study should be performed in the future if possible.
In conclusion, a nomogram with good performance for predicting CSME using systemic variables was developed in our study. It suggested that DPN symptoms and renal function may be crucial risk factors for CSME. The nomogram may be a convenient tool for non-ophthalmic specialists to rapidly recognize CSME in patients and to transfer them to ophthalmologists for early diagnosis and treatment.
References
Cho NH, Shaw JE, Karuranga S et al (2018) IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281
Tan GS, Cheung N, Simó R et al (2017) Diabetic macular oedema. Lancet Diabetes Endocrinol 5(2):143–155
Flaxel CJ, Adelman RA, Bailey ST et al (2020) Diabetic retinopathy preferred practice pattern®. Ophthalmology 127(1):P66-p145
Vié AL, Kodjikian L, Agard E et al (2019) Evaluation of obstructive sleep apnea syndrome as a risk factor for diabetic macular edema in patients with type II diabetes. Retina 39(2):274–280
Merante D, Menchini F, Truitt KE et al (2010) Diabetic macular edema: correlations with available diabetes therapies–evidence across a qualitative review of published literature from MEDLINE and EMBASE. Drug Saf 33(8):643–652
Das R, Kerr R, Chakravarthy U et al (2015) Dyslipidemia and diabetic macular edema: a systematic review and meta-analysis. Ophthalmology 122(9):1820–1827
Kamoi K, Takeda K, Hashimoto K et al (2013) Identifying risk factors for clinically significant diabetic macula edema in patients with type 2 diabetes mellitus. Curr Diabetes Rev 9(3):209–217
Liu E, Craig JE, Burdon K (2017) Diabetic macular oedema: clinical risk factors and emerging genetic influences. Clin Exp Optom 100(6):569–576
Milluzzo A, Maugeri A, Barchitta M et al (2021) Epigenetic mechanisms in type 2 diabetes retinopathy: a systematic review. Int J Mol Sci 22(19):10502
Balachandran VP, Gonen M, Smith JJ et al (2015) Nomograms in oncology: more than meets the eye. Lancet Oncol 16(4):e173–e180
Shi R, Niu Z, Wu B et al (2020) Nomogram for the risk of diabetic nephropathy or diabetic retinopathy among patients with type 2 diabetes mellitus based on questionnaire and biochemical indicators: a cross-sectional study. Diabetes Metab Syndr Obes 13:1215–1229
Jiang S, Fang J, Yu T et al (2020) Novel model predicts diabetic nephropathy in type 2 diabetes. Am J Nephrol 51(2):130–138
Li M, Yuan T, Li S et al (2021) Nomogram analysis of the influencing factors of diabetic foot in patients with diabetes mellitus. Hormones (Athens) 20(2):333–338
Association AD (2015) Classification and diagnosis of diabetes. Diabetes Care 38(Suppl):S8-s16
Li DY, Yin WJ, Yi YH et al (2019) Development and validation of a more accurate estimating equation for glomerular filtration rate in a Chinese population. Kidney Int 95(3):636–646
Solomon SD, Chew E, Duh EJ et al (2017) Diabetic retinopathy: a position statement by the American diabetes association. Diabetes Care 40(3):412–418
Dai P, Chang W, Xin Z et al (2021) Retrospective study on the influencing factors and prediction of hospitalization expenses for chronic renal failure in china based on random forest and lasso regression. Front Public Health 9:678276
Xepapadaki E, Nikdima I, Sagiadinou EC et al (2021) HDL and type 2 diabetes: the chicken or the egg? Diabetologia 64(9):1917–1926
Zander E, Herfurth S, Bohl B et al (2000) Maculopathy in patients with diabetes mellitus type 1 and type 2: associations with risk factors. Br J Ophthalmol 84(8):871–876
Varma R, Bressler NM, Doan QV et al (2014) Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol 132(11):1334–1340
Acan D, Calan M, Er D et al (2018) The prevalence and systemic risk factors of diabetic macular edema: a cross-sectional study from Turkey. BMC Ophthalmol 18(1):91
Mo R, Shi R, Hu Y et al (2020) Nomogram-based prediction of the risk of diabetic retinopathy: a retrospective study. J Diabetes Res 2020:7261047
Chen X, Xie Q, Zhang X et al (2021) Nomogram prediction model for diabetic retinopathy development in type 2 diabetes mellitus patients: a retrospective cohort study. J Diabetes Res 2021:3825155
Bek T (2020) Systemic risk factors contribute differently to the development of proliferative diabetic retinopathy and clinically significant macular oedema. Diabetologia 63(11):2462–2470
Pradhana D, Priya MNS, Surya J et al (2021) Optical coherence tomography-based prevalence of diabetic macular edema and its associated risk factors in urban south India: a population-based study. Ophthalmic Epidemiol. https://doi.org/10.1080/09286586.2021.1907846
Moran EP, Wang Z, Chen J et al (2016) Neurovascular cross talk in diabetic retinopathy: pathophysiological roles and therapeutic implications. Am J Physiol Heart Circ Physiol 311(3):H738-749
