Abstract
Aims
In this retrospective study, we sought to delineate the collateral circulation status of acute ischemic stroke patients by CT perfusion and evaluate 90-day modified Rankin Scale (mRS) scores of patients with good or poor collaterals and its correlation with admission fasting plasma glucose (FPG).
Methods
We enrolled acute ischemic stroke patients who presented to our hospital 4.5 h within an onset of the first episode between January 2009 and December 2015. Neurological assessment was performed using the 90-day mRS scores (0–2 for a favorable and 3–6 for an unfavorable neurologic outcome). Relative filling time delay (rFTD) was evaluated by CT perfusion scan. The primary outcomes were 90-day mRS scores stratified by good (rFTD ≤ 4 s) versus poor collateral circulation (rFTD > 4 s).
Results
Totally 270 patients were included, and 139 (51.5%) patients achieved a favorable neurologic outcome. One hundred eighty-five (68.5%) patients had good collateral circulation. Significantly greater portions of patients with good collateral circulation (60.5%, 112/185) achieved a favorable neurologic outcome compared to those with poor collateral circulation (31.8%, 27/85) (P < 0.05). Patients with good collateral circulation achieving a favorable neurologic outcome had significantly lower baseline FPG (6.6 ± 1.96) than those with good collateral circulation achieving an unfavorable neurologic outcome (8.12 ± 4.02; P = 0.002). Spearman correlation analysis showed that rFTD significantly correlated with 90-day mRS scores (adjusted r = 0.258; P < 0.001) and admission FPG (r = 0.286; P < 0.001).
Conclusion
Higher admission FPG levels are associated with significantly higher rates of unfavorable neurologic outcome of acute ischemic stroke patients with good collateral circulation. FPG and rFTD may serve as useful predictors of short-term patient outcome and could be used for risk stratification in clinical decision making.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stroke is the leading cause of adult disabilities worldwide and the second leading cause of mortalities in industrialized countries. The burden of stroke is particularly high in China, and the total number of stroke patients was estimated at 7 million in China in 2004 and has seen steady increase [1]. Predicting outcomes of acute stroke patients initially presenting with neurologic deficits is crucial in decision making regarding thrombolytic therapy [2, 3]. Admission hyperglycemia is very common in acute ischemic stroke patients with a reported prevalence over 40% [4]. Hyperglycemia has been shown to predict a larger infarct size, poor prognosis, and a higher risk of mortality independent of age, diabetic status, stroke severity or stroke type [5,6,7].
However, it remains controversial that fasting plasma glucose (FPG) levels are independently associated with a poor functional outcome. Stead et al. [8] studied 447 consecutive patients who presented to emergency department with acute ischemic stroke within 24 h of symptom onset and found that hyperglycemia on presentation was associated with significantly poorer outcomes. Osei et al. [9] have recently analyzed the data of 487 patients from the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) and found no significant interaction of either hyperglycemia or admission serum glucose levels with treatment effect on modified Rankin Scale scores. The UK Glucose in Stroke Trial (GIST) [10] and Treatment of Hyperglycemia in Ischemic Stroke (THIS) study [11] also failed to demonstrate beneficial effects of acute glucose control in acute stroke.
Cerebral collaterals are vascular redundancies in the cerebral circulation that can partially maintain blood flow to the ischemic penumbra when primary conduits are blocked [12]. However, the functional outcome of acute ischemic stroke patients with good or poor collaterals has not been well delineated. Ischemic stroke is associated with increased glucose metabolism in the ischemic penumbra and evidence suggests that hyperglycemia may directly generate neuronal toxicity by inducing a pro-oxidative and proinflammatory state in the ischemic penumbra. Hyperglycemia is commonly encountered in both diabetic and non-diabetic patients in acute ischemic stroke. We reasoned that in ischemic stroke patients, good collateral circulation may facilitate transport of plasma glucose to the ischemic penumbra and hyperglycemia in stroke patients with good collateral circulation may worsen glucose neurotoxicity, possibly leading to a poorer neurologic outcome. In patients with poor collateral circulation, plasma glucose levels may not be a prominent factor as poor collateral blood flow hampers glucose transport to the ischemic penumbra.
We were interested in whether admission plasma glucose correlated with the clinical outcome of acute ischemic stroke patients with good or poor collateral circulation. In this retrospective study, we sought to delineate the collateral circulation status of acute ischemic stroke patients by CT perfusion and evaluate the neurologic outcome (90-day modified Rankin Scale scores) of patients with good or poor collaterals and its correlation with admission plasma glucose.
