Abstract
Aims
To evaluate the safety and efficacy of vitrectomy combined with an intraoperative dexamethasone (Ozurdex®) implant in refractory diabetic macular edema (DME).
Methods
Patients who were diagnosed at our institution as having DME refractory to more than 6 months of non-surgical treatment and underwent intravitreal dexamethasone implantation combined with vitrectomy. All patients were followed up for more than 12 months. Best-corrected visual acuity (BCVA, logMAR), central macular thickness (CMT), and intraocular pressure at the initial visit and 1, 3, 4, 6, and 12 months after treatment were recorded.
Results
Twenty-two eyes (22 patients) were included in this study. The mean preoperative BCVA was 0.68 and the mean CMT was 470.80 µm. The total number of the previous injections was 5.1 ± 1.6. The mean BCVA was significantly improved at all visits, and the mean CMT was also significantly reduced (p < 0.05). Sixteen eyes (73%) did not need additional implantations during follow-up.
Conclusions
Vitrectomy combined with an intraoperative dexamethasone (Ozurdex) implant was an effective and safe treatment option in patients with refractory DME.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic macular edema (DME) is a common cause of visual impairment in patients with diabetic retinopathy and occurs in approximately 10% of diabetic patients and 29% of those with a disease duration of more than 20 years [1].
In the Early Treatment Diabetic Retinopathy Study, focal and grid photocoagulation procedures were reported to be effective in DME. However, by 3 years after treatment, macular edema had recurred in 24% of patients and the prognosis was particularly poor in eyes with diffuse macular edema [2, 3]. Intravitreal anti-vascular endothelial growth factor (VEGF) injection is effective therapy for DME, but not effective in all DME patients and requires frequent injection [4]. Triamcinolone is also an effective drug for DME. However, it is difficult to use because of its troublesome side effects, which include endophthalmitis, progression of cataract, and elevation of intraocular pressure (IOP) [5, 6].
Vitrectomy may be considered in patients with DME that is refractory or persistent despite laser or intravitreal injection. Vitrectomy with internal limiting membrane (ILM) peeling can relieve macular edema by reducing traction between the vitreous and retina and by decreasing the levels of cytokines or growth factors in the vitreous that increase vascular permeability [7,8,9,10]. However, there have been reports of persistent or recurrent macular edema after vitrectomy [10] and the clearance rate from the vitreous cavity may increase after vitrectomy, leading to shortening of the drug’s half-life. Therefore, frequent reinjections may be required [11].
Ozurdex® (Allergan Inc., Irvine, CA, USA) is a biodegradable implant that gradually releases low-dose dexamethasone for several months after being injected into the vitreous cavity and can maintain dexamethasone above a certain concentration for 3–6 months. Furthermore, the side effects that tend to appear with frequent injections, such as endophthalmitis, cataract, and elevation of IOP, are reduced [12]. Gillies et al. reported that the number of injections of dexamethasone required to improve visual acuity (VA) in patients with DME was smaller than that required with bevacizumab [13]. There are also several reports of the dexamethasone intravitreal implant being effective for persistent DME [14,15,16]. In particular, prolonged drug release from a dexamethasone implant after vitrectomy can be expected to improve refractory DME.
Thus, we review the medical records of patients who had a history of refractory DME and assess the results achieved by an intravitreal dexamethasone (Ozurdex) implant combined with vitrectomy.
Materials and methods
We retrospectively reviewed our medical records to identify patients who had a history of DME refractory to non-surgical treatment for more than 6 months from October 2014 to April 2016. The study was approved by the Institutional Review Board of Konyang University Hospital, Daejeon, South Korea (KYUH 2017-08-002) and followed the principles of the Declaration of Helsinki.
The outcome measures were best-corrected visual acuity (BCVA), IOP and central macular thickness (CMT) measured on spectral-domain optical coherence tomography (Heidelberg Spectralis®, Heidelberg Engineering, Heidelberg, Germany) at baseline and at 1, 3, 4, 6, and 12 months after treatment.
DME defined clinically and by CMT ≥ 300 µm measured by optical coherence tomography. The inclusion criteria were refractory or persistent DME despite the previous non-surgical treatments, including laser, intravitreal anti-VEGF, or steroid injection or sub-tenon triamcinolone injection, for more than 6 months and Snellen BCVA ≤ 0.5. The exclusion criteria were as follows: treatment for DME in the study eye within the previous 3 months; a history of intravitreal dexamethasone implantation to treat DME; a history of cataract surgery in the study eye within the previous 6 months; a vitrectomized eye; significant visual loss (< 20/40) attributable to severe cataract, corneal disease or optic nerve disease; a history of glaucoma; a history of or active ocular infection or inflammation in the study eye; and a history of any retinal disease that might further compromise VA, such as age-related macular degeneration, retinal vein occlusion, or retinal detachment.