Zhang B, Chou Y, Zhao X et al (2021) Early detection of microvascular impairments with optical coherence tomography angiography in diabetic patients without clinical retinopathy: a meta-analysis. Am J Ophthalmol 222:226–237
Salvi L, Plateroti P, Balducci S et al (2016) Abnormalities of retinal ganglion cell complex at optical coherence tomography in patients with type 2 diabetes: a sign of diabetic polyneuropathy, not retinopathy. J Diabetes Compli 30(3):469–476
Liu ZY, Ma XJ, Liao DY et al (2019) Association of urinary albumin excretion with central foveal thickness and intravitreal conbercept treatment frequency in patients with diabetic macular edema. Int J Ophthalmol 12(10):1598–1604
Yamamoto M, Fujihara K, Ishizawa M et al (2019) Overt proteinuria, moderately reduced egfr and their combination are predictive of severe diabetic retinopathy or diabetic macular edema in diabetes. Invest Ophthalmol Vis Sci 60(7):2685–2689
Lai IP, Huang WL, Yang CM et al (2020) Renal biomarkers for treatment effect of ranibizumab for diabetic macular edema. J Diabetes Res 2020:7239570
Daruich A, Matet A, Moulin A et al (2018) Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res 63:20–68
Klaassen I, Van Noorden CJ, Schlingemann RO (2013) Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 34:19–48
Ciardella AP (2004) Partial resolution of diabetic macular oedema after systemic treatment with furosemide. Br J Ophthalmol 88(9):1224–1225
Krizova L, Kalousova M, Kubena AA et al (2015) Correlation of vitreous vascular endothelial growth factor and uric acid concentration using optical coherence tomography in diabetic macular edema. J Ophthalmol 2015:478509
Zhu DD, Wang YZ, Zou C et al (2018) The role of uric acid in the pathogenesis of diabetic retinopathy based on notch pathway. Biochem Biophys Res Commun 503(2):921–929
Kuwata H, Okamura S, Hayashino Y et al (2017) Serum uric acid levels are associated with increased risk of newly developed diabetic retinopathy among Japanese male patients with type 2 diabetes: A prospective cohort study (diabetes distress and care registry at Tenri [DDCRT 13]). Diabetes Metab Res Rev 33(7):e2905
Pilemann-Lyberg S, Hansen TW, Persson F et al (2019) Uric acid is not associated with diabetic nephropathy and other complications in type 1 diabetes. Nephrol Dial Transplant 34(4):659–666
Wang Y, Lin Z, Zhai G et al (2020) Prevalence of and risk factors for diabetic retinopathy and diabetic macular edema in patients with early and late onset diabetes mellitus. Ophthalmic Res. https://doi.org/10.1159/000508335
Stratton IM, Adler AI, Neil HA et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321(7258):405–412
Nathan DM, Genuth S, Lachin J et al (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329(14):977–986
Wang PH, Lau J, Chalmers TC (1994) Metaanalysis of the effects of intensive glycemic control on late complications of type I diabetes mellitus. RETINA 14(3):287–288
Zhao C, Wang W, Xu D et al (2014) Insulin and risk of diabetic retinopathy in patients with type 2 diabetes mellitus: data from a meta-analysis of seven cohort studies. Diagn Pathol 9:130
Zhang J, Ma J, Zhou N et al (2015) Insulin use and risk of diabetic macular edema in diabetes mellitus: a systemic review and meta-analysis of observational studies. Med Sci Monit 21:929–936
Jingi AM, Tankeu AT, Ateba NA et al (2017) Mechanism of worsening diabetic retinopathy with rapid lowering of blood glucose: the synergistic hypothesis. BMC Endocr Disord 17(1):63
Fei Z, Gao W, Xu X et al (2021) Serum superoxide dismutase activity: a sensitive, convenient, and economical indicator associated with the prevalence of chronic type 2 diabetic complications, especially in men. Free Radic Res 55(3):275–281
Zhao JS, Jin HX, Gao JL et al (2018) serum extracellular superoxide dismutase is associated with diabetic retinopathy stage in chinese patients with type 2 diabetes mellitus. Dis Markers 2018:8721379
Acknowledgements
The research was funded by Science and Technology Projects of Guangzhou, China (3030901006062), and Project of Administration of Traditional Chinese Medicine of Guangdong Province, China (20221077).
Funding
The research was funded by Science and Technology Projects of Guangzhou, China (3030901006062) and Project of Administration of Traditional Chinese Medicine of Guangdong Province, China (20221077).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Deng, X., Zhou, L. et al. Nomogram-based prediction of clinically significant macular edema in diabetes mellitus patients. Acta Diabetol 59, 1179–1188 (2022). https://doi.org/10.1007/s00592-022-01901-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-022-01901-3