Patients and methods
Patients
We reviewed the clinical and radiological records of acute ischemic stroke patients who presented to our hospital between January 2009 and December 2015. Ischemic stroke was diagnosed by a neurologist according to the World Health Organization criteria [13] and confirmed on CT, conventional MRI brain imaging, or both. We included patients if they (1) had a clinical diagnosis of primary acute ischemic stroke with an onset of the first episode within the previous 4.5 h and (2) had an occlusion of the internal carotid artery (ICA) or of the proximal (M1) or distal (M2) segments of the middle cerebral artery (MCA) for this retrospective analysis. Patients were excluded as if they (1) had other intracranial pathologies such as tumor or infection, and (2) had a neurological or psychiatric disease that could complicate neurologic evaluation. The study protocol was approved by the Melbourne Health Human Research Ethics Committee, and patient consent was not required because of the retrospective nature of the study.
Clinical evaluation
Baseline evaluation of patients was performed within 4.5 h after stroke onset and included demographic data, medical history, comorbidities including hypertension, diabetes, dyslipidemia, and atrial fibrillation, physical and neurological examination. Smoking is defined as consumption of > 100 cigarettes/lifetime or > 10 pack year history. Blood pressure was determined using the mean of 3 properly sized automated cuff readings, taken 1 min apart after 5 min of quiet rest without staff in the room. Baseline FPG levels were determined via the glucose oxidase method. Hyperglycemia was defined as blood glucose ≥ 7.8 mmol/L, and type 2 diabetes mellitus was diagnosed according to the 1999 World Health Organization (WHO) criteria. Baseline volume of intracerebral hemorrhage (ICH) was measured from the first available CT scan. Neurological assessment was performed using the modified Rankin Scale [14, 15] and the National Institutes of the Health Stroke Scale (NIHSS) at admission (baseline) and 90 days post-stroke onset. Patients were considered to have a favorable neurologic outcome if they had a 90-day modified Rankin Scale score of 0–2) and an unfavorable neurologic outcome if they had a 90-day modified Rankin Scale score of 3–6 [16].
Imaging analysis
All the patients underwent multimodal CT imaging including non-contrast CT, CT perfusion, and CT angiography before the reperfusion therapy as per our hospital stroke protocol. The images were reviewed by using standard PACS software. One stroke neurologist reviewed CTA images to identify patients with complete occlusion of the ICA, M1 or M2. Relative filling time delay (rFTD) was defined using the unprocessed 4D-CTA images as the time difference of the first contrast pacification in the MCA branches in the Sylvian fissure of the affected hemisphere compared to that in the corresponding contralateral non-affected MCA branches [17]. rFTD was independently assessed by 2 neurologists (F.W. and B.S.J.) blinded to clinical outcomes. rFTD ≤ 4 s was defined as good collateral circulation and > 4 s as poor collateral circulation. The agreement on rFTD assessment between two observers was calculated with a Kappa coefficient of 0.735 (P < 0.001) for inter-observer agreement.
Statistical analysis
The primary outcomes of this study were neurologic outcome based on 90-day modified Rankin Scale scores stratified by good versus poor collateral circulation. The secondary outcomes were 90-day mortality and ICH stratified by good versus poor collateral circulation. For the comparison of variables between two groups, Fisher’s exact test and Chi-square test were used for categorical data, and Wilcoxon two sample tests and Student’s t test were used for continuous data. Spearman’s nonparametric rank correlation was performed to assess the correlation between rFTD and baseline FPG, baseline NIHSS scores, and 90-day modified Rankin Scale scores. Logistic regression analysis was performed to investigate the association among clinical outcome, baseline FPG, and rFTD. The AUC was calculated by plotting the ROC of plasma glucose and adverse outcomes and the cutoff was obtained when Youden’s index was maximal.
A two-sided P value < 0.05 was considered to indicate statistical significance. STATA Version 12 (Stata Corp, College Station, Texas) was used for statistical analyses.