All surgeries were performed under retrobulbar anesthesia by 1 surgeon (YH Lee), and involved 25-gauge transconjunctival sutureless vitrectomy (TSV25) with a Constellation® vision system (Alcon, Fort Worth, TX, USA) and Ultravit® 25-gauge plus (Alcon) vitrectomy instruments. In all eyes, the ILM was stained with 0.25% indocyanine green (LDS Pharm Co., Ltd, Seoul, Korea) and peeled carefully to a radius of 2–3 disc diameters using an intraocular forceps. Just before the end of the surgery, a 0.7-mg dexamethasone implant was placed into the vitreous cavity using a single-use applicator with a 22-gauge needle. Cataract surgery was performed simultaneously as needed.
The BCVA was measured using a Snellen chart and converted to the logarithm of the minimum angle of resolution (logMAR) for the statistical analysis. During follow-up, the dexamethasone implant was re-injected if the CMT increased by > 300 µm or the BCVA decreased by more than two lines. Changes in the preoperative and postoperative BCVA, CMT, and IOP were assessed for statistical significance using the Wilcoxon’s signed-rank test. Binary logistic regression model was established to investigate factors affect to re-treatment. The threshold for statistical significance was set at p < 0.05.
Results
Baseline characteristics
Total 22 patients (22 eyes) were included in this study. The demographic and clinical characteristics of the patients and study eyes at baseline are listed in Table 1. The mean age of the patients was 59.13 years and the mean duration of diabetes was 12.60 years. The mean HbA1c of the patients was 7.42%. Fourteen of the 22 eyes were pseudophakic. The mean numbers of the previous treatments with intravitreal bevacizumab, posterior sub-tenon triamcinolone, and intravitreal triamcinolone were 3.4, 1.1, and 0.5, respectively. The total number of the previous injections was 5.1 ± 1.6. The mean preoperative BCVA (logMAR) was 0.68 and the mean CMT was 470.80 µm (Table 1).
Visual and anatomical outcomes
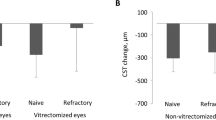
The mean BCVA (logMAR) was improved to 0.39 ± 0.16, 0.36 ± 0.18, 0.37 ± 0.18, 0.40 ± 0.15, and 0.37 ± 0.19 at 1, 3, 4, 6, and 12 months, respectively (p < 0.05; Fig. 1a). The mean change in BCVA was − 0.30 ± 0.20, − 0.32 ± 0.19, − 0.32 ± 0.22, − 0.29 ± 0.21, and − 0.32 ± 0.25 at 1, 3, 4, 6, and 12 months (Fig. 1b). The mean CMT was 342.47 ± 46.72 µm, 303.20 ± 54.95 µm, 334.07 ± 61.86 µm, 331.87 ± 53.20 µm, and 353.33 ± 68.91 µm at 1, 3, 4, 6, and 12 months. It showed a statistically significant reduction in CMT when compared with the preoperative CMT (p < 0.05; Fig. 2a). The mean change in CMT was − 128.33 ± 72.66, − 167.60 ± 95.50, − 136.73 ± 102.30, − 138.93 ± 85.98, and − 117.47 ± 62.08 at 1, 3, 4, 6, and 12 months (Fig. 2b).
Numbers of dexamethasone re-implantations
Sixteen eyes (73%) did not need additional implantations during follow-up. The mean number of dexamethasone re-implantations was 0.4 ± 0.8. Binary logistic regression analysis was performed to identify factors affecting dexamethasome re-implantation. Age, sex, duration f diabetes, HbA1c, hypertension, dyslipidemia, preoperative BCVA, preoperative CMT, initial severity of DR, and PRP status were incorporated into the analysis. None of these variables affected re-treatment.
Safety outcomes
Two eyes (9.1%) showed an increase in IOP (≥ 25 mmHg) at 1 month that reverted to normal after use of an IOP-lowering eyedrop [Cosopt® (Merck & Co., Inc., Kenilworth, NJ, USA)]. No other complications were observed during routine follow-up.
