Abstract
Aims
To investigate changes in retinal vascular plexuses and choriocapillaris in patients with type 2 diabetes mellitus (DM2) without diabetic retinopathy (DR) and healthy controls using optical coherence tomography angiography (OCTA).
Methods
A total of 71 DM2 and 67 healthy control subjects were included. All subjects underwent OCTA examination (RTVue-XR Avanti; Optovue, Fremont, CA, USA). Average vessel density in superficial capillary plexus (SCP), deep capillary plexus (DCP) and choriocapillaris, parafoveal vessel density in SCP and DCP, FAZ area (mm2) in SCP, microaneurysms and capillary nonperfusion were taken into analysis.
Results
Parafoveal vessel density in both SCP and DCP decreased in the eyes without clinical DR compared to normal controls (p < 0.001). Diabetic patients with no signs of DR also had a significant reduction in average vessel density of SCP, DCP and choriocapillaris (p < 0.001, p < 0.001 and p = 0.006, respectively). No significant difference was found in FAZ area of SCP between DM2 eyes and healthy controls (p = 0.253). The average vessel density of SCP and DCP is not correlated with HbA1c or serum creatinine in DM2 patients. Microaneurysms seen in OCTA but not in fundus examination were found in 8 out of the 71 (11.3%) diabetic eyes, and capillary nonperfusion was noted in 18 of 71 diabetic eyes.
Conclusions
We demonstrated that OCTA can identify preclinical DR before the manifestation of clinically apparent retinopathy in diabetic eyes. DM2 patients without DR have SCP, DCP and choriocapillaris impairment. Our results suggested that OCTA might be a promising tool for regular screening of diabetic eyes for DR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid change in lifestyle in modern society, diabetes (DM) has become a major health problem all around the world [1]. In China, a national survey conducted from June 2007 through May 2008 showed that the prevalence of total diabetes and prediabetes was 9.7 and 15.5%, respectively, accounting for 92.4 million adults with DM and 148.2 million adults with prediabetes [2]. Diabetic retinopathy (DR) is a leading cause of visual impairment in working-age adults in China and worldwide. It is estimated that the prevalence rate of DR for all adults with DM aged 40 and older is 34.6% (93 million people) worldwide [3].
The most common early clinically visible manifestations of DR include microaneurysm formation and intraretinal hemorrhage. At present, the ophthalmoscopy and color fundus photography are still the gold standard for the diagnosis and staging of DR. However, the occurrence of microvascular damage is known to occur before findings of retinopathy become apparent on clinical examination or fundus photography [4, 5]. Although fluorescein angiography (FA) is an important modality in revealing capillary leakage and nonperfusion in patients with DM, it is rarely used and not suggested for eyes without visible retinopathy or mild DR [6].
With the development of optical coherence tomography angiography (OCTA), an alternative noninvasive angiographic technique that can demonstrate vessel densities and shape of the vascular arcades of the foveal avascular zone (FAZ), abnormalities in retinal blood flow can now be easily assessed [7,8,9]. The HD 6 × 6 mm OCTA image can provide us with details of microvascular changes as well as quantitative data on different vascular layers of retina with a wider field.
The aim of this study was to investigate retinal microvascular differences between diabetic eyes with no signs of DR and normal controls using OCTA.
Patients and methods
Subjects
Patients with type 2 diabetes mellitus (T2DM) and healthy control (HC) subjects were recruited from Guangdong General Hospital who presented between January 2017 and July 2017. One eye of each subject was randomly selected in the study. In both patients and controls, exclusion criteria were as follows: any other ocular disease that may affect ocular circulation (e.g., glaucoma, age-related macular degeneration, retinal vascular occlusion, refractive error > 3 diopters[D]), intraocular surgery, eye trauma, amblyopia, hypertension exceeding 150/100 mmHg and intraocular pressure (IOP) > 21 mmHg.
Subjects were tested for best corrected visual acuity (BCVA), IOP, and refractive error (autorefractometry). Slit-lamp and fundus examinations using direct and/or indirect ophthalmoscope were performed. ETDRS 35 degree 7-standard fields color retinal photographs (Topcon TRC; Topcon, Tokyo, Japan) were obtained from each participant. DR was graded according to the International Diabetic Retinopathy Severity Scale [10]. Two experienced graders (DC and ZNH) evaluated the fundus photographs and classified both eyes of T2DM patients as no diabetic retinopathy (NDR).