Results
Patient demographic and baseline characteristics
The study flowchart is shown in Fig. 1. Between January 2009 and December 2015, 532 patients with acute ischemic stroke presented to Royal Melbourne Hospital within 4.5 h from onset of stroke symptoms. Two hundred sixty-two patients were excluded because of lack of stroke evidence on CT or MRI (n = 48), occlusion of vessels other than the ICA or MCA (n = 146) or missing data including baseline FPG (n = 68). Finally, 270 patients were included in this retrospective analysis. Patient demographic and baseline characteristics are shown in Table 1. The median age of the patients was 74.00 years, and 54.1% were men. The median baseline NIHSS score was 14.00 (IQR, 9.00–19.00). Occlusion was present in the ICA in 64 (23.70%) patients, M1 MCA in 111 (41.11%) patients, and M2 MCA in 95 (35.19%) patients. The mean baseline FPG was 7.51 ± 2.97 mmol/L for the study population. Seventy-two (26.7%) patients had diabetes. The mean door to needle time was 66.05 ± 45.31 min (range 5.00–224.00 min).
Patient collateral circulation characteristics
The mean rFTD was 3.52 ± 2.97 s (range 0.00–16.00 s). One hundred eighty-five (68.5%) patients had good collateral circulation. Patients with occlusion in the M2 segment had the lowest rFTD (1.48 ± 1.76 s) followed by patients with occlusion in the ICA in (3.91 ± 3.17 s), while patients with occlusion in the M1 segment had the highest rFTD (5.05 ± 2.67 s; P = 0.000). The mean rFTD was comparable between patients with diabetes (3.93 ± 3.4 s) and those without (3.37 ± 2.79 s; P = 0.324) (Fig. 2a).
Patients with good collateral circulation had a significantly higher percentage of males (59.46%) than those with poor collateral circulation (42.35%; P = 0.009) (Table 2). Patients with good collateral circulation had significantly lower baseline FPG (7.20 ± 3.03 mmol/L) than those with poor collateral circulation (8.20 ± 2.73 mmol/L; P < 0.001) (Fig. 2b). They also had significantly lower baseline NIHSS scores (12.28 ± 6.39) than patients with poor collateral circulation (18.27 ± 6.13; P < 0.001). Occlusion occurred most often in M2 in patients with good collateral circulation (48.11%), but in M1 in those with poor collateral circulation (69.41%; P < 0.001). Patients with good and poor collateral circulation were comparable in other demographic and baseline variables (Table 2 and Supplementary Table 1). Baseline FPG in non-diabetic patients with good collateral circulation (6.55 ± 1.91 mmol/L) was also significantly lower than those with poor collateral circulation (7.95 ± 2.72 mmol/L; P < 0.001) (Fig. 2c). However, there was no statistically significant difference in baseline FPG between diabetic patients with good (9.09 ± 4.59 mmol/L) and poor collateral circulation (8.82 ± 2.71 mmol/L; P < 0.001) (Fig. 2d).
Primary outcome
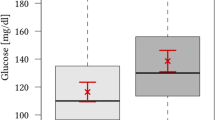
Totally 139 (51.5%) patients achieved a favorable neurologic outcome based on the 90-day modified Rankin Scale scores. Significantly greater portions of patients with good collateral circulation (60.5%, 112/185) achieved a favorable neurologic outcome compared to those with poor collateral circulation (31.8%, 27/85) (P < 0.05) (Table 3). Patients with good collateral circulation achieving a favorable neurologic outcome had significantly lower baseline FPG (6.6 ± 1.96) than those with good collateral circulation achieving an unfavorable neurologic outcome (8.12 ± 4.02; P = 0.002) (Fig. 3a). By contrast, patients with poor collateral circulation achieving a favorable neurologic outcome showed comparable baseline FPG (7.57 ± 2.41) versus those with poor collateral circulation achieving an unfavorable neurologic outcome (8.49 ± 2.84 mmol/L; P = 0.088) (Fig. 3b).
Spearman correlation analysis showed that rFTD significantly correlated with 90-day modified Rankin Scale scores (r = 0.318; P < 0.001) for the study population (Table 4). The correlation between rFTD and 90-day modified Rankin Scale scores still remained statistically significant after adjustment for FPG (r = 0.258; P < 0.001). Furthermore, rFTD correlated with modified Rankin Scale scores both in diabetes patients (r = 0.446; P < 0.001) and non-diabetes patients (r = 0.181; P < 0.001) after adjustment for FPG. In addition, rFTD significantly correlated with baseline FPG (r = 0.286; P < 0.001).