Discussion
Focal and grid photocoagulation has been widely used as a treatment for DME. It is an effective treatment for localized macular edema but less effective for diffuse or cystoid macular edema. In one study, VA improved in only 17% of cases after treatment [3]. Beck et al. reported that more than 15 letters of visual loss were observed in 12% of cases during 3 years of follow-up [17]. Furthermore, adverse effects such as choroidal neovascularization and submacular fibrosis were reported after laser treatment. Therefore, the need for better treatment modalities for refractory DME was recognized.
At present, the most common treatment for DME is intravitreal anti-VEGF injection. In patients with DME, the concentration of intravitreal VEGF is increased in proportion to the severity of the macular edema [18]. The Diabetic Retinopathy Clinical Research Network (DRCR.net) reported a clinically significant decrease in macular thickness at 3–6 weeks after intravitreal bevacizumab injection [19]. However, in a rabbit model, the mean half-life of bevacizumab was 4.32 days after intravitreal injection [20]. Therefore, intravitreal bevacizumab injection has the limitation of needing to be repeated within a relatively short period of time.
Triamcinolone, which was used widely to treat DME prior to anti-VEGF injection, inhibits the arachidonic acid pathway, thereby inhibiting production of the prostaglandins that trigger an inflammatory response. In addition, triamcinolone inhibits production of VEGF and has a potent anti-inflammatory effect on macular edema by inhibiting vascular proliferation and reducing vascular permeability [21]. However, in the study conducted over a period of 3 years by DRCR.net, the rate of adverse effects, including cataract, was high and visual improvement was less than that in a laser treatment group [17].
Dexamethasone is a steroid that exerts potent anti-inflammatory effects via several mechanisms, including reducing vascular permeability, inhibiting fibrin deposition, and migration of white blood cells and inflammatory cells, stabilizing endothelial cell adhesion, and inhibiting synthesis of VEGF, prostaglandins, and other cytokines. Dexamethasone is five times more potent than triamcinolone and can exist in a high concentration in the vitreous because of its high hydrophilicity. Furthermore, it has the advantage of not causing blurring or vitreous floaters after injection [22]. Moreover, because of its short half-life of 3 h, it is less effective after intravitreal injection. However, the recently developed intravitreal dexamethasone (Ozurdex) implant can overcome this limitation by maintaining the concentration of dexamethasone above a certain level in the vitreous cavity for 3–6 months. Ozurdex can be used safely because it decomposes slowly in the vitreous cavity into carbon dioxide and water [23]. Pacella et al. reported significant improvement in VA for 4 months and a decrease in CMT for 6 months after Ozurdex implantation in eyes with DME refractory to laser photocoagulation, intravitreal anti-VEGF, and steroid injections [12]. Gillies et al. reported a significantly greater decrease in CMT in an Ozurdex implant group than in an intravitreal bevacizumab injection group and that the Ozurdex implant group required a mean of 2.7 injections per year compared with 8.6 injections in the bevacizumab group [13]. Catharina et al. reported eyes with DME considered refractory to anti-VEGF therapy after 3 monthly injections which were switched to ozurdex implant and had better visual and anatomical outcomes at 12 months than those that continued treatment with anti-VEGF therapy in a real-world setting [16].
Vitrectomy could be an alternative treatment for refractory DME. The mechanisms by which DME is postulated to improve after vitrectomy include a reduction of vitreomacular traction, removal of VEGF, and improved transvitreal oxygenation of the retina [8]. Rosenblatt et al. reported that vitrectomy and ILM peeling were effective treatments for refractory DME [7]. However, even after vitrectomy, there are some patients in whom there is no significant improvement in VA or a recurrence of macular edema. Therefore, vitrectomy combined with an intraoperative dexamethasone implant may help to improve the prognosis.
After vitrectomy, the vitreous is replaced with an aqueous humor that has lower viscosity than normal vitreous. In a vitrectomized eye, as the circulation increases in the vitreous cavity, agents such as anti-VEGF and triamcinolone are widely distributed and rapidly removed, leading to increased drug clearance and a short half-life. Lee et al reported that drug clearance was 2.49 mL/h in a vitrectomized eye and 0.564 mL/h in a non-vitrectomized eye and that the clearance rate in a vitrectomized eye was about four times higher than that in a non-vitrectomized eye [11]. Ozurdex slowly releases dexamethasone over a period of months after injection, so may be a useful option for vitrectomized eyes.
