Abstract
Background
To investigate the levels of VEGF in the systemic circulation of patients with type 1 ROP who received intravitreal injections of 1 mg (0.025 mL) aflibercept (IVA) or 0.625 mg (0.025 mL) bevacizumab (IVB).
Methods
Patients who had type 1 ROP and received either IVA or IVB were enrolled in this prospective study. Serum and plasma samples were collected prior to and up to 12 weeks after IVB or IVA treatment. The serum and plasma VEGF levels were measured using enzyme-linked immunosorbent assays (ELISAs), and the platelet levels in the blood were also quantified. The serum and plasma levels of VEGF, as well as the ratio of VEGF to platelet count (VEGF/PLT) were measured prior to and up to 12 weeks after anti-VEGF treatment.
Results
In total, 14 patients with type 1 ROP were enrolled in this study; five patients received IVA, and nine patients received IVB. Following either IVA or IVB treatment, all the eyes (100%) showed complete resolution of ROP-induced abnormal neovascularization and presented continued vascularization toward the peripheral retina. Compared to baseline, the serum VEGF levels were significantly reduced in the ROP patients up to 12 weeks after either IVA or IVB treatments (all P < 0.05). At 2, 4, and 8 weeks after intravitreal injection, the serum VEGF levels were more suppressed in the IVB group than in the IVA group (P = 0.039, P = 0.004, and P = 0.003, respectively). The serum VEGF/PLT ratio after IVA or IVB showed similar reductions and trends as the serum VEGF data. Changes in the plasma VEGF levels could not be properly assessed because some of the samples had VEGF levels below the detection limit of the ELISA.
Conclusions
Serum VEGF levels and the VEGF/PLT ratio in patients with type 1 ROP were suppressed for 3 months after treatment with either IVA or IVB, but the suppression of systemic VEGF was more pronounced in patients treated with IVB than those treated with IVA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP) is a leading cause of childhood blindness. As vascular endothelial growth factor (VEGF) has been identified as a primary pathological growth factor that mediates the process of neovascularization, intravitreal injection of VEGF inhibitors have been used in patients with Stage 3+ or Type 1 ROP [1, 2]. VEGF inhibitors, including bevacizumab (Avastin; Genentech Inc., South San Francisco, CA, USA) and ranibizumab (Lucentis; Genentech Inc.), are commonly used in patients with ROP, and most patients experienced resolution of their abnormal neovascularization due to ROP after a single treatment [3, 4].
Aflibercept (Eylea; Regeneron Pharmaceuticals, Tarrytown, NY, USA), the latest VEGF antagonist, has been approved by the US Food and Drug Administration for intraocular use for the treatment of some ocular neovascular diseases in adults and has gained popularity in the treatment of vascular disorders, including age-related macular degeneration (AMD), retinal vascular occlusion and diabetic macular edema [5,6,7]. In contrast to the antibody-based VEGF binding strategy employed by bevacizumab and ranibizumab, aflibercept is a fusion protein consisting of the binding domains of VEGF receptor-1 and VEGF receptor-2 with the ability to bind VEGF-A,VEGF-B, and placental growth factor [8, 9]. However, the clinical application and efficacy of intravitreal injection of aflibercept (IVA) in patients with ROP were seldom reported [8, 10].
Our previous study revealed that bevacizumab was detected in the systemic circulation 1 day after intravitreal injection of bevacizumab (IVB) and that serum VEGF levels were suppressed for 2 months [11]. We also compared the serum VEGF levels after intravitreal injection of bevacizumab or ranibizumab in patients with ROP and observed that VEGF levels were significantly decreased in the patients who received bevacizumab treatment than those who received ranibizumab treatment [12]. Similar reports revealed that aflibercept significantly decreased serum and plasma VEGF levels 1 month after injection in patients with AMD [13]. However, the mechanism by which aflibercept affects serum VEGF levels in patients of ROP remains unclear. Because VEGF is considered an important neurodevelopmental growth factor during the early newborn period, determining the safety of the anti-VEGF treatments in patients with ROP is critical [4, 14, 15].