Secondary outcomes
Forty-seven (17.4%) patients died. The 90-day mortality was significantly lower in patients with good collateral circulation (9.2%, 17/185) than those with poor collateral circulation (35.3%, 30/85; P < 0.05) (Table 4). In patients with good collateral circulation, those who died had significantly higher baseline FPG (10.99 ± 5.61 mmol/L) than those who survived (6.81 ± 2.35 mmol/L; P = 0.000) (Fig. 4a). By contrast, in patients with poor collateral circulation, no statistically significant difference was observed in baseline FPG between those who died (7.65 ± 1.86 mmol/L) and those who did not (8.51 ± 3.08 mmol/L; P = 0.372) (Fig. 4b).
a Baseline FPG in acute ischemic stroke patients with good collateral circulation who died versus those who survived. b Baseline FPG in acute ischemic stroke patients with poor collateral circulation who died versus those who survived. c Baseline FPG in acute ischemic stroke patients with good collateral circulation who experienced ICH within 24 h of stroke onset versus those who did not. d Baseline FPG in acute ischemic stroke patients with poor collateral circulation who experienced ICH within 24 h of stroke onset versus those who did not
In addition, 56 (20.7%) patients experienced ICH within 24 h of stroke onset. Significantly higher proportions of patients with poor collateral circulation (31.8%, 27/85) than those with good collateral circulation (15.7%, 29/185; P < 0.05) experienced ICH (Table 4). In patients with good collateral circulation, no statistically significant difference was observed in baseline FPG between those who developed ICH (8.21 ± 4.52 mmol/L) and those who did not (7.01 ± 2.65 mmol/L; P = 0.324) (Fig. 4c). Meanwhile, in patients with poor collateral circulation, those who developed ICH had higher but statistically insignificant baseline FPG (9.26 ± 3.58 mmol/L) than those who did not (7.71 ± 2.09 mmol/L; P = 0.050) (Fig. 4d).
Subgroup analysis
Totally 138 (138/270, 51.1%) patients were non-diabetic and had good collateral circulation and 63.8% (88/138) of them had a favorable neurologic outcome and 50 (50/138, 36.2%) had an unfavorable outcome. Subgroup analysis further showed non-diabetic patients with good collateral circulation who achieved a favorable neurologic outcome were noticeably younger than those who achieved an unfavorable neurologic outcome (P = 0.009) (Table 5). They also had a significantly lower NIHSS score than those who achieved an unfavorable neurologic outcome (P = 0.012). Furthermore, non-diabetic patients with good collateral circulation who achieved a favorable neurologic outcome had significantly lower baseline FPG than those who achieved an unfavorable neurologic outcome (P = 0.008). In the logistic analysis model using unfavorable 90-day modified Rankin Scale as a dependent variable, after adjustment for DBP, SBP, NIHSS, age and atrial fibrillation, FPG was associated with increased risk of an unfavorable neurologic outcome [OR(95% CI) = 1.247(1.026,1.517); P = 0.027)].
Eight (5.8%) non-diabetic patients with good collateral circulation died. They had significantly higher baseline DBP than those who were alive (P = 0.007) (Supplementary Table 1). Patients who died also had markedly higher baseline FPG than those who were alive (P = 0.001). In the logistic analysis model using death as a dependent variable, after adjustment for DBP, FPG was associated with increased risk of death [OR(95% CI) = 1.532(1.114,2.108); P = 0.009)].
For non-diabetic patients with good collateral circulation, ROC analysis showed that baseline FPG had an AUC of 0.636 (95% CI 0.537–0.735) for an unfavorable neurologic outcome and that at a cutoff of 6.25, the sensitivity was 0.64 and the specificity was 0.682 (Fig. 5a and Supplementary Table 2). In addition, baseline FPG had an AUC of 0.839 (95% CI 0.745–0.933) for 90-day mortality and at a cutoff of 6.45, the sensitivity was 1.00 and the specificity was 0.685 (Fig. 5b).
Discussion
The current study revealed that 68.5% of the study population had good collateral circulation and their baseline FPG was significantly lower than that of patients with poor collateral circulation. Importantly, significantly greater portions of patients with good collateral circulation achieved a favorable neurologic outcome versus those with poor collateral circulation. Furthermore, collateral circulation significantly correlated with 90-day modified Rankin Scale scores, even after adjustment for baseline FPG. Our findings are consistent with earlier studies [17,18,19] and demonstrate that collateral circulation status is associated with the functional outcome of acute ischemic stroke patients.
