Abstract
Aims
To assess the metabolic health of obese and non-obese women at high GDM risk 5 years postpartum.
Methods
This is a secondary analysis of the 5-year follow-up of the RADIEL GDM prevention study including 333 women at high GDM risk (BMI ≥ 30 kg/m2 and/or previous GDM). Five years postpartum metabolic health was assessed including anthropometric measurements, oral glucose tolerance test, lipid metabolism, and body composition as well as medical history questionnaires. For the analysis, we divided the women into four groups based on parity, BMI, and previous history of GDM.
Results
Five years postpartum impaired glucose regulation (IFG, IGT, or diabetes) was diagnosed in 15% of the women; 3.6% had type 2 diabetes. The highest prevalence was observed among obese women with a history of GDM (26%), and the lowest prevalence (8%) among primiparous obese women (p = 0.021). At follow-up 25–39% of the obese women fulfilled the diagnostic criteria for the metabolic syndrome, in the non-obese group 11% (p < 0.001). This was associated with body fat percentage. The non-obese group, however, faced metabolic disturbances (IFG, IGT, diabetes, or metabolic syndrome) at a significantly lower BMI (p < 0.001). Among women who were non-obese before pregnancy, 5 years postpartum, the obesity prevalence based on BMI was 14% and based on body fat percentage 58%.
Conclusions
The prevalence of impaired glucose regulation and metabolic syndrome is high 5 years postpartum among women at high risk of GDM. There are high-risk women also among the non-obese, who develop metabolic derangements already at a lower BMI.
Clinical trial registration
ClinicalTrials.gov, www.clinicaltrials.com, NCT01698385.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, the prevalence of obesity and consequently that of type 2 diabetes is increasing in epidemic proportions. Along with the escalating trend in obesity, there is a similar increase in the prevalence of gestational diabetes (GDM), which in 2016 in Finland reached 18% [1]. Obesity, however, is not the only risk factor for GDM. Other risk factors include advanced age, family history of diabetes, parity, non-Caucasian ethnicity, previous macrosomia, and history of GDM [2].
Type 2 diabetes is known to have multiple underlying pathophysiological mechanisms [3], and the same is true for GDM. The heterogeneity of GDM, however, has received less interest. Already in the 1980s, studies demonstrated differences among women with GDM in insulin secretion, insulin sensitivity, autoimmunity, and adiposity [4, 5]. Women with GDM can be grouped according to their insulin sensitivity and insulin secretion profile; 50% are mainly insulin resistant, and 30% have an impairment in their insulin secretion, while the rest have a combination [6]. Our previous findings have highlighted the heterogeneity of GDM; non-obese participants with a history of GDM had the highest incidence of GDM, despite being metabolically healthy in the first trimester [7].
Women with a history of GDM are at a seven times higher risk of developing type 2 diabetes, the metabolic syndrome, and cardiovascular disease later in life [8,9,10,11,12]. Previous follow-up studies, however, have primarily been performed in an obese population, or the analyses have not taken into account the heterogeneity of GDM. Non-obese women with GDM have an altered insulin secretion profile during pregnancy [13], which is still evident 5 years after delivery [14]. To our knowledge, there are no other published studies focusing on the long-term cardio-metabolic risk profile of non-obese women with a history of GDM.
The aim of this study was to assess the future risk of metabolic disturbances among women in the RADIEL study—i.e., women at high GDM risk—taking into account the heterogeneity of GDM.
Methods
Study design
This is a secondary analysis of women participating in the RADIEL follow-up study, a prospective observational cohort study conducted in Helsinki and Lappeenranta (2013–2017). The original RADIEL study [15] was a GDM prevention study focusing upon women at high GDM risk, conducted in Finland (2008–2014) in the three maternity hospitals in the Helsinki area (University Hospital of Helsinki HUH, Jorvi Hospital, and Kätilöopisto Maternity Hospital) and in the South Karelian Central Hospital (SKCH) in Lappeenranta. In total 720 women were recruited either before or in early pregnancy and randomized to either lifestyle intervention or a control group. Previous publications [15, 16] have presented the details of the original intervention study.
