Abstract
Aims
To investigate whether intravitreal conbercept injection affects contralateral untreated eyes in bilateral diabetic macular edema (DME) patients.
Methods
In this retrospective study, 15 patients (30 eyes) with type 2 diabetes were followed after bilateral DME diagnosis in the Department of Ophthalmology, Peking Union Medical College Hospital from 2015 to 2018. Patients underwent examinations including best corrected visual acuity (BCVA), slit-lamp microscopy, indirect ophthalmoscope, color fundus photography, fundus fluorescein angiography, optical coherence tomography, and glycated hemoglobin (HbA1c). Each patient received conbercept (0.5 mg) intravitreally in the severe eye. Nonparametric Wilcoxon signed-rank tests and Pearson’s correlation coefficient were used to assess changes in BCVA and central retinal thickness (CRT) and relations between BCVA changes in treated and untreated eyes, respectively.
Results
The mean follow-up time was 10.60 ± 2.29 months, and the mean injection number of 15 treated eyes was 9.13 ± 0.68. HbA1c remained below 10% during treatment with no significant changes between the initial and final visits (7.81 ± 1.17 vs 7.62 ± 1.19%) (P = 0.576). In untreated eyes, CRT significantly decreased from the initial to final visits (368.93 ± 125.45 vs 306.27 ± 89.70 μm) (P = 0.028). In untreated eyes, BCVA showed no significant difference between the initial and final visits (0.38 ± 0.30 vs 0.40 ± 0.30 logMAR) (P = 0.937), but BCVA changes in treated and untreated eyes were positively correlated (r = 0.527, P = 0.044).
Conclusions
Intravitreal conbercept injection results in decreased CRT and increased BCVA in untreated eyes, which is consistent with the changes in treated eyes for patients with bilateral DME.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic macular edema (DME) is a major cause of vision loss in patients with diabetes and can occur at any stage of diabetic retinopathy [1]. The main treatment modalities include laser therapy, intravitreal injections of steroids, and vascular endothelial growth factor (VEGF) inhibitors. According to multiple clinical studies [2,3,4,5,6,7,8], intraocular anti-VEGF therapy promotes a rapid alleviation of macular edema and sustained improvement in visual acuity in patients with DME. However, the results of the studies and reports in the literature indicate that only intravitreal bevacizumab injection promotes a significant response in the fellow eye. Conbercept (KH902; Chengdu Kanghong Biotech Co., Sichuan, China), a novel anti-VEGF reagent, is a fully humanized, soluble, VEGF-receptor protein. The most notable characteristic of conbercept is that it binds not only to VEGF-A but also to VEGF-B and placental growth factor, all with high affinity. The present study was performed to evaluate the change in best corrected visual acuity (BCVA) and central retinal thickness (CRT) in untreated eyes following unilateral intravitreal administration of conbercept in patients with bilateral DME.
Materials and methods
Patients
This study was retrospective in nature. Fifteen consecutive patients with type 2 diabetes mellitus were diagnosed at the Department of Endocrinology, Peking Union Medical College (PUMC) Hospital, and all cases with a final diagnosis of nonproliferative diabetic retinopathy (NPDR) and center-involving DME were diagnosed at the Department of Ophthalmology. Data were collected between May 2015 and September 2018. The study was approved by the hospital’s institutional review board and was conducted in accordance with the tenets of the Declaration of Helsinki. The inclusion criteria were as follows: (1) aged 18 or older; (2) glycated hemoglobin (HbA1c) ≤ 10%; and (3) fellow eye CRT ≥ 250 μm. The exclusion criteria were as follows: (1) received intravitreal dexamethasone implant within 6 months, intravitreal injection of steroids (e.g., triamcinolone acetonide) within 3 months or periocular injection of steroids within 1 month; (2) received panphotocoagulation or macular laser within 3 months; (3) received intravitreal injection of any VEGF antagonists within 6 months; (4) a history of vitrectomy or other treatments targeting the macula (e.g., photodynamic therapy); (5) existence of active infection or proliferative diabetic retinopathy; (6) existence of ocular diseases (e.g., retinal vein occlusion, choroidal neovascularization, retinal detachment, macular hole, epiretinal membrane, and vitreomacular traction) causing macular edema or visual damage; (7) coexisting severe cataract that may influence the evaluation of examination results; and (8) existence of severe systemic diseases.
