Abstract
Objective
To determine if differences in outcome exist between diabetic and non-diabetic patients who present to the Emergency Department (ED) with acute ischemic stoke (AIS) and elevated blood glucose.
Methods
The study population consisted of 447 consecutive patients who presented to the ED with AIS within 24 h of symptom onset and had blood glucose measured on presentation. Hyperglycemia was defined as >130 mg/dl. Outcomes studied included infarct volume, stroke severity (NIH Stroke Scale), functional impairment (modified Rankin Score), and 90-day mortality. Patients with hyperglycemia were then stratified into those with and without a prior history of diabetes mellitus (DM) for the purposes of analysis.
Results
Patients with hyperglycemia exhibited significantly greater stroke severity (P = 0.002) and greater functional impairment (P = 0.004) than those with normoglycemia. Patients with hyperglycemia were 2.3 times more likely to be dead at 90 days compared to those with normal glucose (P < 0.001). Stroke severity (P < 0.001) and functional impairment (P < 0.001) were both significantly worse in patients with hyperglycemia and no prior history of DM, when compared to patients with hyperglycemia and previously diagnosed DM. Among the patients without a prior history of DM, patients with hyperglycemia were 3.4 times more likely to die within 90 days (P < 0.001) when compared with patients with normoglycemia. In contrast, the hazard ratio was 1.6 among the patients with DM (P = 0.66).
Conclusion
Hyperglycemia on presentation is associated with significantly poorer outcomes following AIS. Patients with hyperglycemia and no prior history of DM have a particularly poor prognosis, worse than that for patients with known diabetes and hyperglycemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing interest has focused on the role of hyperglycemia in the evolution of acute ischemic stoke (AIS). The role of chronic hyperglycemia in the etiology of stroke is well established. Diabetes mellitus (DM) is an independent risk factor for ischemic stroke, increasing stroke risk by two to threefold. It also confers poor prognosis following stroke, associated with increased mortality and poor functional recovery [1, 2].
In this study we sought to determine if having elevated serum glucose at the time of initial stroke presentation results in worse outcomes. We further sought to evaluate the impact of hyperglycemia at presentation in those with and without a prior diagnosis of DM.
Methods
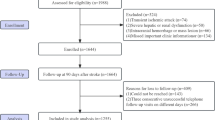
This study was conducted in the Emergency Department (ED) of an academic medical center with an annual ED census of approximately 77,000. The initial study population consisted of 681 consecutive patients who presented to the ED with acute ischemic stroke between December 2001 and March 2004. Among these 681 patients, 19 denied research authorization and were therefore excluded from further study in accordance with Minnesota Statute 144.335, leaving a total of 662 patients. Of the 662 patients, 480 presented within the first 24 h of onset. Of these 480, 447 had a blood glucose measurement done in the ED, and this comprises the final study cohort.
The medical records for all patients were reviewed and details of clinical history, laboratory results, demographic information, risk factor profile, neurological examination, brain-imaging studies, and other diagnostic studies were abstracted. Independent variables included hyperglycemia at admission and a prior diagnosis of DM. Hyperglycemia was defined as a random (non-fasting) serum glucose of 130 mg/dl or greater, consistent with the cutoff used in prior studies [3, 4]. Dependent variables included infarct volume, stroke severity, functional impairment, and death at 90-days.
Infarct volume was measured using the General Electric Medical Systems Advantage Windows workstation and the associated version 4.2 software to measure the areas of interest on the diffusion weighted MRI scans. Images were acquired on a 1.5 Tesla GE Signa scanner. Total volume of the infarct was measured by an algorithmic approach incorporating and adding effective mapped out areas on all MR slices depicting the infarct.
Stroke severity was determined using the National Institutes of Health Stroke Scale (NIHSS) which was calculated by a certified physician, based on the previously validated methods [5, 6]. The scoring was derived from documentation of the neurological examination performed by the neurologist at the time of ED presentation. A logarithmic transformation was applied to the values to obtain a more normal distribution based on visual inspection of histograms and normal probability plots.
Ischemic stroke subtype was assigned based on the review of clinical history, neurological examination, and diagnostic studies in accordance with criteria outlined in the TOAST study [7].
Functional outcome was measured by the modified Rankin Scale (mRS) [8] and a poor Rankin was defined as 3 or greater. Associations between hyperglycemia and poor functional status at hospital discharge (mRS) were evaluated by fitting logistic regression models. The strength of the associations was summarized by calculating odds ratios (OR) and corresponding 95% confidence intervals (CI).
Death at 90 days was ascertained using the date of the last service or dismissal available from registration databases. In addition, dates and causes of death were established from the State of Minnesota Electronic Death Certificate Data. Autopsy reports were also reviewed where available. For the outcome of death at 90 days only, the cohort was limited to those who resided in the local county or the surrounding nine-county area at the time of the ED visit in order to assure recent and consistent follow-up (n = 381). Since not all patients had at least 90 days of follow-up, survival was estimated using the Kaplan–Meier method [9] to take into account the varying length of follow-up. For patients who died within 90 days, the duration of follow-up was calculated from the date of the ED admittance to the date of death. The duration of follow-up for all remaining patients was censored at the date of last follow-up if within 90 days, or at 91 days. Cox proportional hazards models were fit to evaluate associations with survival. These associations were summarized by calculating hazard ratios (HR) and corresponding 95% CIs.