In this study, the ILM was removed in all patients. Yu et al. suggested that the ILM may exert tractional forces on the macula, so peeling of the ILM with vitrectomy may be more effective than vitrectomy alone [24]. However, some authors insist that ILM peeling is not an important factor in DME without macular traction [10, 25]. We suspect that the ILM in patients with diabetes may be associated with macular traction and may function as a scaffold for proliferating astrocytes; therefore, we performed ILM peeling with vitrectomy. In addition, ILM peeling may facilitate penetration of dexamethasone into the retinal tissue by removing a mechanical barrier.
BCVA and CMT were significantly improved up to 12 months after treatment in this study. Lazic et al. reported that BCVA and CMT improved until 3 months after injection, but after that, the treatment effect decreased and the BCVA and CMT at 4 months were similar to those before the treatment [14]. Marco et al. reported improvement in BCVA and CMT until 6 months after ozurdex injection, but the effect decreased slightly after 3 months of treatment [15]. Ozurdex is known to persist for up to 3 months after injection and gradually decrease until 6 months following the injection [11, 12]. Therefore, repeated injection was required to many patients [12,13,14,15]. However, as already reported by Lee et al. [26], our study showed statistically significant improvements in VA and CMT during a year of follow-up. Furthermore, 16 eyes (73%) did not require re-treatment for 12 months. Therefore, vitrectomy combined with an intraoperative dexamethasone implant has the advantage of not only improving BCVA and CMT but also requiring fewer additional injections, which may reduce the risk of intraocular complications associated with frequent injections and be more beneficial in terms of convenience to the patient and financial cost. In addition, intravitreal dexamethasone implant can reduce peripheral retina ischemia in patients with DR and has the potential to not only delay progression of DR and PDR development, but may also improve DR severity over 24 months [27, 28]. These facts can serve potential advantage.
Our study has several limitations in that it had a retrospective design, a relatively short follow-up period, and a small study population that precluded any estimation of long-term efficacy or safety. In addition, we tried to find the factors related to re-treatment through regression analysis, but we could not find any particular association. This is probably due to a small number of patients. Furthermore, the changes in VA after treatment may have been affected in patients with phakic refractory DME, even though the cataracts were not severe. These variables should be controlled for in longer term, larger scale, prospective studies in the future.
In conclusion, our findings demonstrate that vitrectomy combined with intraoperative dexamethasone implantation may produce a clinically meaningful and statistically significant benefit in the treatment of refractory DME. This treatment strategy may have the additional benefit of entailing fewer repeat intravitreal injections, thereby decreasing side effects and increasing patient convenience.
References
Pelzek C, Lim KI (2002) Diabetic macular edema: review and update. Ophthalmol Clin N Am 15:555–563
Early Treatment Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular edema Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 103:1796–1806
Early Treatment Diabetic Retinopathy Study Research Group (1995) Focal photocoagulation treatment of diabetic macular edema Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol 113:1144–1155
Diabetic Retinopathy Clinical Research Network (DRCR.net) (2016) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 123:1351–1359
Massin P, Audren F, Haouchine B et al (2004) Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology 111:218–224. https://doi.org/10.1016/j.ophtha.2003.05.037
Vasconcelos-Santos DV, Nehemy PG, Schachat AP, Nehemy MB (2008) Secondary ocular hypertension after intravitreal injection of 4 mg of triamcinolone acetonide: incidence and risk factors. Retina 28:573–580. https://doi.org/10.1097/IAE.0b013e31816079e8
Rosenblatt BJ, Shah GK, Sharma S, Bakal J (2005) Pars plana vitrectomy with internal limiting membranectomy for refractory diabetic macular edema without a taut posterior hyaloid. Graefes Arch Clin Exp Ophthalmol 243:20–25. https://doi.org/10.1007/s00417-004-0958-z
Kumagai K, Furukawa M, Ogino N, Larson E, Iwaki M, Tachi N (2009) Long-term follow-up of vitrectomy for diffuse nontractional diabetic macular edema. Retina 29:464–472. https://doi.org/10.1097/IAE.0b013e31819c632f