Participants with a live birth, and their children, were invited to a follow-up visit 4–6 years after delivery. In this study, they comprised a cohort at high diabetes risk without considering the previous assignment into an intervention or a control group.
Participants
Originally the RADIEL study included women who were 18 years of age or older, with a BMI ≥ 30 kg/m2 and/or a history of GDM in a previous pregnancy. Exclusion criteria were current diabetes, medications altering glucose metabolism, multiple pregnancy, severe psychiatric problems, physical disabilities, and communication problems based on inadequate language skills. Among the participants, 228 entered the study in pre-pregnancy and 492 in early pregnancy before 20 gestational weeks. In total 348 women participated in the follow-up, but in this study, we included those 333 women who attended an oral glucose tolerance test (OGTT) or had a physician-diagnosed diabetes after the index pregnancy. All participants gave written informed consent and the Ethics Committees of HUH and SKCH approved the study design.
Outcomes
The main outcome was the cumulative incidence of impairment in glucose regulation. A secondary outcome was presence of the metabolic syndrome and its components. Diagnosis of diabetes was either self-reported (diagnosed by a physician and/or use of antidiabetic drugs) or based on the following laboratory tests: fasting plasma glucose ≥ 7.0 mmol/l or 2-h glucose in a 2-h 75 g OGTT ≥ 11.1 mmol/l. Impaired glucose tolerance (IGT) was defined as 2-h glucose 7.8–11.0 mmol/l and impaired fasting glucose (IFG) as fasting plasma glucose 6.1–6.9 mmol/l. Meeting the criteria for either IFG, IGT, or type 2 diabetes resulted in the composite outcome of impaired glucose regulation. In addition to glucose metabolism, the laboratory tests performed in conjunction with the study visit included assessment of insulin, glycated hemoglobin, lipids (cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol), thyroid function (TSH and free thyroxine), alanine aminotransferase, and highly sensitive C-reactive protein. In the analysis of hs-CRP, we excluded values exceeding 10 mmol/l. Previous reports include description of the methods used for laboratory analysis [7].
For assessing the metabolic syndrome, we used the National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATP III) criteria [17]. Meeting three out of five criteria resulted in the diagnosis of the metabolic syndrome: waist circumference > 88 cm, fasting plasma glucose ≥ 5.6 mmol/l, triglycerides ≥ 1.7 mmol/l, HDL < 1.29 mmol/l, and systolic blood pressure > 130 mmHg or diastolic blood pressure > 85 mmHg. Additional markers were the use of medication influencing blood pressure, HDL, or triglyceride levels. We also analyzed a composite metabolic outcome defined as having either impaired glucose regulation (IFG, IGT, or type 2 diabetes) and/or metabolic syndrome.
The study visit included anthropometric measurements as well as assessment of blood pressure in the sitting position from the right arm with a sphygmomanometer. Body composition was assessed by a multi-frequency bio-impedance measurement method (InBody3.0, Biospace Co., Ltd, Seoul, Korea) [18]. American Council on exercise guidelines [19] provided the definition of obesity based on body fat percentage of 32% or over. Questionnaires provided data on chronic diseases, regular medications, and family history of diabetes. Educational attainment was reported as years of education. Alcohol consumption and smoking were self-reported as well as physical activity, which was reported as duration of moderately strenuous physical activity in minutes per week.
For the analysis of this study, we divided the participants into four groups (A, B, C, D) according to their pre-pregnancy BMI, parity, and GDM history before the index pregnancy, similarly to our previous study [7]. Group A: obese primiparous women, group B: multiparous obese women without GDM history, group C: multiparous non-obese women with previous GDM, and group D: multiparous obese women with previous GDM. Diagnosis of GDM in the index pregnancy was based on a 2-h 75 g OGTT either in the first or second trimester with diagnostic thresholds of 5.3–10.0–8.6 mmol/l (0, 1 and 2 h).