This cross-sectional observational study was performed according to the Declaration of Helsinki and was approved by the Research Ethics Committee of Guangdong General Hospital (registration number: gdrec2016232A). Informed consent was obtained from all study participants before examination.
OCTA image collection and analysis
OCTA images were obtained after pupillary dilation in a dark room by using AngioVue OCTA system (RTVue-XR Avanti; Optovue, Fremont, CA, USA, version 2016.2.035). Split-spectrum amplitude decorrelation angiography (SSADA) software algorithm was used for evaluation of vessel density and FAZ area [11]. With a 70-kHz spectral OCT system and 200+ A-scans per cross-sectional B-scan, SSADA should be sensitive to even the slowest flow at the capillary level, where flow speeds have been estimated at between 0.4 and 3 mm/s.
Two sets of imaging were performed at 1 assessment. Each image set comprised 2 raster volumetric patterns (1 vertical priority and 1 horizontal priority) covering HD 6 × 6 mm. An orthogonal registration algorithm (built-in software, which has the ability to correct some of the motion artifacts) is used to merge 3D OCT angiograms. Each volume was composed of 400 scan lines. The vessel density is the percentage of signal positive pixels per total pixels in an area of interest. FAZ area (mm2) was evaluated in the superficial vascular plexus by using the nonflow area tool of the software that delineated it automatically after selecting a segment of the FAZ (Fig. 1). The superficial retinal, deep retinal and choroidal vascular networks were generated by using automated software algorithm. The boundaries for each layer were as follows: a slab extending from 3 to 15 μm from the internal limiting membrane was generated for detecting the superficial capillary plexus (SCP), a slab extending from 15 to 70 μm below the internal limiting membrane for the deep capillary plexus (DCP) and a slab extending from 30 to 60 μm below retinal pigment epithelium reference for choriocapillaris. The 6 × 6 mm OCTA images were evaluated for the following characteristics: average vessel density in SCP, DCP and choriocapillaris, parafoveal vessel density in SCP and DCP (Fig. 2), FAZ alterations (size and irregularity) and microaneurysms. FAZ irregularities were defined as any of the following changes: capillary nonperfusion adjacent to the FAZ, the presence of arteriolar stumps at the FAZ margin or loss of the normal spider web-like architecture of capillaries in the perifoveal area (Fig. 1). Microaneurysms were defined as focal dilatations or loops along or at the end of capillaries. Two readers (DC and ZNH) evaluated the angiographic features on OCTA images. Image quality was considered by including images having signal strength of at least 40. In patients with poor images, we repeated the scans until an image with at least fair quality could be obtained.
Example images of vessel density measurement in a control subject (a, superficial capillary plexus; b, deep capillary plexus). Vessel densities in SCP and DCP (whole image and parafovea) were calculated automatically (red box). OCTA data between the blue rings (ETDRS contour) were used to calculate parafoveal vessel density at the level of superficial and deep capillary plexus
Statistical analysis
Statistical analysis was performed with SPSS 19.0 software (SPSS. Inc, Chicago, IL, USA). Qualitative variables are presented as number and percentage. Quantitative variables are presented as means and standard deviations. Data were confirmed to be normally distributed with Shapiro–Wilk test, and independent, two-tailed Student’s t tests were applied to compare means of the DM2 and control subjects. Pearson correlation coefficients were calculated to assess correlations between HbA1c, serum creatinine and vessel density in T2DM group. p < 0.05 was considered statistically significant.
A sample size calculation determined that 71 eyes of each group were required to detect a 0.50% vessel density difference (sample size calculation was performed using a web-based simple power/sample size calculation, UCSF Biostatistics: Power and Sample Size Programs, https://www.stat.ubc.ca/~rollin/stats/ssize/, α = 0.05, power 0.80, standard deviation 1.29%, two-sided test).
Results
A total of 71 T2DM and 67 HC subjects were included. Baseline demographics were comparable in the two cohorts (Table 1). T2DM patients had an average HbA1c of (8.6 ± 3.0) % or (70 ± 9) mmol/ml and average serum creatinine of (81.32 ± 24.97) mmol/L. Nine of 71 T2DM patients had impaired renal function with elevated serum creatinine. The average duration of diabetes for T2DM patients was (6.6 ± 1.9) years.
