Abstract
Purpose
To evaluate the capillary flow density (CFD) of choriocapillary (CC) microvasculature using optical coherence tomography angiography (OCT-A) in diabetic eyes and the association of CFD and systemic and metabolic factors.
Methods
Cross-sectional study. This study enrolled 282 eyes of 146 subjects, including 43 healthy control eyes, 56 diabetic eyes without diabetic retinopathy (DR), 43 eyes with mild nonproliferative DR (NPDR), 54 eyes with moderate NPDR, 38 eyes with severe NPDR, and 48 eyes with proliferative DR (PDR). CFD was measured in the CC layer. Clinical data were collected. Multiple linear regression analyses were performed to identify associated clinical variables.
Results
CFD in the CC layer presented a downward trend with DR progression. Comparisons of CFD in the CC layer between adjacent stages of DR revealed significant differences between severe NPDR and PDR using both 3-mm and 6-mm scan patterns (P = 0.003, P = 0.001). CFD in the CC layer in DR with diabetic macular edema (DME) was less than that in DR without DME using both 3-mm and 6-mm scan patterns (P < 0.001, P < 0.001). Coronary artery disease and atherosclerosis in other locations, estimated glomerular filtration rate, and increased HbA1c were associated with CFD in the CC layer using both 3-mm and 6-mm scan patterns (all P values < 0.05).
Conclusions
OCT-A revealed decreased CFD in the CC layer in the PDR stage and the presence of DME. Diabetic patients with apparently decreased CFD should be assessed carefully under general conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is a leading cause of blindness in the active population, affecting almost 35% of all diabetic patients worldwide [1]. The progression of diabetes mellitus (DM) can lead to microvascular damage, vessel occlusion, increased permeability, retinal ischemia, macular edema, and neovascularization [2]. Retinal damage, especially macular dysfunction, contributes to visual impairment. The choroid is responsible for the blood supply to the outer retina, including the retinal pigmented epithelium (RPE) and photoreceptors, and is the only source of metabolic exchange for the avascular fovea [3]. Choriocapillary (CC) dropout, luminal narrowing and obstruction, and microaneurysms have been documented in the human diabetic choroids [4, 5]. Therefore, diabetic choroidopathy (DC) is associated with macular dysfunction. Indocyanine green angiography (ICGA) and laser Doppler flowmetry can image choroidal vascular abnormalities [6], but cannot provide any information concerning depth-resolved images of the choroid.
Optical coherence tomography (OCT) angiography (OCT-A) has recently enabled dye-less in vivo depth-resolved visualization of microvasculature. Compared with fluorescein angiography (FA) and ICGA, OCT-A allows ophthalmologists to analyze various layers of retinal and choroidal capillary networks with more accuracy and higher resolution [7, 8]. This newly developed imaging method enables more precise, quantitative evaluation of retinal and choroidal vasculature. Since the advent of OCT-A, several studies have reported changes in the microvasculature in patients with DM [9,10,11,12]. Compared with FA, OCT-A can provide quantitative analysis of enlargement of the foveal avascular area and retinal capillary drop-off in a detailed manner. However, most of these studies had small sample sizes and did not consider the effect of previous treatment on microvascular density obtained via OCT-A. In addition, the correlation between systemic conditions and CC has not been investigated.
The primary purpose of this study was to assess CC capillary flow density (CFD) changes in patients with diabetes and healthy subjects using OCT-A. The secondary purpose was to investigate the correlation between CFD and systematic conditions.
Methods
This observational, cross-sectional study was approved by the Peking Union Medical College Hospital Institutional Review Board (S-K631) and was conducted in accordance with the Declaration of Helsinki. All participants provided signed informed consent. This study enrolled 282 eyes of 146 subjects who presented in the Department of Ophthalmology, Peking Union Medical College Hospital, Beijing, China from August 1, 2017 to May 1, 2018. All enrolled diabetic eyes were untreated.