Statistics
Continuous data are presented as means with standard deviations (SD) or as medians with interquartile range (IQR). Counts with percentages are reported for frequencies. Between-group comparisons were made using analysis of variance (ANOVA) and the Kruskal–Wallis test for continuous variables and using Chi-squared tests for categorical variables. When using adjusted models, analysis of covariance or logistic regression model was applied. In the case of violation of the assumptions (e.g., non-normality), a bootstrap-type test was used (10,000 replications). The normality of the variables was tested using the Shapiro–Wilk W test. The adjustment for multiplicity was performed by Hommel’s multiple comparison procedure to identify significant differences in at least one of the two between-group comparisons (p = 0.05) if needed. All analyses were performed using STATA software (version 14.0, StataCorp, LP, TX).
Results
In total 333 women were included in this study. Median follow-up time after delivery was 65 months (IQR 62–68) with no difference between the groups. GDM was diagnosed in the index pregnancy in 33% of the women in group A, in 25% in group B, in 60% in group C, and in 66% in group D. Weight change during follow-up was similar in all groups (p = 0.90), and it was not associated with GDM status of the index pregnancy (p = 0.42), (interaction p = 0.95).
Table 1 presents the baseline characteristics of the participants in the first trimester of the index pregnancy according to previously defined groups based on parity, pre-pregnancy BMI, and history of GDM (A, B, C, and D). These groups showed marked differences in the parameters related to metabolic health. In general, both in the first trimester and 5 years postpartum, women belonging to group C (defined as non-obese women with GDM prior to the index pregnancy) were metabolically healthier, except for their glucose metabolism.
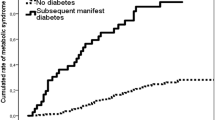
At follow-up, 3.6% of the women were diagnosed with diabetes and altogether 15% of the women had impaired glucose regulation (IFG/IGT/diabetes) (Fig. 1). The highest prevalence of abnormalities in glucose metabolism was observed in group D (26%), and it was significantly different from group A (8%) (p = 0.021) after adjustment for age and educational attainment. Marked differences were observable at follow-up between the groups in the assessed metabolic parameters (Table 2).
Prevalence of IFG, IGT, and type 2 diabetes according to ABCD grouping as well as the overall prevalence of impairment in glucose regulation (p = 0.013). Group A: primiparous BMI ≥ 30 kg/m2. Group B: multiparous, BMI ≥ 30 kg/m2, no history of GDM. Group C: multiparous, BMI < 30 kg/m2, history of GDM. Group D: multiparous, BMI ≥ 30 kg/m2, history of GDM
Women with GDM in the index pregnancy had higher fasting and 2-h glucose concentrations as well as HbA1c compared to those with normal glucose tolerance. There was no interaction between history of GDM and ABCD grouping (supplementary figure).
Metabolic syndrome was present in 32% of the participants in group A, in 25% in group B, in 11% in group C, and in 39% in group D (p < 0.001, adjusted for age and educational attainment). The difference was localized between group C and the other groups (p < 0.001). Figure 2 shows the prevalence of the individual pathological components of the metabolic syndrome according to ABCD grouping. Prevalence of the composite metabolic outcome defined as either impaired glucose regulation and/or metabolic syndrome was similar between the groups (p = 0.24, adjusted for age and educational attainment).
Components of the metabolic syndrome according to the National Cholesterol Education Program Adult Treatment Panel III (ATP III) in ABCD groups. Group A: primiparous BMI ≥ 30 kg/m2. Group B: multiparous, BMI ≥ 30 kg/m2, no history of GDM. Group C: multiparous, BMI < 30 kg/m2, history of GDM. Group D: multiparous, BMI ≥ 30 kg/m2, history of GDM
Women with metabolic disturbances at follow-up had a higher BMI compared to those considered metabolically healthy (Fig. 3). Women in group C showed metabolic disturbances at a significantly lower BMI than women in the other groups; in all groups, the metabolic syndrome was associated with higher body fat percentage (Fig. 4). The prevalence of obesity defined according to body fat percentage (i.e., ≥ 32%) was lowest among participants in group C (prevalence 58%, p < 0.001).