Ophthalmic examinations and laboratory assessments
At the initial and final visits, patients underwent comprehensive ophthalmic examinations, including measurement of BCVA measurement with a Snellen chart, intraocular pressure evaluations by noncontact tonometry, anterior segment slit-lamp examination, fundus examination, color fundus photography, fluorescein angiography, and CRT measurement by spectral-domain optical coherence tomography (Spectralis, Heidelberg, Germany). HbA1c levels were also detected to monitor glycemic control over a 3-month period.
Therapeutic regimen and intravitreal injection protocol
Intravitreal injections were performed in the eye exhibiting more severe macular edema in 15 patients, and they were treated with a single injection of conbercept (0.5 mg/0.05 mL), followed by treatment as needed with monthly assessments. Eyes were retreated if deemed necessary depending on changes in BCVA and CRT. The interval of injections was a minimum of 4 weeks. All patients were informed of the potential side effects and complications of intravitreal drug therapy and signed an informed consent form prior to each intraocular injection.
The intravitreal injection procedure was carried out under the aseptic principle in accordance with intraocular surgery in the ophthalmic operating room. Patients were instructed to apply levofloxacin eye drops to both eyes four times a day for 3 days before the injection. Oxybuprocaine hydrochloride eye drops were administered three times prior to the injection, followed by the application of 5% povidone-iodine in the conjunctival sac that was washed 30 s later. A 30-gauge needle was inserted 4 mm posterior to the superior or inferior temporal limbus through the pars plana; then, conbercept 0.5 mg (0.05 mL) was injected. Ofloxacin eye ointment was administered, and sterile gauze was applied after confirming that intraocular pressure was normal. All patients returned to the ophthalmic outpatient facility for follow-up 1 day after the surgery and were instructed to apply levofloxacin eye drops to both eyes four times a day for 7 days.
Statistical analysis
Statistical analyses were conducted using SPSS statistical software version 23.0 (IBM, Armonk, NY, USA). BCVA scores were converted to the logarithm of the minimum angle of resolution (logMAR) equivalents. All data are presented as the mean ± standard deviation (SD). A nonparametric Wilcoxon signed-rank test was performed to compare BCVA and CRT between treated eyes and untreated eyes and to compare those parameters between the initial and final visits. The relation between BCVA changes in treated eyes and untreated eyes was assessed by Pearson’s correlation coefficient. The differences in data are reported with 95% confidence intervals (CIs). A two-tailed P value ≤ 0.05 was considered statistically significant for all analyses.
Results
Demographics and clinical characteristics
Fifteen patients (30 eyes) with bilateral DME were included in the study. Of these patients, ten were male and five were female. Their mean age was 59.40 ± 4.88 years (range, 51–68 years). The mean follow-up time was 10.60 ± 2.29 months (range, 5–12 months), and the mean number of conbercept injections during follow-up was 9.13 ± 0.68 injections (range, 5–12 injections). HbA1c was controlled below 10% during treatment, and there was no significant change between the initial and final visits (7.81 ± 1.17 vs 7.62 ± 1.19%) (P = 0.576).
The anterior segment of 30 eyes was normal. Fundus examinations revealed numerous microangiomas and flake hemorrhages, as well as soft and hard exudations. FFA showed that the capillary nonperfusion area was greater than 4 papillary diameters, and the late phase showed a macular area of hyperfluorescence due to leakage. OCT showed retinal edema and thickening in the macular area.
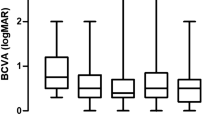
Comparison of BCVA and CRT between treated and untreated eyes at the initial visit
The mean BCVAs of treated and untreated eyes were 0.59 ± 0.31 logMAR and 0.38 ± 0.30 logMAR, respectively (Table 1). Eleven treated eyes (73%) and nine untreated eyes (60%) showed a BCVA > 0.3 logMAR by comparison. Comparing the BCVA of treated eyes with that of untreated eyes revealed no significant difference (P = 0.107) (Table 1, Figs. 1, 2). However, notably, there was a tendency for a better BCVA in untreated eyes than in treated eyes. In addition, the mean CRT of treated and untreated eyes was 470.60 ± 192.63 μm and 368.93 ± 125.45 μm, respectively (Table 1). A CRT > 450 μm was observed in 7 treated eyes (47%) and 4 untreated eyes (27%), and CRT was significantly different between untreated and treated eyes (P = 0.033) (Table 1, Figs. 1, 2).