Examinations, medical history, and biochemical markers
All treatment-naïve eyes were included in the study if there was no apparent media opacity or hard exudates affecting slit-lamp examination and OCT capture. The exclusion criteria included pregnancy, any other previous disease or treatment involving the retina or choroid, glaucoma, and other diseases that might affect CFD. Poor-quality OCT-A images with an image quality index lower than 7 were also excluded [13]. All patients underwent a comprehensive ophthalmic examination, including assessment of best-corrected visual acuity which was converted to logarithm of the minimal angle of resolution (logMAR), slit-lamp biomicroscopy, axial length measurement using IOLMaster (Version 4.08, Carl Zeiss Meditec AG, Jena, Germany), and OCT-A. The severity of DR was categorized into healthy without DM, DM without DR, mild nonproliferative DR (NPDR), moderate NPDR, severe NPDR, and proliferative DR (PDR). Similarly, the presence of diabetic macular edema (DME) was classified based on the same scale.
Clinical information, including a history of hypertension, dyslipidemia, coronary arterial diseases, and atherosclerosis in other locations, which were documented or diagnosed by physicians in Peking Union Medical College Hospital, was obtained. Hypertension was defined as a blood pressure of 140/90 mmHg or more on more than three occasions after sufficient rest on different days or anti-hypertensive treatment. Dyslipidemia was defined as total cholesterol ≥ 200 mg/dl, triglycerides ≥ 150 mg/dl, low-density lipoprotein cholesterol ≥ 130 mg/dl, or high-density cholesterol ≤ 40 mg/dl (AU5821 automatic biochemical analyzer, Beckman Coulter K.K., Japan). Coronary arterial diseases and atherosclerosis in other locations included a positive result obtained by ultrasound, computed tomography, or magnetic resonance imaging; a history of relevant symptoms or revascularization, such as coronary artery bypass surgery or angioplasty. The laboratory data were reviewed within a time window of 1 week from the acquisition of OCT-A images. After overnight fasting, blood samples were taken, and hemoglobin A1c (HbA1c) (AU5800 automatic biochemical analyzer, Beckman Coulter K.K., Japan) and creatinine (AU5821 automatic biochemical analyzer, Beckman Coulter K.K., Japan) were measured. The value of estimated glomerular filtration rate (eGFR) value was calculated using the equation utilized in the Chronic Kidney Disease Epidemiology Collaboration [14].
OCT-A image acquisition
Eyes were imaged with an Optovue Avanti RTVue XR SD-OCT (Optovue, Fremont, California, USA). Both Angio Retina 3.0 mm and HD Angio Retina 6.0 mm, centered on fovea, were acquired in each patient. Patients also underwent OCT scans using the raster and cross-line pattern for structural information. The Avanti system employs motion correction technology and 3D projection artifact reduction. The built-in software calculates the CFD of the retinal superficial capillary plexus, retinal deep capillary plexus, and CC layer by computing the percentage area occupied by detected OCT-A-vasculature. Manual corrections were made if any scan errors were identified.
Two trained graders (JY and SX) were masked to all clinical information; these graders diagnosed and categorized DR as well as reviewed OCT-A images independently. In cases with disagreement, a third retinal specialist (YC) made the final decision.
Statistical analysis
Continuous variables are presented as the mean (standard deviation, SD). Categorical variables are given as numbers or percentages. Differences in CFD among the stages of DR and DME were evaluated with ANOVA. Pairwise comparisons were investigated with the LSD test as the post hoc tests. Pearson’s correlation coefficient (r) was calculated for correlation analysis. Interscan pattern reliability was evaluated with the intraclass correlation coefficient (ICC) and 95% confidence interval (CI) based on a single rating, concordance, two-way mixed-effect model. ICC values between 0 and 0.5 indicate poor reliability, moderate reliability between 0.5 and 0.75, good reliability between 0.75 and 0.9, and excellent reliability above 0.9. Multiple linear regression analysis was performed on all of the clinical variables (history of hypertension, dyslipidemia, coronary arterial diseases, and atherosclerosis in other locations as independent variables), and CFD in the CC layer from the right eyes of subjects was used as the dependent variable using the stepwise model with the threshold P value = 0.05 for entry and 0.10 for removal. Statistical analyses were performed using SPSS software version 25.0 (IBM-SPSS, Chicago, Il, USA). Results with P < 0.05 were considered statistically significant.