Body mass index (BMI) in women according to presence of metabolic disturbances (MD+) postpartum (IFG, IGT, T2D, or MetS) and in women without metabolic disturbances (MD−) based upon ABCD grouping. Group A: primiparous BMI ≥ 30 kg/m2. Group B: multiparous, BMI ≥ 30 kg/m2, no history of GDM. Group C: multiparous, BMI < 30 kg/m2, history of GDM. Group D: multiparous, BMI ≥ 30 kg/m2, history of GDM
A. Relationship between body fat percentage and diagnosis of metabolic syndrome in ABCD groups. MetS p < 0.001, group p < 0.001, interaction p = 0.06. B. Prevalence of obesity according to body fat percentage ≥ 32% in ABCD groups. p < 0.001. Group A: primiparous BMI ≥ 30 kg/m2. Group B: multiparous, BMI ≥ 30 kg/m2, no history of GDM. Group C: multiparous, BMI < 30 kg/m2, history of GDM. Group D: multiparous, BMI ≥ 30 kg/m2, history of GDM
Discussion
At 5 years postpartum, the cumulative incidence of impaired glucose regulation among these high-risk women was 15%. The highest prevalence was seen in obese women with a history of GDM before the index pregnancy. They were additionally at high risk of the metabolic syndrome; the incidence 5 years postpartum was 39%. As anticipated, women with GDM during the index pregnancy had at follow-up higher glucose concentrations during the OGTT. Overall, the prevalence of impaired glucose regulation was high. History of GDM before the index pregnancy seemed to be a more important risk factor for impaired glucose regulation than degree of adiposity based on BMI.
The original RADIEL study included a group of non-obese women with a history of GDM, who despite being metabolically healthier at first trimester of pregnancy, showed a high recurrence of GDM, i.e., 60%. At 5 years postpartum, they showed additionally a high prevalence (18%) of derangements in glucose metabolism, despite an otherwise rather normal metabolic profile. The cumulative incidence of metabolic syndrome was lower than in the other groups. The women belonging to this group developed, however, metabolic derangements at a significantly lower BMI and with a lower body fat percentage compared to the other groups. Diabetes-related autoantibodies measured in the first trimester did not provide an explanation to the high GDM incidence [7].
The future risk of diabetes among women with a history of GDM varies according to study design, population, ethnicity, and diagnostic criteria. A 5-year follow-up [20] on women with GDM based on IADPSG criteria reported a 26% cumulative incidence of prediabetes or diabetes. The cumulative incidence in our study reached 23% among women with GDM in the index pregnancy. In Sweden, where cut-offs for GDM diagnosis are markedly higher, 30% developed diabetes already 5 years postpartum and 51% had some form of impaired glucose metabolism [21].
Many studies have assessed the determinants of future diabetes risk in women with prior GDM. A systematic review [22] demonstrated higher risk ratios for BMI, family history of diabetes, certain ethnicities, age, multiparity, early onset of GDM, fasting blood glucose, and HbA1c, as well as insulin use and hypertension during pregnancy. Based on a German study including 304 women, Köhler and colleagues created a risk score for predicting risk of diabetes after GDM [23]. Risk markers included BMI, insulin treatment of GDM, family history of diabetes, and lactation, and the C-statistic reached 0.75. Additionally, severity of glucose intolerance during pregnancy seems to be associated with later risk of type 2 diabetes [20, 24]. Although many studies highlight an elevated risk for type 2 diabetes with increasing adiposity, our study demonstrates that there are high-risk individuals also among the non-obese. Findings from the US Nurses’ Health Study similarly indicate an increase in diabetes risk already among women with normal BMI [25].
In addition to being susceptible for type 2 diabetes, women with a history of GDM are also at high risk of the metabolic syndrome; 5 years after delivery it was diagnosed in one-fourth of the women in our study. One year after delivery in a cohort similar to the RADIEL study [9], the incidence of metabolic syndrome was 16%. Another study [10] reported increasing rates according to 1 or 2 pathological values in OGTT during pregnancy: in the 10-year follow-up, the rates were 46 and 63%, respectively. The importance of the metabolic syndrome lies in its association with future risk of cardiovascular disease and type 2 diabetes [16, 26]. Although metabolic disturbances during early postpartum period are associated with future diabetes risk [27], in our study the non-obese high-risk women were metabolically healthy both early in pregnancy as well as 5 years postpartum, except for their glucose metabolism. This suggests that there is heterogeneity in the pathophysiology concerning both GDM and the future development of diabetes.