Cao et al. [17] hypothesized that delayed filling of the middle cerebral artery (MCA) in the Sylvian fissure due to poor collateral flow may be associated with worse radiologic and functional outcome after ischemic stroke, and using CT perfusion, they demonstrated that rFTD was a useful independent predictor of clinical outcome after ischemic stroke. Dankbarr et al. [18] analyzed a prospective cohort of 188 acute ischemic stroke patients with a M1 MCA occlusion and found that patients with poor collateral filling by CT perfusion had a significantly worse outcome (90-day modified Rankin Scale 3–6; 80% vs. 52%, P = 0.001). Similar findings were also reported by van Seeters et al. [19]. We also demonstrated that rFTD correlated with the neurologic outcome of acute ischemic stroke patients (r = 0.318; P < 0.001; good collaterals: 60.5% vs. poor collaterals: 31.8%).
Hyperglycemia is commonly encountered in both diabetic and non-diabetic patients in acute ischemic stroke and is found in 30–40% of patients with acute ischemic stroke [16, 20]. Although admission plasma glucose has been found to be an independent prognostic predictor of neurologic outcome of acute ischemic stroke patients, this statistical predictor function has not been proven therapeutically. Clinical investigations aiming for glucose control in acute ischemic stroke have failed to improve functional outcome or reduce mortality [11, 21]. We speculate that this failure can be partially attributed to the heterogeneity of acute ischemic stroke patients. This study delineated the collateral circulation status of acute ischemic patients by CT perfusion and defined a subset of non-diabetic acute ischemic stroke patients with good collateral circulation. This subset constituted a significant proportion (51.1%) the study population. Moreover, 63.8% of this subset of patients achieved a favorable neurologic outcome versus 51.5% of the total study population and 36.2% of non-diabetic acute ischemic stroke patients with poor collateral circulation. Non-diabetic acute ischemic stroke patients with good collateral circulation also had a higher rate of favorable neurologic outcome than non-diabetic acute ischemic stroke patients (56.6%) and non-diabetic acute ischemic stroke patients in general (37.5%) (Supplementary Table 3). These findings indicate that non-diabetic acute ischemic stroke patients with good collateral circulation represent a subset of acute ischemic stroke patients with the best 90-day neurologic outcome.
Higher admission plasma glucose is an independent predictor of poor neurologic outcome and mortality in acute ischemic stroke patients [22]. We further investigated the association of baseline FPG with neurologic outcome of non-diabetic acute ischemic stroke patients with good collateral circulation. We found that baseline FPG correlated with 90-day modified Rankin Scale scores of these patients, even after adjustment for rFTD, and was associated with increased risk of an unfavorable neurologic outcome [OR(95% CI) = 1.247(1.026,1.517); P = 0.027)]. ROC analysis further showed that admission plasma glucose had an AUC of 0.636 (95% CI 0.537–0.735) for an unfavorable neurologic outcome. Earlier studies have indicated that hyperglycemia in acute ischemic stroke is associated with increased morbidity and mortality [14, 23, 24]. The results of the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS-ISTR) showed that admission hyperglycemia was an independent predictor of higher mortality and lower independence [25]. An earlier systemic review of more than twenty studies revealed that acute hyperglycemia predicts increased risk of in-hospital mortality after ischemic stroke in non-diabetic patients and increased risk of poor functional recovery in non-diabetic stroke survivors [20]. The results of our study support the proposition that admission PFG levels predict short-term outcome of non-diabetic acute ischemic stroke patients with good collateral circulation. It currently remains unclear whether hyperglycemia in acute ischemic stroke is an epiphenomenon of underlying stroke severity or if itself is directly harmful to the ischemic brain [16]. It remains to be investigated whether lowering plasma glucose in this subset of acute ischemic stroke patients translates into improved functional outcome of these patients.
Furthermore, rFTD correlated with the short-term neurologic outcome of acute ischemic stroke patients, and in both diabetes and non-diabetes patients. These findings suggest that rFTD may be a useful predictor of short-term neurologic outcome of acute ischemic stroke patients. Collateral circulation plays an important role in maintaining tissue viability during large vessel occlusion. Good collateral circulation can limit infarct volume expansion and improve functional status in ischemic stroke patients, while poor collateral circulation is insufficient to sustain cerebral perfusion in the penumbra and increased infarct volume [26]. Previous studies have indicated that variations in collateral circulation anatomy might be associated with infarct volume expansion in high glucose patients with good collateral circulation. Prado et al. [27] reported that cortical infarct regions were vulnerable to the deleterious effects of high glucose in the presence of collateral circulation. We also observed that FPG correlated with rFTD in non-diabetic acute ischemic stroke patients.