VEGF levels in both serum and plasma samples have been used in clinical studies. However, which sample is more closely correlated to clinical disorders remains uncertain. Plasma has been suggested to be a more accurate assessment of circulating VEGF because it eliminates the influence of platelets on VEGF measurements [16]. However, because citrated plasma VEGF levels are low and usually lie at the lower detection limit of currently available enzyme-linked immunosorbent assays (ELISAs), serum assessments might have greater sensitivity [17]. Other investigators suggest the use of the ratio of serum VEGF levels to platelet count (PLT) (VEGF/PLT) to reflect the level of systemic VEGF per platelet and account for the contribution of VEGF from platelet activation during thrombosis. This parameter has been suggested be more indicative of clinical pathology [17, 18].
In this prospective study, we aimed to investigate the serum and plasma concentrations of VEGF in the systemic circulation prior to and up to 3 months after either IVA or IVB. In addition, we calculated the VEGF/PLT ratio to reflect the effect of platelets on the VEGF levels. To the best of our knowledge, this is the first study to evaluate the systemic levels VEGF after IVA in patients with ROP. Changes in the serum and plasma VEGF levels up to 3 months after IVA or IVB were compared in patients with type 1 ROP.
Methods
Patients
This investigation was a prospective, non-randomized cohort study that assessed the serum VEGF levels in type 1 ROP patients before and after either IVA or IVB. Patients with type 1 ROP as defined by the ETROP study [19] and who received either IVA or IVB was enrolled. Patients who received prior laser treatment, laser treatment after IVA or IVB, or whole blood transfusions before or after IVA or IVB were excluded. This study was conducted from September 2014 to August 2016 at Chang Gung Memorial Hospital in Taoyuan, Taiwan and was approved by the institutional review board at the hospital (IRB100-4294A3) and adhered to the tenets of the Declaration of Helsinki. The status of the off-label use of IVA and IVB for ROP treatment was thoroughly explained to the parents of the patients. The parents were well informed about the efficacy and possible complications of both treatments, including the risk of endophthalmitis, retinal detachment, systemic VEGF suppression, and the possible neurodevelopmental impact following ocular anti-VEGF treatment. Standard treatments using peripheral retinal laser photocoagulation were also explained to the parents of the patients. Neither the IVA nor IVB treatment was covered by the national insurance, and the parents covered all treatment costs. The choice of laser photocoagulation or IVA/IVB treatment was made by the parents. All the parents signed informed consent before IVA or IVB administration, and written informed consent was obtained from the parents prior to enrollment of their child in this study.
Intravitreal injection of anti-vascular endothelial growth factor drugs
The technique used for intravitreal injection of anti-VEGF agents was performed as previously described [3]. Anesthesia was achieved by an intravenous injection of either midazolam (Dormicum; Cenexi SAS, Fontenay-sous-Bois, France) or fentanyl (Fentanyl-Fresenius; Bodene Limited, Port Elizabeth, South Africa) to sedate the infant before treatment administration, and vital signs were monitored throughout the entire procedure. The eyes were prepared in a sterile manner using 5% povidone/iodine and topical antibiotics; then 1 mg (0.025 mL) of aflibercept or 0.625 mg (0.025 mL) of bevacizumab was intravitreally injected 1.5 mm posterior to the limbus via the pars plicata while the patient was under intravenous sedation. The injection was performed using a 30-gauge needle directed perpendicularly to the globe initially and then directed slightly toward the center of the globe after the tip of the needle passed the lens equator of the eye. Retinal artery perfusion was monitored after the injection, and the patients received the topical antibiotic levofloxacin (Cravit; Santen Pharmaceutical Co., Osaka, Japan) for 7 days.
VEGF measurement after IVA or IVB
Blood samples were collected prior to and at 2, 4, 8, and 12 weeks after IVA or IVB, with the baseline blood samples collected 1 to 2 days before intravitreal injection. The tested serum and plasma target was VEGF, which was measured using ELISAs. The procedures were performed as described by a previous study with some modifications [20]. Serum was collected into a serum separator tube, and the samples were allowed to clot for 30 min prior to centrifugation. Plasma was collected with citrate as an anticoagulant, and the blood samples were centrifuged at 3000 rpm for 10 min until a clear separation between the serum or plasma and the cell components was observed. The serum or plasma was then transferred into sterile tubes and stored at −20 °C until further use. The serum and plasma concentrations of VEGF were measured using an ELISA kit that could detect the 121 and 165 isoforms of human VEGF (Human VEGF Immunoassay, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. The minimum detectable concentration of the assay was 9.0 pg/mL. The ratio of serum VEGF to platelet count (i.e., VEGF molecules per platelet; VEGF/PLT, pg/106) was further examined to account for the VEGF contribution from platelet activation in the serum sample during thrombosis [17, 18]. The assay used only measured free and not total VEGF levels [21]. That is, the VEGF bound to bevacizumab would not be detected. All measurements were performed twice to obtain an average value.