Both type 2 diabetes and the metabolic syndrome are strongly associated with adiposity [28], and correspondingly our results demonstrate an association between metabolic syndrome and higher body fat percentage. This concerns also the normal weight population [29], and therefore BMI should not be the only marker of adiposity when assessing the cardiovascular risk profile. In our study, waist circumference correlated more strongly with body fat percentage than BMI, especially in the non-obese group. Unfortunately, we can only speculate on the amount of visceral adiposity based on waist circumference, which in group C was above the 88 cm limit among 58% of the participants.
Among the group C participants, who were non-obese before the index pregnancy, prevalence of obesity at 5-year follow-up based on BMI was 14%. The prevalence of obesity based on body fat percentage, however, was 58%. This resembles “normal weight obesity” (NWO) [30], defined commonly as BMI in the normal range (18.5–24.9 kg/m2) and body fat percentage above normal (≥ 32%). The NWO prevalence rates range from 0.1 to 41.1% [30], depending on the criteria applied. Studies have demonstrated associations between NWO and insulin resistance, high blood pressure, dyslipidemia, and increased risk of cardiovascular disease and type 2 diabetes, while showing no typical signs of chronic inflammation usually associated with obesity [31, 32]. According to Lorenzo [33], this condition could be the “pro-inflammatory step” to the chronic inflammatory state of obesity. In our study these NWO women showed merely derangements in their glucose metabolism.
One of the proposed underlying causes explaining the elevated diabetes risk observed in GDM women is impaired insulin secretion [34]. Interestingly, during pregnancy secretory defects are more characteristic in normal weight women whereas obese women are more frequently insulin resistant [6]. To our knowledge, there are no longitudinal studies focusing on future diabetes risk in non-obese women with prior GDM. Damm and his colleagues demonstrated that the reduced and delayed insulin secretory profile observed in non-obese women during pregnancy persisted 5 years postpartum [14]. We have similarly shown that the non-obese women with GDM are at high risk of type 2 diabetes and to a lesser degree also for metabolic syndrome, already at a significantly lower BMI.
We hypothesize that there could be a common underlying denominator for GDM and type 2 diabetes not based purely on adiposity. Genetic predisposition and gut microbiota are potential underlying factors, but also intrauterine programming [35, 36] could offer an explanation. Metabolic memory extends from intrauterine life into adulthood [37] and women born small for gestational age tend to have a higher body fat percentage regardless of their BMI [38, 39]. Maternal diet, physical activity, hyperglycemia, adiposity, and stress can affect fetal size, body composition, and risk of non-communicable diseases later in life [36]. Supporting this hypothesis, the women in groups C and D, with highest prevalence of glycemic disturbances, had lower birth weight compared to the other groups.
The potential long-term consequences of fetal programming [40] are not the only reason for the importance of diagnosing GDM in non-obese women. Frequently, history of GDM is the only marker of increased diabetes risk in these women. Therefore, IADPSG and WHO recommend universal screening [41], but as the EBCOG report [42] shows, strategies vary highly even inside Europe. Many countries use risk factor-based screening focusing primarily on obese women, certain ethnic groups, or women with history of macrosomia, GDM, or family history of diabetes. This approach seems insufficient [43], but it reflects both financial issues and also the underestimated consequences of GDM in non-obese women.
Strengths of this study include the relatively high number of participants and inclusion of non-obese women. We have detailed knowledge on the metabolic status during pregnancy, creating excellent opportunities for long-term follow-up. The lack of a control group of non-obese women without previous GDM can be considered a weakness of the study. Additionally, we do not have data on diabetes-related autoantibodies at follow-up. We also lack more sophisticated measures of insulin secretion and insulin sensitivity. Further, all participants were of Caucasian origin, limiting the generalizability to other populations.