Comparison of BCVA and CRT between the initial and final visits in treated eyes
The final BCVA of treated eyes was 0.47 ± 0.26 logMAR. Compared with the initial BCVA (P = 0.286), visual acuity improved in 8 eyes (53%), remained unchanged in 3 eyes (20%), and decreased in 4 eyes (27%) (Table 1, Fig. 1). The mean CRT of treated eyes was 470.60 ± 192.63 μm at the initial visit and was reduced to 325.20 ± 156.76 μm at the final visit; CRT decreased in 11 eyes (74%), remained unchanged in 2 eyes (13%), and increased in 2 eyes (13%). CRT was significantly different between the initial and final visits (P = 0.016) (Table 1, Fig. 2).
Comparison of BCVA and CRT in untreated eyes between the initial and final visits
The final BCVA of untreated eyes was 0.40 ± 0.30 logMAR. Compared with the initial BCVA (P = 0.937), visual acuity improved in 7 eyes (47%), remained unchanged in 3 eyes (20%), and decreased in 5 eyes (33%) (Table 1, Fig. 1). In untreated eyes, the mean CRT was 368.93 ± 125.45 μm at the initial visit and was reduced to 306.27 ± 89.70 μm at the final visit; the CRT decreased in 8 eyes (54%), remained unchanged in 5 eyes (33%), and increased in 2 eyes (13%). There was a significant difference in CRT between the initial and final visits (P = 0.028) (Table 1, Fig. 2).
Among two untreated eyes with CRT decrease exceeding 150 μm, CRT significantly decreased by 455 μm in one eye. The initial logMAR BCVA of the treated eye and untreated eye was 0.6 and 0.5, respectively. Bilateral fundus presented severe hemorrhages, microaneurysms, hard exudates, and cotton wool spots. The CRT was 938 μm in the treated eye and 647 μm in the untreated eye, and for the FFA, the capillary nonperfusion area was greater than 4 papillary diameters. After treatment with nine intravitreal injections of conbercept (0.5 mg/0.05 mL), the final logMAR BCVA of treated and untreated eyes was improved to 0.3 and 0.2, respectively. The fundus hemorrhages, hard exudates, and cotton wool spots had been absorbed, and the final CRT of treated and untreated eyes was significantly decreased to 168 μm and 192 μm, respectively (Fig. 3).
The bilateral eye of 51-year-old male patient with nonproliferative diabetic retinopathy (NPDR) stage III and diabetic macula edema (DME). Nine intravitreal injections of conbercept were administered to the right eye. a, c Fundus photographs of treated and untreated eyes at the initial visit show severe hemorrhages, microaneurysms, hard exudates, and cotton wool spots. b, d SD-OCT reveals significant macular edema in both eyes. The CRT was 938 μm in the right eye and 647 μm in the left eye. c, d Fundus photographs of treated and untreated eyes at the final visit show that fundus hemorrhages, hard exudates, and cotton wool spots had been absorbed. g, h SD-OCT reveals alleviated macular edema and significantly decreased CRT in both eyes. The final CRT was 168 μm in the right eye and 192 μm in the left eye
Comparison of BCVA and CRT between treated and untreated eyes at the final visit
The final CRT of treated and untreated eyes was significantly decreased, and the final increased or unchanged BCVA of treated and untreated eyes was 73% and 67%, respectively. There was no significant difference between untreated and treated eyes in BCVA and CRT (P = 0.396, 0.394) (Table 1).
Relation between BCVA changes in treated eyes and untreated eyes
Statistical analysis showed a positive correlation between BCVA changes in treated eyes and untreated eyes (r = 0.527, P = 0.044). A positive correlation between treated and untreated eyes was noted in 10 eyes (67%). Among these eyes, visual acuity improved in 5, remained unchanged in 2 and decreased in 3 (Table 2).