Results
In total, 282 eyes of 146 subjects were reviewed. The mean (SD) age of the 146 patients was 54.27 (12.59). The clinical characteristics of the diabetic patients are summarized in Table 1. For the DR severity level, 56 (19.86%) eyes had no DR, while 43 (15.25%), 54 (19.15%), 38 (13.48%), and 48 (17.02%) eyes had mild NPDR, moderate NPDR, severe NPDR, and PDR, respectively. Of those eyes, 96 (34.04%) eyes had DME. In addition, 43 (15.25%) eyes were nondiabetic control eyes. Ten eyes were excluded owing to poor OCT-A image quality.
As shown in Fig. 1, the mean (SD) CFD in the CC layer using the 3-mm scan pattern for the control, no DR, mild NPDR, moderate NPDR, severe NPDR, and PDR was 65.66 (0.50), 63.50 (0.61), 62.07 (0.67), 58.13 (1.04), 57.31 (1.13), and 53.79 (0.67), respectively (Fig. 1). The pairwise comparisons of CFD using the 3-mm scan pattern between mild NPDR and moderate NPDR and between severe NPDR and PDR showed significant differences (P = 0.001, P = 0.003), while the other comparisons between adjacent stages showed no significant differences. Moreover, using the 6-mm scan pattern, the mean (SD) CFD values in the CC layer were 68.33 (0.40), 67.46 (0.54), 66.48 (0.54), 62.59 (0.84), 61.42 (0.80), and 58.19 (0.60) for the control, no DR, mild NPDR, moderate NPDR, severe NPDR, and PDR, respectively (Figs. 1 and 2). The pairwise comparisons of CFD using the 6-mm scan pattern between mild NPDR and moderate NPDR and between severe NPDR and PDR showed significant differences (P < 0.001, P = 0.001), while the other comparisons between adjacent stages showed no significant differences. With the exception of the PDR stage, the Pearson correlation test revealed a significant correlation between 3-mm and 6-mm scan patterns in the CC layer in each DR stage. The ICC (95% CI) of the 3-mm scan pattern and the 6-mm scan pattern in the CC layer was 0.889 (0.864–0.991), which presents good reliability.
Choriocapillary flow density in the choriocapillary layer. Bar graph of mean choriocapillary flow density in the choriocapillary layer with 95% confidence intervals. The mean choriocapillary flow density shows a decreasing trend with the progression of diabetic retinopathy. The mean choriocapillary flow density in the 6-mm scan pattern is greater than that in the 3-mm scan pattern
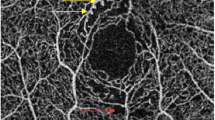
Nonsegmented OCT-A images at CC layer. Representative OCT-A images in the CC layer of a healthy eye (a), a diabetic eye without DR (b), an eye with mild NPDR (c), an eye with moderate NPDR (d), an eye with severe NPDR (e), and an eye with PDR (f). Areas of choriocapillary flow are shown here in yellow. Reduced flow areas are present in eyes with severe NPDR and PDR
CFD with and without DME in the CC layer at various stages of DR is shown in Table 2. Using the 3-mm scan pattern, the mean (SD) CFD values for DR without DME and DR with DME group were 62.39 (0.58) and 53.98 (0.60) in the CC layer, respectively. The pairwise comparisons of CFD using the 3-mm scan pattern between the DR without DME group and DR with DME group showed significant differences (P < 0.001), while the other comparisons between adjacent groups showed no significant differences. In addition, using the 6-mm scan pattern, the mean (SD) CFD values for DR without DME and DR with DME group were 66.19 (0.49) and 58.80 (0.48) in the CC layer, respectively (Fig. 1). The pairwise comparisons of CFD using the 6-mm scan pattern between the DR without DME group and DR with DME group showed a significant difference (P < 0.001), while the other comparisons between adjacent groups showed no significant differences.
In the multiple linear regression analysis results after controlling for age, gender, severity of DR, and axial length of eyes, the CFD in the CC layer using both 3-mm and 6-mm scan patterns was significantly associated with the axial length of eyes (P < 0.001, P < 0.001), coronary artery disease (P = 0.015, P = 0.001), atherosclerosis in other locations (P = 0.006, P < 0.001), eGFR (P < 0.001, P < 0.001), and HbA1c (P = 0.001, P < 0.001) (Table 3). CFD in the CC layer using 6-mm scan patterns was also significantly associated with dyslipidemia (P = 0.042).