Mitchell et al. [28] demonstrated that a significant proportion of patients admitted to hospital with stroke or transient ischemic attack had undiagnosed diabetes mellitus with 37% patients experiencing hyperglycemia on at least one occasion during the first 5 days of admission. Bravata et al. [29] demonstrated that hyperglycemic stroke patients without a previous diagnosis of diabetes are not routinely screened for diabetes. Gray et al. [30] studied 582 consecutive acute stroke patients and found that at 12 weeks post-admission impaired glucose tolerance or diabetes mellitus were present in two-thirds of survivors presenting with post-stroke hyperglycemia. Masrur et al. [31] identified 58,265 acute ischemic stroke patients receiving tPA therapy and found that hyperglycemia was associated with a poorer neurologic outcome. Given the common occurrence of hyperglycemia in both diabetic and non-diabetic stroke patients, it is important to be vigilant in the detection of hyperglycemia in acute ischemic stroke patients and also important to identify the subpopulation of hyperglycemic acute ischemic stroke patients that are truly at risk of an adverse neurologic outcome.
We know that cerebral collaterals can partially maintain blood flow to the ischemic penumbra in acute ischemic stroke. We theorized that in ischemic stroke, good collateral circulation may facilitate transport of plasma glucose to the ischemic penumbra, thus worsening glucose neurotoxicity and neurologic outcome, which may not occur in ischemic stroke patients with poor collateral circulation. The current study found that, indeed, higher admission FPG levels are associated with significantly higher rates of unfavorable neurologic outcome of acute ischemic stroke patients with good collateral circulation, but not in those with poor collateral circulation. Our findings also showed significant correlation between baseline FPG and rFTD, suggesting that baseline FPG and rFTD may serve as useful predictors of short-term patient outcome and for risk stratification of hyperglycemic acute ischemic stroke patients. Hyperglycemia remains under-monitored, undertreated and underreported in acute ischemic stroke patients. Our study highlights the clinical significance of collateral circulation status in hyperglycemic acute ischemic stroke patients and shows that the subpopulation of acute ischemic stroke patients with acute hyperglycemia who have good collateral circulation should be more closely monitored and promptly treated to lessen an adverse neurologic outcome.
This study had several limitations. First, it is an observational exploratory study based on retrospective analysis. Second, there were a relatively low number of patients in poor collateral group. Third, we do not have any time course data on glucose levels. FPG was only registered once at admission. Nitaios et al. [32] showed that FPG at 24–48 h post-stroke onset did not predict a worse function outcome of acute ischemic stroke patients. Luitse et al. [33] found that chronic hyperglycemia was associated with a poor functional outcome independent of acute hyperglycemia. It would be worthwhile to further delineate the relation of FPG at different time points with patient outcome. Furthermore, A1c was not used to assess patient diabetic status. Hyperglycemia itself may be due to stress or undiagnosed diabetes and it remains possible that a previous undiagnosed diabetic status cannot be excluded without A1c dosage at admission or during hospital stay. Lastly, for observing the effect of collateral status on the correlation between admission FPG and outcome, patients included in our study were those with large artery occlusion and within 4.5 h from symptom onset.
In conclusion, higher admission FPG levels are associated with significantly higher rates of unfavorable neurologic outcome of non-diabetic acute ischemic stroke patients with good collateral circulation. FPG and rFTD may serve as useful predictors of short-term patient outcome and could be used for risk stratification in clinical decision making.