Based on the Shapiro–Wilk test results, the average vessel density in SCP/DCP/choriocapillaris and parafoveal vessel density in SCP/DCP had normal distribution (p > 0.05 for all comparisons). Parafoveal vessel density in both SCP and DCP decreased in the eyes with NDR compared to normal controls (p < 0.001). T2DM patients with NDR also had a significant reduction in average vessel density of SCP, DCP and choriocapillaris (p < 0.001, p < 0.001 and p = 0.006, respectively). There was no significant difference in FAZ area in the SCP between T2DM eyes and HC (p = 0.253) (Table 2).
Correlation between average vessel density of SCP, DCP and HbA1c was unremarkable (p = 0.125 and 0.246, respectively). The average vessel density of SCP and DCP is not correlated with serum creatinine (p = 0.151 and 0.667, respectively). No significant correlation was found between duration of diabetes and vessel density in SCP and DCP, either (p = 0.734 and 0.766, respectively).
FAZ irregularity was noted in 22 out of 71 diabetic eyes (30.9%). Microaneurysms were found in eight of the 71 (11.3%) diabetic eyes (Fig. 3), while capillary nonperfusion presented in 18 diabetic eyes (Fig. 4). We further analyzed the vessel density of T2DM patients with and without microaneurysms on OCTA. The average vessel density of SCP, DCP and choriocapillaris in diabetic eyes with microaneurysms was (52.74 ± 3.16), (58.39 ± 4.54) and (66.58 ± 0.83) % compared to (50.55 ± 4.53), (57.28 ± 4.78) and (66.04 ± 2.02) % in eyes without OCTA-detected microaneurysms.
Typical T2DM patient with NDR in the left eye. a No visible microaneurysms were delineated in color fundus photography. 6 × 6 mm OCT angiogram (b, superficial capillary plexus; c, deep capillary plexus) showed microaneurysms adjacent to the fovea (yellow circle). d–f Another diabetic eye without clinical DR. e Capillary nonperfusion was seen directly adjacent to the FAZ. f A few microaneurysms were seen in deep capillary plexus angiogram superior-temporal to the fovea (yellow circle) (e, superficial capillary plexus; f, deep capillary plexus)
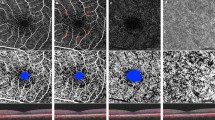
6 × 6 mm OCT angiogram of a control nondiabetic eye (a), a diabetic eye with NDR (b) and a diabetic eye with preclinical DR (c) (superficial capillary plexus, deep capillary plexus and choriocapillary from top to bottom). c. Capillary nonperfusion adjacent to the FAZ (yellow arrow) was noted in superficial capillary plexus, and several microaneurysms were seen in deep capillary plexus angiogram (yellow circle)
Discussion
DR is a progressive microvascular disease. OCTA has the capability to advance our understanding of DR by providing high-resolution images of retinal and choroidal microvasculature blood flow and structure [12]. OCTA can also provide us with quantitative measurements of capillary perfusion of the macula and nerve [13].
Our study showed that T2DM patients with no signs of DR have reduced vessel density in SCP, DCP and choriocapillaris compared to normal controls. However, FAZ area was comparable in diabetic eyes with NDR and controls. Also, we found that the vessel density of SCP and DCP was not associated significantly with duration of diabetes, HbA1c or serum creatinine.
In a cross-sectional study done by Dimitrova et al. [14], in which 33 control subjects and 29 patients with NDR were enrolled, a reduction in parafoveal superficial and deep retinal vessel density was noted in diabetic eyes compared to healthy subjects. Simonett et al. and Carnevali et al. found that patients with type 1 diabetes mellitus (T1DM) without any signs of DR showed a reduction in vessel density in DCP but not in SCP [15,16,17]. In our cohorts, T2DM patient with NDR had decreased parafoveal and average superficial, deep vessel density as well as average choriocapillaris vessel density in comparison with the control group, indicating that both retinal and choroidal circulation may be affected before clinical manifestation of DR.
Previous studies have documented subtle changes in color perception and contrast sensitivity in diabetic eyes without clinical retinopathy [18, 19], which was not fully understood. Another study that investigated retinal blood circulation, using video fluorescein angiography, had reported decreased mean circulation time in T2DM patients without DR [20]. And researches using retinal oximetry to measure oxygen saturation (SO2) in major peripapillary arteries and veins in patients with diabetes but no DR suggested that a maldistribution of oxygen even occurred in patients with diabetes without DR [21]. The above phenomenon might be explained by early retinal microvascular alterations seen on OCTA such as decreased retinal and choroidal vessel density.