Discussion
VEGF is considered a key factor in the pathogenesis of DME, which impairs the blood–retinal barrier and induces extracellular fluid accumulation in the macular region [9]. VEGF inhibitors are effective in reducing retinal thickness and improving visual acuity by reducing the VEGF level of vitreous humor and retinal vascular leakage, which have become the first-line treatment of DME [10]. Several studies have investigated the efficiency of unilateral intravitreal bevacizumab injections in fellow eyes in DME [11, 12]. In this paper, 15 patients with bilateral DME who received unilateral conbercept injections were included, and analyses were performed to compare the change in BCVA and CRT between the initial and the final visits and the difference between treated and untreated eyes. A significant decrease in CRT in untreated eyes was found, and increased and unchanged BCVA accounted for a high proportion of eyes (67%) after treatment.
Bakbak et al. performed a study on 55 patients with bilateral DME who received unilateral intravitreal bevacizumab injections and found that CRT decreased from 417 to 372 μm after treatment in 4 weeks [11]. Hanhart et al. also found a decrease in CRT by 49 μm after treatment in 8 months through a study of unilateral intravitreal administration of bevacizumab in 35 patients [12]. In addition, the significant decrease in CRT in untreated eyes after intravitreal injection of anti-VEGF was found in cases of bilateral macular edema caused by other ocular diseases with blood–retinal barrier destruction, such as uveitis and retinal vein occlusion [13, 14]. In this study, the CRT of untreated eyes significantly decreased from 368.93 ± 125.45 to 306.27 ± 89.70 μm (P = 0.028); among these eyes, 13 (87%) had decreased and unchanged CRT. This effect is facilitated by the escape of conbercept to the systemic circulation; this escape may be enhanced by the breakdown of the blood–retinal barrier in eyes with all types of retinal diseases. Avery et al. reported on systemic exposure to ranibizumab, aflibercept, and bevacizumab, along with associated reduced free VEGF levels, following intravitreal administration in 56 consecutive patients with neovascular age-related macular degeneration [15]. Similarly, significantly reduced levels of systemic VEGF were also reported after the intravitreal injection of aflibercept and bevacizumab in 14 infants with retinopathy of prematurity [16]. Conbercept and aflibercept are fusion protein VEGF inhibitors. Therefore, it was speculated that an impaired blood–retinal barrier function in patients with diabetes may alter penetrance to the fellow eye. Bakbak et al. found that even although anatomic findings were improved, changes in visual acuity did not reach significance after treatment with bevacizumab [11]. Consistent with the previous study, although we found increased and unchanged BCVA in approximately 67% of eyes, the change in BCVA did not reach significance between the measurements made at the initial and the final visits. This lack of significance may be related to photoreceptor damage due to longstanding macular edema.
Nevertheless, other studies have provided conflicting data on the effect of anti-VEGF treatment on fellow eyes. In animal experiments, Bakri et al. reported on the pharmacokinetics of intravitreal bevacizumab and ranibizumab in a rabbit model [17, 18]. These authors found that a low concentration of bevacizumab enters systemic circulation and can be detected 4 weeks later in the aqueous humor of untreated eyes in contrast to intravitreal ranibizumab, which was not detected in untreated eyes at follow-up. Furthermore, according to Dias et al., molecular weight is a determining factor for ocular pharmacokinetics [19]. The rate of diffusion is approximately inversely proportional to the cube root of the molecular weight; therefore, high molecular weights are thought to prolong vitreous half-life. In the present study, we used conbercept, a humanized fusion protein with a higher molecular weight (149 kD) than that of ranibizumab (48 kD), which is a monoclonal antibody. Therefore, we speculate that conbercept has a long half-life, which promotes its effect on untreated eyes. In addition, Hanhart et al. noted that the effect of bevacizumab on untreated eyes is due to the fragment crystallizable portion and the active transport of the compound into the systemic circulation [12]. Conbercept includes the fragment crystallizable region of the IgG1 immunoglobulin, which may also promote its exposure to systemic circulation. However, it may increase the risk of systemic serious adverse events when patients have the highest exposure to intravitreal anti-VEGF. In the present study, we found no arteriothrombotic events in our patients, which may be due to the small number of samples. In addition, our study excluded patients with severe systemic diseases and strictly controlled the blood glucose of patients during the treatment, which was also conducive to reducing the risk of systemic adverse events. Nevertheless, in clinical trials, Mentoya et al. failed to identify a contralateral eye effect in a prospective study of 23 patients with bilateral DME who received unilateral intravitreal bevacizumab followed by 4 weeks of follow-up [20]. Similarly, Meyer et al. reported undetectable levels of unbound bevacizumab in the aqueous humor of untreated eyes following one injection of bevacizumab [21]. Several factors may be responsible for these contradictory findings, including the short-term design of the studies and a small number of injections. At present, many studies have shown that patients with DME should receive the initial intensive treatment of 5 intravitreal anti-VEGF injections at an early stage to achieve good therapeutic effects [22,23,24,25]. Thus, our longer follow-up time (mean 10.60 ± 2.29 months) and larger number of injections (5–12 injections, mean 9.13 ± 0.68 injections) facilitate the detection of the effect on untreated eyes.