Based on our results, postpartum follow-up should not exclude non-obese women as they are also at high risk of diabetes. Current screening strategies need further strengthening since they are rather suboptimal in most countries [44, 45]. Future studies need to further investigate the underlying pathophysiology of GDM in non-obese women and aim at creating preventive strategies tailored for them. As they seldom show easily identifiable risk factors, this emphasizes in accordance to IADPSG guidelines, the need for universal GDM screening. Early diagnosis, improved identification of non-obese individuals at risk, and preferably early preventive strategies are needed to fight the epidemic of diabetes.
References
National Institute for Health and Welfare (2016) Official Statistics of Finland; Health; 2014
Lamain-de Ruiter M, Kwee A, Naaktgeboren CA et al (2016) External validation of prognostic models to predict risk of gestational diabetes mellitus in one Dutch cohort: prospective multicentre cohort study. BMJ 354:4338. https://doi.org/10.1136/bmj.i4338
Tuomi T, Santoro N, Caprio S et al (2014) The many faces of diabetes: a disease with increasing heterogeneity. Lancet 383(9922):1084–1094. https://doi.org/10.1016/S0140-6736(13)62219-9
Freinkel N, Metzger BE, Phelps RL et al (1985) Gestational diabetes mellitus. Heterogeneity of maternal age, weight, insulin secretion, HLA antigens, and islet cell antibodies and the impact of maternal metabolism on pancreatic B-cell and somatic development in the offspring. Diabetes 34(Suppl 2):1–7
Bo S, Menato G, Pinach S et al (2003) Clinical characteristics and outcome of pregnancy in women with gestational hyperglycaemia with and without antibodies to beta-cell antigens. Diabet Med 20(1):64–68
Powe CE, Allard C, Battista M-C et al (2016) Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care 39(6):1052–1055. https://doi.org/10.2337/dc15-2672
Huvinen E, Grotenfelt NE, Eriksson JG et al (2016) Heterogeneity of maternal characteristics and impact on gestational diabetes (GDM) risk—implications for universal GDM screening? Ann Med 48(1–2):52–58. https://doi.org/10.3109/07853890.2015.1131328
Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373(9677):1773–1779. https://doi.org/10.1016/S0140-6736(09)60731-5
Puhkala J, Kinnunen TI, Vasankari T, Kukkonen-Harjula K, Raitanen J, Luoto R (2013) Prevalence of metabolic syndrome one year after delivery in Finnish women at increased risk for gestational diabetes mellitus during pregnancy. J Pregnancy 2013:139049
Hakkarainen H, Huopio H, Cederberg H, Paakkonen M, Voutilainen R, Heinonen S (2016) The risk of metabolic syndrome in women with previous GDM in a long-term follow-up. Gynecol Endocrinol. https://doi.org/10.1080/09513590.2016.1198764
Lauenborg J, Mathiesen E, Hansen T et al (2005) The prevalence of the metabolic syndrome in a danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab 90(7):4004–4010. https://doi.org/10.1210/jc.2004-1713
McKenzie-Sampson S, Paradis G, Healy-Profitos J, St-Pierre F, Auger N (2018) Gestational diabetes and risk of cardiovascular disease up to 25 years after pregnancy: a retrospective cohort study. Acta Diabetol. https://doi.org/10.1007/s00592-017-1099-2
Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA (1991) Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol 165(6 Pt 1):1667–1672
Damm P, Kuhl C, Hornnes P, Molsted-Pedersen L (1995) A longitudinal study of plasma insulin and glucagon in women with previous gestational diabetes. Diabetes Care 18(5):654–665
Rono K, Stach-Lempinen B, Klemetti MM et al (2014) Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled multicenter trial (RADIEL). BMC Pregnancy Childbirth 14:70. https://doi.org/10.1186/1471-2393-14-70
Koivusalo SB, Rono K, Klemetti MM et al (2016) gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish Gestational Diabetes Prevention Study (RADIEL): a randomized controlled trial. Diabetes Care 39(1):24–30. https://doi.org/10.2337/dc15-0511
Alberti KGMM, Eckel RH, Grundy SM et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644