Results
Demographics of the study cohort is summarized in Table 1. A total of 153 patients (34.2%) had blood glucose >130 mg/dl on presentation. This hyperglycemic group was noted to have significantly greater stroke severity (P = 0.002); other parameters such as stroke subtype and volume of infarct were not significantly different from the normoglycemic group (Table 2). Interestingly, this association of hyperglycemia with stroke severity is only noted in those without a prior diagnosis of DM (P < 0.001), while in the diabetic subgroup, there is no statistically significant association (P = 0.086). Furthermore, there is a significantly higher frequency of cardioembolic strokes in non-diabetics with hyperglycemia (P = 0.005) (Table 3).
There were 381 patients in the survival analysis. Seventy-nine deaths occurred in the first 90 days. Overall, patients with hyperglycemia at presentation were significantly more likely to be dead at 90 days (HR = 2.3, 95% CI 1.5–3.6, P < 0.001). Even after adjusting for the major confounders of age and stroke severity, AIS patients with hyperglycemia at presentation were 1.8 times more likely to die within 90 days (95% CI 1.1–2.9, P = 0.012). Looking at the subgroup without the history of DM, patients with hyperglycemia were 3.4 times more likely to die within 90 days (95% CI 2.1–5.8, P < 0.001) when compared with patients with normoglycemia (Fig. 1). This association persisted after adjusting for age and NIHSS (HR = 2.0, 95% CI 1.1–3.3, P = 0.004). In contrast, there was no statistically significant association between hyperglycemia and death among the patients with DM (HR = 1.6, 95% CI 0.5–5.0, P = 0.66).
When the outcome of functional impairment (mRS) was assessed overall, it appeared that those with normoglycemia did worse (poor mRS = 53.7% [normoglycemic] vs. 39.5% [hyperglycemic], P = 0.004). As this is counterintuitive, we searched for effect modification. Indeed, there is an interaction effect between DM and elevated glucose, in that the relationship between elevated glucose and Rankin is different depending on whether or not the patients have DM. In particular, among the patients without a history of DM, patients with elevated glucose were more likely to have a poor mRS (OR 3.6; 95% CI 2.0–6.5, P < 0.001), whereas among the patients with a history of DM there was no clear impact of hyperglycemia on poor functional outcome (mRS) (OR = 0.7; 95% CI 0.3–1.5, P = 0.32).
Discussion
In this study we found that hyperglycemia at the time of presentation to the ED with AIS conferred a worse prognosis in unselected patients. This has been shown in numerous previous studies [10] but debate continues as to if it is a contributing factor to the more severe stroke or merely a stress response to a larger infarct. In this study the group with hyperglycemia had significantly more severe strokes (higher NIHSS), and the association with increased risk of death persisted after adjustment for age and severity. The volume of infarct did not differ, and was neither a confounder nor an effect modifier. The regression model results were similar whether volume was included or not. This would suggest that the hyperglycemia does in fact contribute in some way to the worsening of the initial infarct, irrespective of volume, and severity.
The overwhelming majority of basic science research supports the theory that elevated blood glucose at the time of infarction worsens the ultimate outcome [11–13]. Numerous animal studies have examined this and have postulated mechanisms as to how hyperglycemia may be toxic to the brain tissue and in particular the vulnerable ischemic penumbra. Theories include the accumulation of lactic acid due to anaerobic metabolism, enhanced glutamate release, and increased cerebral edema among others [14–16]. More recently work has been done to demonstrate the effect of hyperglycemia on the ischemic penumbra in humans. Parsons et al. [17] using MRI and magnetic resonance spectroscopy, established that hyperglycemia in patients with acute perfusion–diffusion mismatch was correlated with reduced salvage of mismatched tissue, greater final infarct size, and worse functional outcome.
In our study we further subdivided our patients and found that it is only the patients with hyperglycemia and no prior known history of DM that have a worse prognosis. Capes et al. [18] had similar findings in a meta-analysis of 32 cohort studies. In non-diabetic patients the relative risk of in-hospital or 30-day mortality in those with admission glucose of over 126 mg/dl was increased threefold compared to non-diabetic patients with normal serum glucose. Interestingly, among diabetic patients admission hyperglycemia was not associated with a significantly higher short-term mortality. One explanation is that the patients with hyperglycemia at presentation may have impaired glucose metabolism or undiagnosed DM. However in order to decipher whether undiagnosed diabetes was at play, when we reviewed the glycosylated hemoglobin (HbA1c) levels in our cohort, we found that patients with hyperglycemia and no known history of DM had a median HbA1c of 6.0% which is under the abnormal cutoff of 7.0%, suggesting that the hyperglycemia at stroke presentation was likely not due to underlying, albeit undiagnosed diabetes.