FAZ alterations including size and shape change have been studied recently using OCTA. For FAZ size, previous researches have reached different conclusions about it in diabetic eyes with NDR [15, 22,23,24]. De Carlo et al. [22] observed FAZ area measured 0.348 mm2 in diabetic eyes and 0.288 mm2 in control eyes (p = 0.04). Scarinci et al. [15] did not find differences between T1DM patients with NDR and normal controls in FAZ area of both superficial and deep capillary plexus. Our study also showed no significant differences in FAZ area between eyes with NDR and HC (0.32 ± 0.18 mm2 in NDR and 0.35 ± 0.09 mm2 in controls). The inconsistency may be due to high variability of the FAZ area in healthy individuals; therefore, FAZ size may not be a sensitive imaging biomarker to define early DR. On the other hand, irregular FAZ contour with notchings and indentations were seen in 30.9% T2DM eyes with NDR. Recent studies demonstrated that the presence of irregularities in FAZ boundaries might be one of the earliest signs of diabetic damage to the retinal vasculature [15, 22].
In the 71 diabetic eyes without any signs of DR, we found eight eyes presented with microaneurysms in OCTA images. At present, both the early treatment diabetic retinopathy study (ETDRS) grading system or the international clinical DR and diabetic macular edema severity scale use visible microaneurysms as the first clinical sign of retinopathy [10, 25], so the eight eyes with microaneurysms seen in OCTA but not in fundus examination may still be diagnosed as NDR. We hereby suggest the disease classification ‘preclinical DR’ for this kind of patients, as these microaneurysms on OCTA presented before any visible microvascular findings upon dilated ophthalmoscopy. De Carlo et al. also reported that microaneurysms were delineated in five of the 61 diabetic eyes without DR (8%) [22]; however, they did not include color fundus photographs in their study.
In the current study, we emphasized the concept ‘preclinical DR,’ which means microaneurysms found in OCT angiogram preceding any apparent abnormalities in fundus examination. The importance of this finding lies in that using OCTA we can detect early subtle changes in retinal vessels of diabetic eyes even before they become visible on fundus images, quickly and noninvasively. Usually, the screening strategy for T2DM patients without retinopathy would be dilated pupil fundus examination using the slit-lamp biomicroscopy, and FA is not recommended for this kind of patients. In fact, a subset of patients might be overlooked if we do not look at the retinal vasculature at the capillary level. By using OCTA, we can quantify vessel density in multiple retinal layers and detect the onset of DR even though the patient is asymptomatic. T2DM individuals at risk of developing retinopathy could be informed that they may have good vision, but that they may still have to examine their eyes regularly.
In addition, we found capillary nonperfusion in 18 out of 71 diabetic eyes. Previous studies using FA techniques have already demonstrated macular capillary nonperfusion in patients with no DR. Therefore, capillary nonperfusion or capillary dropout is another microvascular change that may precede any abnormalities happening in diabetic eyes, which can now be easily assessed by OCTA without the need of injecting contrast agent [26].
Taken together, the microvascular alterations detected by OCTA in eyes with NDR (microaneurysms, enlargement of FAZ, irregular FAZ and macular capillary dropout) were more reliable indicators of preclinical DR than was reduction in vessel density alone. Ophthalmologist could make use of OCTA to diagnose preclinical DR and establish a close partnership with the primary care physicians to ensure optimal patient care. T2DM patients with preclinical DR should have a stricter control of blood glucose, serum lipid levels and blood pressure.
There were a few limitations of the current study. Firstly, FA examinations were not available in the eyes with preclinical DR. Secondly, the HD 6 × 6 mm OCT angiograms can only include a small field in the posterior pole, which may limit our understanding of the microvascular changes in the peripheral retina in eyes with NDR. Thirdly, we only evaluated a single HbA1c value for each T2DM patient, which would not be useful to retrospectively assess the disease control for a longer period. Fourthly, OCTA is not able to recognize blood flow below the lowest detectable speed, so areas detected as capillary nonperfusion in OCT angiogram may simply be areas of slow or turbulent flow. And fifth, a sample size calculation determined that 71 eyes of each group were required; however, we only included 67 control eyes in the study, so this study may not be powered enough to draw any conclusion. Last but not the least, the algorithms used in this study were not projection resolved, so the projection artifacts from superficial blood vessels onto DCP and choriocapillaris were inevitable. The density measures of the deeper structures may be affected by these artifacts.