Statistical analysis
Data were presented as either the median (range) or the mean ± standard deviation (SD). The pairwise comparisons of the serum VEGF levels at baseline and each time point within the group were conducted using the Wilcoxon signed-rank test. Additionally, trends regarding the changes in the VEGF levels at various time points within a group were evaluated using the Friedman test. A Mann-Whitney U test was conducted to compare the percent reduction in the VEGF levels from baseline at each follow-up time between the two treatment groups. Statistical Analysis System (SAS) software version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for all data analyses. A P value less than 0.05 was considered statistically significant.
Results
In total, 14 patients (six boys and eights girls) with type 1 ROP were enrolled in this study, with five patients receiving IVA and nine patients receiving IVB. Among these patients, 10 (71%) had a history of transfusions of packed red blood cells, which should have had either a minimal or no effect on the serum protein composition [22]. The median time between blood transfusion and blood collection was 8 days (range, 1–14 days). None of the enrolled patients received a whole blood transfusion. The demographics of the patients are presented in Table 1. Of the five patients who received IVA, four (80%) received it in both eyes, and one (20%) received it in one eye. Eight of the nine IVB patients (88%) received the treatment in both eyes, and the ninth patient (12%) received the treatment in one eye. The mean gestational age of the infants was 26 ± 2.4 weeks (range, 23.6–31.7 weeks), and the mean birth weight was 764 ± 214.1 g (range, 476–1160 g). All the patients received either IVA or IVB as the primary treatment, and none of the infants had undergone laser photocoagulation of the peripheral avascular retina prior to IVA or IVB. The mean postmenstrual age of the infants at the time of the initial IVA or IVB treatment was 37.2 ± 2.9 weeks. All the treated eyes showed complete resolution of abnormal neovascularization and continued vascularization toward the peripheral retina following a single IVA or IVB treatment. At the end of the follow-up, all the eyes had resolved the ROP, and none showed any recurrence. Additionally, no obvious adverse systemic complications were noted in the patients after a follow-up of 11 ± 6.72 months (range, 4–27 months).
In patients treated with IVA, the serum VEGF levels were significantly reduced for 12 weeks, as shown in Table 2. The median (range) serum VEGF levels were 373.31 (315.51–513.75) pg/mL before IVA (n = 5), which dropped to 43.29 (28.35–85.75) pg/mL 2 weeks after IVA (n = 5; P = 0.04). The levels remained lower at 4, 8, and 12 weeks after IVA [77.62 (37.18–116.75) pg/mL, n = 5; P = 0.04; 41.27 (30.65–143) pg/mL, n = 5, P = 0.04; and 49.19 (20.17–100.95) pg/mL, n = 5; P = 0.04; respectively]. In the ROP patients who underwent IVA treatment, the serum VEGF levels were significantly decreased between baseline and up to 12 weeks after treatment (P = 0.017). At 2, 4, 8 and 12 weeks after IVA, the serum VEGF/PLT ratio was also suppressed compared to the baseline (P = 0.04, P = 0.04, P = 0.04 and P = 0.04, respectively). The results are shown in Table 3. The serum VEGF/PLT ratio in patients treated with IVA was also significantly reduced between baseline and up to 12 weeks after treatment (P = 0.006).
In patients treated with IVB, the serum VEGF levels were significantly decreased up to 12 weeks after treatment compared to the baseline levels, as shown in Table 4. The median (range) serum VEGF levels were 470.69 (332.44–1540.44) pg/mL before IVB (n = 9), but the levels decreased to 22.94 (7.40–88.87) pg/mL 2 weeks after IVB (n = 9; P = 0.01). This reduction was maintained at 4, 8, and 12 weeks after IVB treatment [17.03 (12.74–56.25) pg/mL, n = 7, P = 0.02; 23.65 (8.31–48.09) pg/mL, n = 8, P = 0.01; and 29.32 (13.19–114.93) pg/mL, n = 6, P = 0.03; respectively]. Overall, in the ROP patients who underwent IVB treatment, the serum VEGF levels were significantly decreased between baseline and up to 12 weeks after treatment (P = 0.017). At 2, 4, 8 and 12 weeks after IVB, the serum VEGF/PLT ratios were also suppressed (P = 0.01, P = 0.02, P = 0.02 and P = 0.03, respectively). The results are shown in Table 5. However, the serum VEGF/PLT ratio in patients who received IVB was not significantly reduced between baseline and up to 12 weeks (P = 0.066).