In addition, several studies have indicated an association between the level of blood glucose and ocular complications [26, 27]. In this study, the HbA1c of the patients was 7.81 ± 1.17% at the initial visit and 7.62 ± 1.19% at the final visit and was controlled below 10% during treatment. Regular ophthalmic examinations and systemic blood glucose control during treatment are important factors in alleviating bilateral DME and even diabetic retinopathy (DR).
One limitation is the lack of a larger group. The small confidence provided by this small group makes it impossible to draw concrete conclusions regarding whether there is an effect in the untreated eye. To have a 95% confidence level, the study group should be at least doubled. Only then can the changes observed in the study be truly significant. Another major limitation is the retrospective nature. Therefore, prospective and multicenter studies are needed to address these limitations.
Although a successive anti-VEGF treatment for binocular DME patients was not provided in this study, the existence of a clinical meaningful effect on both eyes was detected. In addition, there are several advantages in treating one eye rather than both, among them being reduced discomfort associated with intravitreal injection, reduced risk for ocular complication, and reduced cost. Potential dose-dependent, drug-related systemic adverse events may also be reduced.
Conclusions
In conclusion, this study indicates that the intravitreal injection of conbercept resulted in decreased CRT and increased BCVA in the untreated eye, which was consistent with the changes in treated eyes, in patients with bilateral DME. However, a large number of patients is required before more specific conclusions can be drawn. In addition, long-term systemic blood glucose management in patients with diabetes is essential to control the progress of DME and DR.
References
Ting DS, Cheung GC, Wong TY (2016) Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol 44:260–277
Korobelnik JF, Do DV, Schmidt-Erfurth U et al (2014) Intravitreal aflibercept for diabetic macular edema. Ophthalmology 121:2247–2254
Brown DM, Schmidt-Erfurth U, Do DV et al (2015) Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 122:2044–2052
Staurenghi G, Feltgen N, Arnold JJ et al (2018) Impact of baseline diabetic retinopathy severity scale scores on visual outcomes in the VIVID-DME and VISTA-DME studies. Br J Ophthalmol 102:954–958
Laiginhas R, Silva MI, Falcão MS (2018) Reply to the letter to the editor: aflibercept in diabetic macular edema refractory to previous bevacizumab: outcomes and predictors of success. Graefes Arch Clin Exp Ophthalmol 256:1355–1356
Herbaut A, Fajnkuchen F, Qu-Knafo L et al (2017) Switching to aflibercept in diabetic macular edema not responding to ranibizumab and / or intravitreal dexamethasone implant. J Ophthalmol 2017:8035013
Li F, Zhang Le, Wang Y et al (2018) One-year outcome of combercept therapy for diabetic macular edema. Curr Eye Res 43:218–223
Funatsu H, Yamashita H, Nakamura S et al (2006) Vitreous levels of pigment epithelial-derived factor and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology 113:294–301
Wells JA, Glassman AR, Ayala AR et al (2015) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372:1193–1203
Xu X, Lu Y (2018) Focusing on the diagnosis and treatment of diabetic macular edema. Chin J Ocul Fundus Dis 34:313–316
Bakbak B, Ozturk BT, Gonul S et al (2013) Comparison of the effect of unilateral intravitreal bevacizumab and ranibizumab injection on diabetic macular edema of the fellow eye. J Ocul Pharmacol Ther 29:728–732
Hanhart J, Tiosano L, Averbukn E et al (2014) Fellow eye effect of unilateral intavitreal bevacizumab injection in eyes with diabetic macular edema. Eye 28:646–653