References
Gao X et al (2012) Admission clinical characteristics and early clinical outcomes among acute ischemic stroke patients. J Biomed Res 26(3):152–158
Jeng JS et al (2008) Predictors of survival and functional outcome in acute stroke patients admitted to the stroke intensive care unit. J Neurol Sci 270(1–2):60–66
Kwak HS et al (2013) Predictors of functional outcome after emergency carotid artery stenting and intra-arterial thrombolysis for treatment of acute stroke associated with obstruction of the proximal internal carotid artery and tandem downstream occlusion. AJNR Am J Neuroradiol 34(4):841–846
Scott JF et al (1999) Prevalence of admission hyperglycaemia across clinical subtypes of acute stroke. Lancet 353(9150):376–377
Bruno A, Williams LS, Kent TA (2004) How important is hyperglycemia during acute brain infarction? Neurologist 10(4):195–200
Weir CJ et al (1997) Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ 314(7090):1303–1306
Williams LS et al (2002) Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology 59(1):67–71
Stead LG et al (2009) Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care 10(2):181–186
Osei E et al (2017) Admission glucose and effect of intra-arterial treatment in patients with acute ischemic stroke. Stroke 48(5):1299–1305
Gray CS et al (2007) Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol 6(5):397–406
Bruno A et al (2008) Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke 39(2):384–389
Winship IR (2015) Cerebral collaterals and collateral therapeutics for acute ischemic stroke. Microcirculation 22(3):228–236
Hatano S (1976) Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 54(5):541–553
Cox NH, Lorains JW (1986) The prognostic value of blood glucose and glycosylated haemoglobin estimation in patients with stroke. Postgrad Med J 62(723):7–10
Uyttenboogaart M et al (2005) Optimizing cutoff scores for the Barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke 36(9):1984–1987
Uyttenboogaart M et al (2007) Moderate hyperglycaemia is associated with favourable outcome in acute lacunar stroke. Brain 130(Pt 6):1626–1630
Cao W et al (2014) Relative filling time delay based on CT perfusion source imaging: a simple method to predict outcome in acute ischemic stroke. AJNR Am J Neuroradiol 35(9):1683–1687
Dankbaar JW et al (2017) Internal carotid artery stenosis and collateral recruitment in stroke patients. Clin Neuroradiol. https://doi.org/10.1007/s00062-017-0568-x
van Seeters T et al (2015) The prognostic value of CT angiography and CT perfusion in acute ischemic stroke. Cerebrovasc Dis 40(5–6):258–269
Capes SE et al (2001) Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32(10):2426–2432
Rosso C et al (2012) Intensive versus subcutaneous insulin in patients with hyperacute stroke: results from the randomized INSULINFARCT trial. Stroke 43(9):2343–2349
Kruyt ND et al (2010) Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol 6(3):145–155
Bruno A et al (2002) Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology 59(5):669–674
Pulsinelli WA et al (1983) Increased damage after ischemic stroke in patients with hyperglycemia with or without established diabetes mellitus. Am J Med 74(4):540–544
Ahmed N et al (2010) Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: results from the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS-ISTR). Arch Neurol 67(9):1123–1130
Kim JJ et al (2004) Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke 35(6):1340–1344
Prado R et al (1988) Hyperglycemia increases infarct size in collaterally perfused but not end-arterial vascular territories. J Cereb Blood Flow Metab 8(2):186–192
Mitchell EA et al (2012) Hyperglycaemia monitoring and management in stroke care: policy vs. practice. Diabet Med 29(9):1108–1114
Bravata DM et al (2003) Hyperglycaemia in patients with acute ischaemic stroke: how often do we screen for undiagnosed diabetes? QJM 96(7):491–497
Gray CS et al (2004) Prevalence and prediction of unrecognised diabetes mellitus and impaired glucose tolerance following acute stroke. Age Ageing 33(1):71–77
Masrur S et al (2015) Association of acute and chronic hyperglycemia with acute ischemic stroke outcomes post-thrombolysis: findings from get with the guidelines-stroke. J Am Heart Assoc 4(10):e002193
Ntaios G et al (2011) Persistent hyperglycemia at 24–48 h in acute hyperglycemic stroke patients is not associated with a worse functional outcome. Cerebrovasc Dis 32(6):561–566
Luitse MJ et al (2017) Chronic hyperglycemia is related to poor functional outcome after acute ischemic stroke. Int J Stroke 12(2):180–186
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights disclosure
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Melbourne Health Human Research Ethics Committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Patient consent was not required because of the retrospective nature of the study.
Additional information
Managed by Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
592_2018_1139_MOESM1_ESM.tif
Supplementary Figure 1 ROC curve of baseline FPG for 90-day mortality in diabetic patients with good collateral circulation. (TIFF 299 kb)
Rights and permissions
About this article
Cite this article
Wang, F., Jiang, B., Kanesan, L. et al. Higher admission fasting plasma glucose levels are associated with a poorer short-term neurologic outcome in acute ischemic stroke patients with good collateral circulation. Acta Diabetol 55, 703–714 (2018). https://doi.org/10.1007/s00592-018-1139-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1139-6