In conclusion, our study suggested that OCTA can identify preclinical DR before the manifestation of clinically apparent retinopathy. T2DM patients without DR have SCP, DCP and choriocapillaris impairment. OCTA may be a promising tool for screening of diabetic eyes for DR.
References
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87(1):4–14
Yang W, Lu J, Weng J et al (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362(12):1090–1101
Yau JW, Rogers SL, Kawasaki R et al (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35(3):556–564
Durham JT, Herman IM (2011) Microvascular modifications in diabetic retinopathy. Curr Diab Rep 11(4):253–264
Barot M, Gokulgandhi MR, Patel S, Mitra AK (2013) Microvascular complications and diabetic retinopathy: recent advances and future implications. Future Med Chem 5(3):301–314
Kwiterovich KA, Maguire MG, Murphy RP et al (1991) Frequency of adverse systemic reactions after fluorescein angiography: results of a prospective study. Ophthalmology 98(7):1139–1142
de Carlo TE, Romano A, Waheed NK, Duker JS (2015) A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous 1:5
Jia Y, Bailey ST, Hwang TS et al (2015) Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci USA 112(18):E2395–E2402
Kashani AH, Chen CL, Gahm JK et al (2017) Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retinal Eye Res 60:66–100
Wilkinson CP, Ferris FL 3rd, Klein RE et al (2003) Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110(9):1677–1682
Huang D, Jia Y, Gao SS, Lumbroso B, Rispoli M (2016) Optical coherence tomography angiography using the optovue device. Dev Ophthalmol 56:6–12
Bandello F, Corbelli E, Carnevali A, Pierro L, Querques G (2016) Optical coherence tomography angiography of diabetic retinopathy. Dev Ophthalmol 56:107–112
Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH (2016) Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57(9):Oct362–Oct370
Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K (2017) Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Invest Ophthalmol Vis Sci 58(1):190–196
Scarinci F, Picconi F, Giorno P et al (2017) Deep capillary plexus impairment in patients with type 1 diabetes mellitus with no signs of diabetic retinopathy revealed using optical coherence tomography angiography. Acta Ophthalmol. https://doi.org/10.1111/aos.13510 [ahead of print]
Simonett JM, Scarinci F, Picconi F et al (2017) Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol 95(8):e751–e755. https://doi.org/10.1111/aos.13404
Carnevali A, Sacconi R, Corbelli E et al (2017) Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol 54(7):695–702
Trick GL, Burde RM, Gordon MO, Santiago JV, Kilo C (1988) The relationship between hue discrimination and contrast sensitivity deficits in patients with diabetes mellitus. Ophthalmology 95(5):693–698
Kurtenbach A, Wagner U, Neu A, Schiefer U, Ranke MB, Zrenner E (1994) Brightness matching and colour discrimination in young diabetics without retinopathy. Vis Res 34(1):115–122
Arend O, Wolf S, Jung F et al (1991) Retinal microcirculation in patients with diabetes mellitus: dynamic and morphological analysis of perifoveal capillary network. Br J Ophthalmol 75(9):514–518
Blair NP, Wanek J, Felder AE et al (2017) Retinal oximetry and vessel diameter measurements with a commercially available scanning laser ophthalmoscope in diabetic retinopathy. Invest Ophthalmol Vis Sci 58(12):5556–5563
de Carlo TE, Chin AT, Bonini Filho MA et al (2015) Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina (Philadelphia, PA) 35(11):2364–2370
Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S (2016) Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol 254(6):1051–1058
Nesper PL, Roberts PK, Onishi AC et al (2017) Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 58(6):307–315
Early Treatment Diabetic Retinopathy Study Research Group (1991) Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 98(5 Suppl):786–806
Soares M, Neves C, Marques IP et al (2017) Comparison of diabetic retinopathy classification using fluorescein angiography and optical coherence tomography angiography. Br J Ophthalmol 101(1):62–68
Funding
This study was funded by National Natural Science Foundation of China (Grant Number 81500737) and Guangzhou Science and Technology Program (Grant Number 201607010343).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Managed by Massimo Federici.
Rights and permissions
About this article
Cite this article
Cao, D., Yang, D., Huang, Z. et al. Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol 55, 469–477 (2018). https://doi.org/10.1007/s00592-018-1115-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1115-1