There was no significant difference in the baseline serum VEGF levels between the IVA and IVB treatment groups (P = 0.257). The comparison of the percentage of serum VEGF reduction from baseline to up to 12 weeks in both treatment groups is shown in Table 6. Both the IVA and IVB groups showed a reduction in VEGF levels. At 2, 4 and 8 weeks after intravitreal treatment, the reduction of serum VEGF levels was significantly more apparent in the IVB group than the IVA group (P = 0.039, P = 0.004, and P = 0.003, respectively). The concentration of IVB (0.625 mg, 0.025 mL) was lower than IVA (1 mg, 0.025 mL), but the suppression of systemic VEGF was even more pronounced in patients treated with IVB than those treated with IVA. However, at 12 weeks after intravitreal treatment, the reduction of VEGF levels between the two groups was not significantly different (P = 0.273). The reduction of the VEGF/PLT ratio was also more apparent in the IVB group than the IVA group with significant differences observed at 2, 4 and 8 weeks (P = 0.004, P = 0.004, P = 0.004, respectively); however, there was no significant difference at 12 weeks.
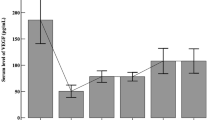
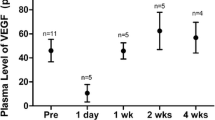
(P = 0.144). The results are shown in Table 7. Figures 1 and 2 illustrate the significant reduction of the VEGF levels and the VEGF/PLT ratio for 12 weeks in both the IVA and IVB groups.
Box plot showing the serum levels of vascular endothelial growth factor (VEGF) following intravitreal injections of aflibercept (IVA) or bevacizumab (IVB) in patients with retinopathy of prematurity (ROP). At baseline, the mean serum VEGF levels in the patients who received IVA or IVB were comparable. After IVA or IVB, the serum VEGF levels in both groups were reduced significantly for 3 months. * P < 0.05 versus baseline
Box plot showing the VEGF levels per platelet (VEGF/PLT, pg/106) following intravitreal injections of aflibercept (IVA) or bevacizumab (IVB) in patients with retinopathy of prematurity (ROP). Both the IVA and IVB groups presented a significant reduction in the VEGF/PLT ratio for 3 months. * P < 0.05 versus baseline
The plasma VEGF levels in patients who received either IVA or IVB could not be properly assessed because some of the measurements were below the detection level of the ELISA.
Discussion
In this prospective study, we observed that both the IVA and IVB groups presented a significant reduction of serum VEGF levels for 3 months compared to their respective baseline levels (P = 0.017 for both groups). Interestingly, the serum VEGF was more suppressed in the IVB group than in the IVA group at 2, 4, and 8 weeks after intravitreal injection. To account for the VEGF released during platelet activation during thrombosis, the ratio of serum VEGF levels to the platelet count (VEGF/PLT, pg/106) was calculated and compared, and the results revealed similar reductions in patients treated with either IVB or IVA. Although the safe range of serum VEGF levels in premature babies remains unknown, our data suggest that both aflibercept and bevacizumab have a profound impact on systemic VEGF and that clinicians should take these results into consideration if using these treatments for patients with ROP.
VEGF plays an important role in the development of ROP. Anti-VEGF therapies have revolutionized the treatment of ROP in recent years, but some systemic effects were observed after their use. Sato et al. found that IVB resulted in bevacizumab entering the systemic circulation and corresponding serum VEGF suppression for up to 2 weeks after treatment [20]. Our previous study revealed that bevacizumab was present in the systemic circulation 1 day after IVB and that serum VEGF levels were suppressed for 2 months [11]. We also compared the effects of intravitreal injection of either bevacizumab or ranibizumab in patients with ROP and observed that bevacizumab led to significantly decreased VEGF levels for 2 months whereas ranibizumab had a minimal effect [12]. In adult patients with AMD, Wang et al. [13] reported that aflibercept significantly decreased serum and plasma VEGF concentrations 1 month after injection, but that ranibizumab had no significant effect on either the serum or plasma VEGF levels. Consistent with previous reports, the current study revealed that both bevacizumab and aflibercept led to longer periods of VEGF suppression in ROP patients than ranibizumab. In addition, systemic VEGF suppression was extended in ROP patients compared to that in adults who receive anti-VEGF treatment. This might be related to different pharmacokinetics of these anti-VEGF drugs between newborns and adults [23].