Discussion
In the present study, we described the application of OCT-A to study diabetic eyes in different stages and evaluated the relationship between CC flow density and a variety of measures with systemic and metabolic vascular factors. Our study suggested that OCT-A is a quantitative, noninvasive tool to evaluate the CC microvasculature in diabetic eyes.
We reported several significant findings that might advance our understanding of DC. First, this study showed that the CFD in the CC layer significantly decreased with worsening severity of DR at the stage of moderate NPDR and PDR and with the presence of DME, which suggested that the severity of DC did not entirely agree with the severity of DR. Second, more importantly, we showed that a variety of systemic vascular and metabolic vascular factors, including dyslipidemia, eGFR, and HbA1c, were associated with CC CFD, especially using the 6-mm scan pattern. Third, although the CFD in the CC layer using a 6-mm scan pattern was greater than that using a 3-mm scan pattern, the two scan areas showed good reliability and correlation. The differences in CFD values between the two scan patterns might result from differences in resolution and distinguishability. However, one of the advantages of the 6-mm scan pattern was that the CFD was associated with more systemic and metabolic factors.
Compared with previous studies, the present study used a built-in software to assess the CFD in the CC layer in treatment-naïve diabetic eyes of a larger sample size in an automated manner with 3D projection artifact reduction. In contrast, in a cross-sectional study of 44 eyes, Nesper et al. used OCT-A without 3D projection artifact reduction to compare the nonflow area in the CC layer in diabetic eyes and similarly found an increased nonflow area in diabetic eyes [11]. A projection artifact reduction algorithm is more likely to provide reliable results [15]. Choi et al. also reported CC flow impairment qualitatively using a swept-source OCT-A prototype, and motion errors were not corrected [9]. Our study provided CC flow density quantitative results, and motion errors were corrected using the built-in algorithm, which made measurements more reliable. In addition, Conti et al. reported a decreased trend of CFD in the CC layer in 99 diabetic eyes among which some had been treated [10]. Since the effect of treatment including photocoagulation and anti-vascular endothelial growth factor (anti-VEGF) injection on OCT angiograms of the CC layer have not been thoroughly studied and interpreted, we enrolled diabetic eyes with no previous treatment. However, compared with the aforementioned studies, the present study came to a generally similar conclusion as follows: decreased CFD in the CC layer in diabetic eyes. Alternatively, Carnevali et al. used OCT-A to assess CC in only 25 diabetic eyes without DR and found no significant difference in the vessel density of CC between diabetic patients and healthy subjects [12]. In contrast, our study revealed a downward trend of CFD in the CC layer with worsening DR severity.
Histopathological studies of diabetic eyes reported degeneration of the CC layer with loss of endothelial cells, obstruction and choroidal aneurysms, and RPE degeneration, ultimately resulting in choroidal neovascularization [16]. As DR progresses, the level of VEGF increases, especially in eyes with diffuse diabetic DME and PDR [17,18,19]. DME, partially resulting from a relatively high level of VEGF, as well as the stage of PDR, which was related to neovascularization resulting from VEGF, suggests similar significant pathophysiologic alterations [17, 18]. In our study, a significant decrease in the CFD of the CC layer was observed in eyes in the PDR stage and in eyes with DME. Therefore, decreased CFD in the CC layer was associated with the VEGF level. However, Conti et al. reported no significant difference was found in CFD in the CC layer between the NPDR and PDR groups [10]. The present study with a larger sample size and NPDR classification strongly suggested that CFD in the CC layer in eyes with PDR was indeed less than that in eyes with NPDR.