Malavolti M, Mussi C, Poli M et al (2003) Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann Hum Biol 30(4):380–391. https://doi.org/10.1080/0301446031000095211
USA: The American council on exercise (2016) What are the guidelines for percentage of body fat loss? http://www.acefitness.org/acefit/healthy-living-article/60/112/what-are-the-guidelines-for-percentage-of-body-fat. Accessed 22 Aug 2016
Noctor E, Crowe C, Carmody LA et al (2016) Abnormal glucose tolerance post-gestational diabetes mellitus as defined by the International Association of Diabetes and Pregnancy Study Groups criteria. Eur J Endocrinol 175(4):287–297. https://doi.org/10.1530/EJE-15-1260
Ekelund M, Shaat N, Almgren P, Groop L, Berntorp K (2010) Prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetologia 53(3):452–457. https://doi.org/10.1007/s00125-009-1621-3
Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S (2016) Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia 59(7):1403–1411. https://doi.org/10.1007/s00125-016-3927-2
Kohler M, Ziegler AG, Beyerlein A (2016) Development of a simple tool to predict the risk of postpartum diabetes in women with gestational diabetes mellitus. Acta Diabetol 53(3):433–437. https://doi.org/10.1007/s00592-015-0814-0
Hakkarainen H, Huopio H, Cederberg H, Paakkonen M, Voutilainen R, Heinonen S (2015) Post-challenge glycemia during pregnancy as a marker of future risk of type 2 diabetes: a prospective cohort study. Gynecol Endocrinol 31(7):573–577. https://doi.org/10.3109/09513590.2015.1032926
Colditz GA, Willett WC, Rotnitzky A, Manson JE (1995) Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 122(7):481–486
Cho NH, Ahn CH, Moon JH, Kwak SH, Choi SH, Lim S, Park KS, Metzger BE, Jang HC (2016) Metabolic syndrome independently predicts future diabetes in women with a history of gestational diabetes mellitus. Medicine 95(35):e4582. https://doi.org/10.1097/MD.0000000000004582
Gobl CS, Bozkurt L, Prikoszovich T, Winzer C, Pacini G, Kautzky-Willer A (2011) Early possible risk factors for overt diabetes after gestational diabetes mellitus. Obstet Gynecol 118(1):71–78. https://doi.org/10.1097/AOG.0b013e318220e18f
Xu T, Liu J, Liu J, Zhu G, Han S (2017) Relation between metabolic syndrome and body compositions among Chinese adolescents and adults from a large-scale population survey. BMC Public Health 17(1):337. https://doi.org/10.1186/s12889-017-4238-3
Shah RV, Murthy VL, Abbasi SA et al (2014) Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA study. Jacc Cardiovasc Imaging 7(12):1221–1235. https://doi.org/10.1016/j.jcmg.2014.07.017
Franco LP, Morais CC, Cominetti C (2016) Normal-weight obesity syndrome: diagnosis, prevalence, and clinical implications. Nutr Rev 74(9):558–570. https://doi.org/10.1093/nutrit/nuw019
Romero-Corral A, Somers VK, Sierra-Johnson J et al (2010) Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 31(6):737–746. https://doi.org/10.1093/eurheartj/ehp487
Marques-Vidal P, Pecoud A, Hayoz D et al (2010) Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc Dis 20(9):669–675. https://doi.org/10.1016/j.numecd.2009.06.001
De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L (2007) Normal-weight obese syndrome: early inflammation? Am J Clin Nutr 85(1):40–45
Huopio H, Hakkarainen H, Paakkonen M et al (2014) Long-term changes in glucose metabolism after gestational diabetes: a double cohort study. BMC Pregnancy Childbirth 14:296. https://doi.org/10.1186/1471-2393-14-296
Barker DJ (1990) The fetal and infant origins of adult disease. BMJ 301(6761):1111
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1):61–73. https://doi.org/10.1056/NEJMra0708473
Eriksson JG, Forsen T, Tuomilehto J, Jaddoe VWV, Osmond C, Barker DJP (2002) Effects of size at birth and childhood growth on the insulin resistance syndrome in elderly individuals. Diabetologia 45(3):342–348. https://doi.org/10.1007/s00125-001-0757-6
Sarr O, Yang K, Regnault TRH (2012) In utero programming of later adiposity: the role of fetal growth restriction. J Pregnancy 2012:134758. https://doi.org/10.1155/2012/134758