Ranibizumab, bevacizumab and aflibercept are three anti-VEGF agents that vary in their molecular size, structure and half-life; their ability to penetrate the neural retina and retinal pigment epithelial (RPE); their pharmacokinetics and their affinity to VEGF [24, 25]. In the bloodstream, Fc-containing molecules such as bevacizumab and aflibercept are recycled by binding to endothelial cell FcRn receptors, which protects these molecules from the default degradation pathway within endosomes [26]; this recycling decreases the rate of systemic clearance. By contrast, ranibizumab was specifically designed without the Fc domain to allow for rapid systemic clearance. Avery et al. [27] reported that after intravitreal injection in AMD patients, all three agents rapidly dispersed into the bloodstream, but ranibizumab was cleared the quickest, whereas bevacizumab and aflibercept demonstrated greater systemic exposure and produced a marked reduction in plasma-free VEGF. Their results were consistent with the current study and our previous reports [11, 12].
Avery RL et al. also reported the systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab and ranibizumab [28]. Their result revealed that aflibercept had greatest reduction in plasma free-VEGF than bevacizumab and ranibizumab. In our study, we had greater serum VEGF suppression in bevacizumab than aflibercept. Difference may result from the following reasons: First, the measurement of serum VEGF or plasma free-VEGF may lead to different results. Secondly, the basic VEGF status in different age groups (newborns vs. adults) or different disease entities (ROP vs. AMD, DME, RVO) may contribute to different results. Third, the treatment protocols were different between ROP and adult retinal disorders. ROP patients usually received only one injection, but long-term multiple injections of anti-VEGFs may be needed in adult patients. Finally, we may need more patient numbers for further investigation in the future.
VEGF is an important mitogen for vascular endothelial cells and plays a critical role for promoting physiological angiogenesis, wound repair following injury, and the development of various vital organs in the body, as well as maintaining organ health [29,30,31,32,33,34,35,36,37,38,39]. It also promotes pathological angiogenesis and is regulated by tissue hypoxia [29, 40]. Thus, blocking VEGF activity might reduce the vascular activity associated with ROP [34, 35]. Retinal ablation therapies such as cryotherapy or laser photocoagulation reduce VEGF production by completely ablating the peripheral avascular retina [2]. On the other hand, anti-VEGF drugs neutralize VEGF levels in both the retina and vitreous fluid with limited tissue destruction. However, the prolonged VEGF inhibition raises concerns that the normal physiological effects associated with VEGF will be affected and result in abnormal organogenesis or neurodevelopment [14]. Selecting an anti-VEGF agent with reduced systemic VEGF interference, reducing the dose of the anti-VEGF therapy or administering a single dose of anti-VEGF treatments in ROP patients appear to be safer choices because of the abovementioned concerns.
Though our result showed a sustained suppression of VEGF concentration for 12 weeks, there is no clear answer when the normalization of serum VEGF level will reach. Lingkun K et al. reported the pharmacokinetics of bevacizumab and its effect on serum VEGF in infants of ROP [41]. Their study showed that systemic exposure to bevacizumab was variable among the subjects and clearance of bevacizumab from the blood stream in premature infants took at least 2 months. Their finding demonstrated suppression of VEGF level up to 2 months in ROP patients. It is, therefore, difficult to predict or correctly estimate the time when the systemic VEGF returns to normal because of the high variability among the subjects. In the clinical practice, we usually perform laser photocoagulation for the patient if there are signs of recurrence of ROP, rather than the use of secondary injection of anti-VEGF because of the uncertainty of long-term VEGF suppression. Further studies with a larger study cohort and an extended period beyond 3 months to measure the systemic VEGF level are warranted to answer this question.
Both serum and plasma samples have been used in clinical studies; however, it is still unclear which sample is more representative of the peripheral VEGF level. VEGF is stored in platelet alpha granules and is released upon platelet activation during thombosis [42, 43]. Therefore, serum VEGF levels are usually higher than the plasma VEGF levels, and the wide confidence interval in measured serum VEGF levels could be related to this factor. Plasma has been suggested to more accurately reflect the circulating VEGF levels [43]. Because the levels of citrated plasma VEGF lie at the lower limit of detection of currently available ELISAs, the use of serum samples may have greater clinical applications [16]. Therefore, the current study used both the serum and plasma to assess the VEGF levels in peripheral blood.