Another explanation for Capes et al. findings and ours could be that pre-conditioning by chronic exposure to elevated blood sugar levels may offset adverse metabolic effects, which may influence prognosis in non-diabetics. Since diabetic patients have a higher baseline glucose level we may need to use higher cutoff levels to detect stress hyperglycemia in this population when compared with the non-diabetic population. Finally, it is possible that certain medications that diabetics are frequently prescribed such as statins, antihypertensive, and antiplatelet agents may in some way confer a protective effect.
In our study it is clear that elevated glucose levels at the time of ED presentation lead to a higher short-term mortality. We found this effect most marked in our non-diabetic population. It is clear from recent literature that hyperglycemia during acute stroke is not consistently controlled. In one study of 253 patients with acute cerebral infarction and admission hyperglycemia, 76% remained hyperglycemic throughout their hospital stay and 40% received no hypoglycemic drugs [19]. Whether treating hyperglycemia in the acute phase of stroke beginning in the ED improves outcome remains to be seen. Clinical trials are currently underway evaluating the effect of achieving euglycemia in the acute phase of ischemic stroke. Meanwhile, guidelines regarding the management of acute ischemic stroke published by the American Stroke Association [20] and the European Stroke Initiative [21] recommend gradual lowering of high blood glucose levels with insulin infusion.
The limitations of the present study are those attributable to historical cohort designs, in a single center located in an area with low ethnic diversity with predominantly Caucasian population.
Summary
In summary, we undertook a study of ED patients presenting with acute ischemic stroke and find that hyperglycemia on presentation is associated with significantly poorer outcomes following acute ischemic stroke. Patients with hyperglycemia but no prior history of DM have a particularly poor prognosis, worse than that for patients with known diabetes and hyperglycemia. Whether early intervention with measures to aggressively control blood glucose levels in these patients may favorably influence their clinical course awaits clarification from randomized clinical trials.
References
Toni D, Sacchetti ML, Argentino C, Gentile M, Cavalletti C, Frontoni M, et al. Does hyperglycaemia play a role on the outcome of acute ischaemic stroke patients? J Neurol. 1992;239:382–6.
Olsson T, Viitanen M, Asplund K, Eriksson S, Hagg E. Prognosis after stroke in diabetic patients. A controlled prospective study. Diabetologia. 1990;33:244–9.
Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow-up study. BMJ. 1997;314:1303–6.
Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, et al. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;59:67–71.
Kasner SE, Chalela JA, Luciano JM, Cucchiara BL, Raps EC, McGarvey ML, et al. Reliability and validity of estimating the NIH stroke scale score from medical records. Stroke. 1999;30:1534–7.
Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31:858–62.
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.
Rankin J. Cerebral vascular accidents in patients over age 60. Scott Med J. 1957;2:200–15.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Gilmore RM, Stead LG. The role of hyperglycemia in acute ischemic stroke. Neurocrit Care. 2006;5:153–8.
Myers RE, Yamaguchi S. Nervous system effects of cardiac arrest in monkeys. Preservation of vision. Arch Neurol. 1977;34:65–74.
Pulsinelli WA, Waldman S, Rawlinson D, Plum F. Moderate hyperglycemia augments ischemic brain damage: a neuropathologic study in the rat. Neurology. 1982;32:1239–46.
Lin B, Ginsberg MD, Busto R. Hyperglycemic exacerbation of neuronal damage following forebrain ischemia: microglial, astrocytic and endothelial alterations. Acta Neuropathol. 1998;96:610–20.
Hoxworth JM, Xu K, Zhou Y, Lust WD, LaManna JC. Cerebral metabolic profile, selective neuron loss, and survival of acute and chronic hyperglycemic rats following cardiac arrest and resuscitation. Brain Res. 1999;821:467–79.
Benveniste H. The excitotoxin hypothesis in relation to cerebral ischemia. Cerebrovasc Brain Metab Rev. 1991;3:213–45.
Kawai N, Keep RF, Betz AL. Effects of hyperglycemia on cerebral blood flow and edema formation after carotid artery occlusion in Fischer 344 rats. Acta Neurochir Suppl. 1997;70:34–6.
Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–8.
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–32.
Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, et al. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;59:67–71.
Adams HP Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, et al. Stroke Council of the American Stroke Association. Guidelines for the early management of patients with ischemic stroke: a scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056–83.
Hack W, Kaste M, Bogousslavsky J, Brainin M, Chamorro A, Lees K, et al. European Stroke Initiative Recommendations for Stroke Management—update 2003. Cerebrovasc Dis. 2003;16:311–37.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ethics committee approval: The article is part of the project “Emergency Department Stroke Registry” with the Institutional Review Board approval number 1056-04.
Rights and permissions
About this article
Cite this article
Stead, L.G., Gilmore, R.M., Bellolio, M.F. et al. Hyperglycemia as An Independent Predictor of Worse Outcome in Non-diabetic Patients Presenting with Acute Ischemic Stroke. Neurocrit Care 10, 181–186 (2009). https://doi.org/10.1007/s12028-008-9080-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-008-9080-0