Entringer S, Buss C, Swanson JM et al (2012) Fetal programming of body composition, obesity, and metabolic function: the role of intrauterine stress and stress biology. J Nutr Metab 2012:632548. https://doi.org/10.1155/2012/632548
Nehring I, Chmitorz A, Reulen H, von Kries R, Ensenauer R (2013) Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabet Med 30(12):1449–1456. https://doi.org/10.1111/dme.12286
Catalano PM, McIntyre HD, Cruickshank JK et al (2012) The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 35(4):780–786. https://doi.org/10.2337/dc11-1790
Benhalima K, Mathieu C, Van Assche A et al (2016) Survey by the European Board and College of Obstetrics and Gynaecology on screening for gestational diabetes in Europe. Eur J Obstet Gynecol Reprod Biol 201:197–202. https://doi.org/10.1016/j.ejogrb.2016.04.003
Farrar D, Simmonds M, Bryant M et al (2017) Risk factor screening to identify women requiring oral glucose tolerance testing to diagnose gestational diabetes: a systematic review and meta-analysis and analysis of two pregnancy cohorts. PLoS ONE [Electronic Resource] 12(4):e0175288. https://doi.org/10.1371/journal.pone.0175288
Goueslard K, Cottenet J, Mariet AS, Sagot P, Petit JM, Quantin C (2017) Early screening for type 2 diabetes following gestational diabetes mellitus in France: hardly any impact of the 2010 guidelines. Acta Diabetol 54(7):645–651. https://doi.org/10.1007/s00592-017-0986-x
Ferrara A, Peng T, Kim C (2009) Trends in postpartum diabetes screening and subsequent diabetes and impaired fasting glucose among women with histories of gestational diabetes mellitus: a report from the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 32(2):269–274. https://doi.org/10.2337/dc08-1184
Acknowledgements
The study was funded by Ahokas Foundation, the Finnish Foundation for Cardiovascular Disease, Academy of Finland, Special state subsidy for health science research of Helsinki University Hospital (HUH), Samfundet Folkhälsan, Finska Läkaresällskapet, Juho Vainio Foundation, Viipuri Tuberculosis Foundation, The Finnish Diabetes Research Foundation, State Provincial Office of Southern Finland, Health Promotion Grant (Ministry of Social Affairs and Health) EU H2020-PHC-2014-DynaHealth Grant No. 633595, and The Social Insurance Institution of Finland. The funders have not had any role in designing or conducting the study; nor in collection, management, analysis, or interpretation of the data; nor in preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
EH participated in the design and implementation of the study, literature search, data interpretation, and the drafting and editing of the article. JGE is the principal investigator of the study, participated in the implementation of the study, analysis of the results, and advised on drafting and editing the article. SBK initiated, participated in the design of the study, coordinated the study, and helped in the editing of the article. NG participated in the design of the study and helped with the editing of the article. AT participated in the design of the study and helped with the editing of the article. BS-L participated in the design of the study, coordinated the study in Lappeenranta, and helped with editing the article. KR participated in the design of the study and helped in the statistical analysis and drafting and editing of the article. All authors have read and approved the final version of the manuscript. EH is the guarantor of this work and, as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committees of HUH and SKCH and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed written consent was obtained from all individual participants included in the study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Managed By Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huvinen, E., Eriksson, J.G., Koivusalo, S.B. et al. Heterogeneity of gestational diabetes (GDM) and long-term risk of diabetes and metabolic syndrome: findings from the RADIEL study follow-up. Acta Diabetol 55, 493–501 (2018). https://doi.org/10.1007/s00592-018-1118-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1118-y