There were several limitations to this study. First, we had a small cohort due to the difficulty of enrolling patients with severe, acute ROP. In addition, the small cohort size might have contributed to the wide confidence intervals in the measurement of serum growth factors in this study. The uneven distribution of both groups were mainly caused by the economic issue and parents’ preference. Since bevacizumab had a longer history of clinical use with a more affordable price, most of parents chose this drug. Second, there were few blood samples because the systemic condition of a newborn might not always be suitable to allow for blood collection at each time point to obtain an adequate sample. Third, the choice of treatments was not random. Despite these limitations, our data showed that systemic VEGF levels were significantly inhibited after either IVA or IVB treatment in ROP patients.
In conclusion, both the serum VEGF levels and the VEGF/PLT ratio in patients with type 1 ROP were suppressed for 3 months after treatment with either IVA or IVB. The suppression of systemic VEGF was more pronounced in patients receiving IVB than those receiving IVA, and the duration of VEGF suppression after anti-VEGF treatment was longer in these pediatric patients compared to adult patients with neovascular or ischemic retinal disorders. Because systemic VEGF has been shown to play an important role in the neurodevelopment of newborns, anti-VEGF treatment for ROP patients should be used with caution, and surgeons should be aware of the potential long-term impact of these treatments on ROP patients. These data highlight the importance of long-term neurodevelopmental outcome study in these infants who were treated with anti-VEGFs.
References
Stone J, Chan-Ling T, Pe'er J et al (1996) Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci 37:290–299
Mutlu FM, Sarici SU (2013) Treatment of retinopathy of prematurity: a review of conventional and promising new therapeutic options. Int J Ophthalmol 6:228–236
Wu WC, Kuo HK, Yeh PT et al (2013) An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in Taiwan. Am J Ophthalmol 155:150–158
Mintz-Hittner HA, Kennedy KA, Chuang AZ (2011) BEAT-ROP cooperative Group.Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 364:603–615
Heier JS, Brown DM, Chong V et al (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548
Brown DM, Heier JS, Clark WL et al (2013) Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol 155:429–437
Korobelnik JF, Do DV, Schmidt-Erfurth U et al (2014) Intravitreal aflibercept for diabetic macular edema. Ophthalmology 121:2247–2254
Salman AG, Said AM (2015) Structural, visual and refractive outcomes of intravitreal aflibercept injection in high-risk prethreshold type 1 retinopathy of prematurity. Ophthalmic Res 53:15–20
Stewart MW (2011) Aflibercept (VEGF trap-eye): the newest anti-VEGF drug. Inflamm Allergy Drug Targets 10:497–508
Sukgen EA, Söker G, Koçluk Y, Gülek B (2017) Effect of intravitreal aflibercept on central retinal arterial blood flow in type 1 retinopathy of prematurity. Eur J Ophthalmol. https://doi.org/10.5301/ejo.5000938
Wu WC, Lien R, Liao PJ et al (2015) Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol 133:391–397
Wu WC, Shih CA, Lien R et al (2017) Serum vascular endothelial growth factor after bevacizumab or ranibizumab treatment for retinopathy of prematurity. Retina 37:694–701
Wang X, Sawada T, Sawada O et al (2014) Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol 158:738–744
Lien R, Yu MH, Hsu KH et al (2016) Neurodevelopmental outcomes in infants with retinopathy of prematurity and bevacizumab treatment. PLoS One 11:e0148019
Carmeliet P, Ruiz de Almodovar C (2013) VEGF ligands and receptors:implications in neurodevelopment and neurodegeneration. Cell Mol Life Sci 70:1763–1778
Banks RE, Forbes MA, Kinsey SE et al (1998) Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer 77:956–964
George ML, Eccles SA, Tutton MG, Abulafi AM, Swift RI (2000) Correlation of plasma and serum vascular endothelial growth factor levels with platelet count in colorectal cancer: clinical evidence of platelet scavenging? Clin Cancer Res 6:3147–3152
Enjoji M, Nakamuta M, Yamaguchi K et al (2005) Clinical significance of serum levels of vascular endothelial growth factor and its receptor in biliary disease and carcinoma. World J Gastroenterol 11:1167–1171