Acharya NR, Sittivarakul W, Qian Y et al (2011) Bilateral effect of unilateral ranibizumab in patients with patients with uveitis-related macular edema. Retina 31:1871–1876
Zq Wu, Sadda SR (2018) Effects on the contralateral eye after intravitreal bevacizumab and ranibizumab injections: a case report. Ann Acad Med Singap 37:591–593
Avery RL, Castellarin AA, Steinleetal NC et al (2014) Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 98:1636–1641
Huang CY, Lien R, Wangetal NK et al (2018) Changes in systemic vascular endothelial growth factor levels after intravitreal injection of aflibercept in infants with retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 256:479–487
Bakri SJ, Snyder MR, Reid JM et al (2007) Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 114:855–859
Bakri SJ, Snyder MR, Reid JM et al (2007) Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 114:2179–2182
Dias CS, Mitra AK (2000) Vitreal elimination kinetics of large molecular weight FITC-labeled dextrans in albino rabbits using a novel microsampling technique. J Pharm Sci 89:572–578
Montoya RV, Guerra JF, Burgos O et al (2009) The effect of unilateral intravitreal bevacizumab (Avastin), in the treatment of diffuse bilateral diabetic macular edema a polot study. Retina 29:20–26
Meyer CH, Krohne TU, Holz FG (2012) Concentrations of unbound bevacizumab in the aqueous of untreated fellow eyes after single intravitreal injection in humans. Acta Ophthalmol 90:68–70
Brown DM, Nguyen QD, Marcus DM et al (2013) Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 120:2013–2022
Heier JS, Korobelnik JF, Brown DM et al (2016) Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology 123:2376–2385
Diabetic Retinopathy Clinical Retinopathy Network (2018) Protocol T: a comparative effectiveness study of intravitreal aflibercept, bevacizumab and ranibizumab for diabetic macular edema [EB/OL]. https://drcrnet.jaeb.org. 2018-10-18/2019-4-30. Accessed 30 Apr 2019
Diabetic Retinopathy Clinical Retinopathy Network (2013) Protocol I: intravitreal ranibizumab or triamcinolone acetonide in combination with laser photocoagulation for diabetic macular edema [EB/OL]. https://drcrnet.jaeb.org. 2013-12-31/2019-4-30. Accessed 30 Apr 2019
Hammes HP, Welp R, Kempe HP et al (2015) Risk factors for retinopathy and DME in type 2 diabetes-results from the German/Austrian DPV Database. PLoS ONE 10:e0132492
Yau JW, Rogers SL, Kawasaki R (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564
Author information
Authors and Affiliations
Contributions
YD and ZL carried out the entire procedure including the collection of medical records, statistical analysis, drafting the manuscript, and manuscript revision. JY conceived of the study, coordinated and participated in the entire process of drafting, and revised the manuscript. LL contributed to manuscript revision. All authors read and approved the final manuscript. BL and RY contribute to the collection of medical records and statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
All authors have contributed significantly and agree with the content of the manuscript. All authors have no relevant financial relationships to disclose.
Ethical approval
The study was approved by the hospital’s institutional review board and was conducted in accordance with the tenets of the Declaration of Helsinki. The reference number for the ethics approval is NO. S-K848.
Informed consent
The authors ensured that patients’ privacy is well protected in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques.
Rights and permissions
About this article
Cite this article
Di, Y., Li, Z., Ye, J. et al. The fellow eye effect of unilateral intravitreal conbercept injections in eyes with diabetic macular edema. Acta Diabetol 57, 1001–1007 (2020). https://doi.org/10.1007/s00592-020-01511-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01511-x