Dillinger P, Mester U (2004) Vitrectomy with removal of the internal limiting membrane in chronic diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol 242:630–637. https://doi.org/10.1007/s00417-003-0849-8
Flaxel CJ, Edwards AR, Aiello LP et al (2010) Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: diabetic retinopathy clinical research network. Retina 30:1488–1495. https://doi.org/10.1097/IAE.0b013e3181e7974f
Lee SS, Ghosn C, Yu Z et al (2010) Vitreous VEGF clearance is increased after vitrectomy. Investig Ophthalmol Vis Sci 51:2135–2138. https://doi.org/10.1167/iovs.09-3582
Pacella F, Ferraresi AF, Turchetti P et al (2016) Intravitreal injection of Ozurdex implant in patients with persistent diabetic macular edema, with six-month follow-up. Ophthalmol Eye Dis 8:11–16. https://doi.org/10.4137/OED.S38028
Gillies MC, Lim LL, Campain A et al (2014) A randomized clinical trial of intravitreal bevacizumab versus dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 121:2473–2481. https://doi.org/10.1016/j.ophtha.2014.07.002
Lazic R, Lukic M, Boras I et al (2014) Treatment of anti-vascular endothelial growth factor-resistant diabetic macular edema with dexamethasone intravitreal implant. Retina 34:719–724. https://doi.org/10.1097/IAE.0b013e3182a48958
Dutra Medeiros M, Postorino M, Navarro R, Garcia-Arumí J, Mateo C, Corcóstegui B (2014) Dexamethasone intravitreal implant for treatment of patients with persistent diabetic macular edema. Ophthalmologica 231:141–146. https://doi.org/10.1159/000356413
Catharina B, Dinah Z, Samantha FB et al (2018) Shall we stay, or shall we switch? Continued anti-VEGF therapy versus early switch to dexamethasone implant in refractory diabetic macular edema. Acta Diabetol 55:789–796
Diabetic Retinopathy Clinical Research Network, Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, Hartnett E, Ip MS, Kim JE, Kollman C (2009) Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol 127:245–251. https://doi.org/10.1001/archophthalmol.2008.610
Aiello LP, Avery RL, Arrigg PG et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487. https://doi.org/10.1056/NEJM199412013312203
Diabetic Retinopathy Clinical Research Network, Scott IU, Edwards AR et al (2007) A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 114:1860–1867. https://doi.org/10.1016/j.ophtha.2007.05.062
Bakri SJ, Snyder MR, Reid JM et al (2007) Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 114:855–859. https://doi.org/10.1016/j.ophtha.2007.01.017
Shimura M, Nakazawa T, Yasuda K et al (2008) Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol 145:854–861. https://doi.org/10.1016/j.ajo.2007.12.031
Thakur A, Kadam R, Kompella UB (2001) Trabecular meshwork and lens partitioning of corticosteroids: implications for elevated intraocular pressure and cataracts. Arch Ophthalmol 129:914–920. https://doi.org/10.1001/archophthalmol.2011.39
Chang-Lin JE, Burke JA, Peng Q et al (2011) Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Investig Ophthalmol Vis Sci 52:4605–4609. https://doi.org/10.1167/iovs.10-6387
Yu IC, Song S, Seo MS (2002) Peeling of internal limiting membrane for diabetic macular edema with severe hard exudates. J Korean Ophthalmol Soc 43:630–637 (242)
Patel JI, Hykin PG, Schadt M et al (2006) Pars plana vitrectomy with and without peeling of the inner limiting membrane for diabetic macular edema. Retina 26:5–13
Lee DH, Kim YJ, Yoon YH (2016) Minimally invasive microincision vitrectomy surgery with an intraoperative dexamethasone implant for refractory diabetic macular edema. Ophthalmologica 235:150–156. https://doi.org/10.1159/000443751
Lea Q, Mariacristina P, Riccardo S et al (2017) Ischemic index changes in diabetic retinopathy after intravitreal dexamethasone implant using ultra-widefield fluorescein angiography: a pilot study. Acta Diabetol 54:769–773
Matias I, Dinah Z, Catharina B, Mali O, Anat L (2018) Progression of diabetic retinopathy severity after treatment with dexamethasone implant: a 24-month cohort study the ‘DR–Pro–DEX Study’. Acta Diabetol 55:541–547
Acknowledgements
This research was supported by a Grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number HI17C2412).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Review Board of Konyang University Hospital, Daejeon, South Korea (KYUH 2017-08-002) and followed the principles of the Declaration of Helsinki.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
None.
Additional information
Managed by Antonio Secchi.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jung, Y.H., Lee, Y. Efficacy of vitrectomy combined with an intraoperative dexamethasone implant in refractory diabetic macular edema. Acta Diabetol 56, 691–696 (2019). https://doi.org/10.1007/s00592-019-01305-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01305-w