Early Treatment For Retinopathy of Prematurity Cooperative Group (2003) Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Opthalmol 121:1684–1694
Sato T, Wada K, Arahori H et al (2012) Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol 153:327–333
Jelkmann W (2001) Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem 47:617–623
Ergenekon E, Bozkaya D, Goktas T et al (2010) Are serum nitric oxide and vascular endothelial growth factor levels affected by packed red blood cell transfusions? Hematology 15:170–173
Hard AL, Hellstrom A (2011) On safety, pharmacokinetics and dosage of bevacizumab in ROP treatment: a review. Acta Paediatr 100:1523–1527
Yoshihara N, Terasaki H, Shirasawa M et al (2017) Permeability and anti-vascular endothelial growth factor effects of bevacizumab, ranibizumab and aflibercept in polarized retinal pigment epithelial layer in vitro. Retina 37:179–190
Cam D, Berk AT, Micili SC et al (2016) Histological and Immunohistochemical retinal changes following the intravitreal injection of Aflibercept, bevacizumab and Ranibizumab in newborn rabbits. Curr Eye Res 42:315–322
Ternant D, Paintaud G (2005) Pharmacokinetics and concentration-effect relationships of therapeutic monoclonal antibodies and fusion proteins. Expert Opin Biol Ther 5(Suppl 1):S37–S47
Avery RL, Castellarin AA, Steinle NC et al (2014) Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 98:1636–1641
Avery RL, Castellarin AA, Steinle NC et al (2017) Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizuman, and ranibizumab. Retina 37:1847–1858
Been JV, Zoer B, Kloosterboer N et al (2010) Pulmonary vascular endothelial growth factor expression and disaturated phospholipid content in a chicken model of hypoxia- induced fetal growth restriction. Neonatology 97:183–189
Dvorak HF (2002) Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20:4368–4380
Ferrara N (2002) VEGF and the quest for tumor angiogenesis factors. Nat Rev Cancer 2:795–803
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25:581–611
Ishii M, Tanaka E, Imaizumi T et al (2009) Local VEGF administration enhances healing of colonic anastomoses in a rabbit model. Eur Surg Res 42:249–257
McColm JR, Geisen P, Hartnett ME (2004) VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis 10:512–520
Young TL, Anthony DC, Pierce E, Foley E, Smith LE (1997) Histopathology and vascular endothelial growth factor in untreated and diode laser-treatedretinopathy of prematurity. JAAPOS 1:105–110
Sakowski SA, Heavener SB, Lunn JS et al (2009) Neuroprotection using gene therapy to induce vascular endothelial growth factor-a expression. Gene Ther 16:1292–1299
Yamamoto C, Yagi S, Hori T et al (2010) Significance of portal venous VEGF during liver regeneration after hepatectomy. J Surg Res 159:e37–e43
Yancopoulos GD, Davis S, Gale NW et al (2000) Vascular-specific growth factors and blood vessel formation. Nature 407:242–248
Zisa D, Shabbir A, Suzuki G, Lee T (2009) Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun 390:834–838
Sato T, Kusaka S, Shimojo H, Fujikado T (2009) Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology 116:1599–1603
Kong L, Bhatt AR, Demny AB et al (2015) Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci 56:956–961
Wartiovaara U, Salven P, Mikkola H et al (1998) Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost 80:171–175
Maloney JP, Silliman CC, Ambruso DR et al (1998) In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Phys 275:H1054–H1061
Acknowledgments
We would like to thank Juang Hsiao-Ting, who was supported by grants from the Center for Big Data Analytics and Statistics (Grant CLRPG3D0043) and the Research Services Center for Health Information (Grant CIRPD1D0031) from Chang Gung Memorial Hospital, for providing statistical guidance.
Funding
This study was supported by research grants from Chang Gung Memorial Hospital Grants (CMRPG3E0521~ − 2 and CMRPG3F0191~3) and a grant from the National Science Council Research (MOST 104–2314-B-182A-100-MY2).
The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Chang Gung Memorial Hospital) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Huang, CY., Lien, R., Wang, NK. et al. Changes in systemic vascular endothelial growth factor levels after intravitreal injection of aflibercept in infants with retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 256, 479–487 (2018). https://doi.org/10.1007/s00417-017-3878-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3878-4