Furthermore, in the multivariate analyses, low CFD in the CC layer was associated with coronary artery disease, atherosclerosis in other locations, and HbA1c and eGFR levels. Previous clinical studies revealed that DR severity was positively associated with TC, TG, and LDL-C and negatively associated with HDL-C [20, 21]. Similarly, in our study, the severity of DC showed a similar correlation with lipid metabolism [22]. Notably, CFD in the CC layer using a 6-mm scan pattern rather than a 3-mm scan pattern was associated with dyslipidemia. Therefore, evaluation of CFD in the CC layer with a larger scan area was recommended because it presented more correlations with systemic conditions. In addition, DM is a disease concerning inflammation [23, 24], and coronary artery disease and atherosclerosis in other locations also involve inflammation [25]. Therefore, we investigated the relationship between artery diseases and DC. We found that DC is associated with coronary artery disease and atherosclerosis in other locations using both 3-mm and 6-mm scan patterns. In addition, a previous study reported that the production of VEGF secondary to renal glomerular injury might increase the VEGF level in systemic circulation, which might lead to the progression of DC [26]. In the present study, decreased CFD in the CC layer was associated with increased eGFR. Moreover, there is no established relationship between DC and the level of HbA1c, and our study indicates a positive association of the severity of DC and the level of HbA1c, which suggests that several hyperglycemia-triggered metabolic pathways lead to abnormalities in the retinal capillary bed as well as in the CC bed [27, 28]. For other systemic factors, hypertension was reportedly positively associated with the prevalence and progression of DR [29]. However, in our study, no clear association between hypertension with DC was established. Different histological structures of the retinal capillaries and CC might contribute to different correlations with hypertension. In summary, diabetic patients with apparently decreased CFD should be assessed carefully under general conditions.
We acknowledge several limitations of this study. First, the lack of follow-up data did not allow us to analyze the role of HbA1c for a longer period. Second, large-sample longitudinal studies are warranted for the evaluation of the relationship between systemic and metabolic factors and DC onset and progression. Third, the OCT-A scan area was limited to the central macula, which does not represent the entire CC layer. Fourth, the relationship between CFD in the CC layer and some factors, including choroidal thickness and smoking history, were not analyzed. Different studies presented conflicting results on the choroidal thickness in diabetic eyes [18], which made it difficult to explain the relationship between CFD in the CC layer and choroidal thickness. Similarly, the impact of tobacco smoking on choroid is uncertain [30, 31]. And the calculation of cumulative smoking exposure requires exact parameters which are difficult for ophthalmic patients to recall sometimes, while semiquantitative metrics might introduce bias [32]. Therefore, the relationship between smoking history and CFD in the CC layer was not investigated in the present study.
In conclusion, we demonstrated decreased CC flow density associated with DR severity using OCT-A in diabetic patients compared to that in healthy controls. The CC flow density showed a correlation with systemic metabolic and vascular factors. Diabetic patients with lower CFD in the CC layer should be evaluated under general condition. OCT-A has the potential to be used in prospective studies and interventional trials, to deepen our understanding of DC in a noninvasive manner.
References
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564. https://doi.org/10.2337/dc11-1909
Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. N Engl J Med 366:1227–1239. https://doi.org/10.1056/NEJMra1005073
Hayreh SS (2001) Blood flow in the optic nerve head and factors that may influence it. Prog Retin Eye Res 20:595–624
McLeod DS, Lutty GA (1994) High-resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci 35:3799–3811
Lutty GA (2013) Effects of diabetes on the eye. Invest Ophthalmol Vis Sci 54:Orsf81–Orsf87. https://doi.org/10.1167/iovs.13-12979
Takahashi A, Nagaoka T, Sato E, Yoshida A (2008) Effect of panretinal photocoagulation on choroidal circulation in the foveal region in patients with severe diabetic retinopathy. Br J Ophthalmol 92:1369–1373. https://doi.org/10.1136/bjo.2007.136028
Agrawal R, Xin W, Keane PA, Chhablani J, Agarwal A (2016) Optical coherence tomography angiography: a non-invasive tool to image end-arterial system. Expert Rev Med Devices 13:519–521. https://doi.org/10.1080/17434440.2016.1186540
Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, Potsaid B, Liu JJ, Lu CD, Kraus MF, Fujimoto JG, Huang D (2014) Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 121:1435–1444. https://doi.org/10.1016/j.ophtha.2014.01.034
Choi W, Waheed NK, Moult EM, Adhi M, Lee B, De Carlo T, Jayaraman V, Baumal CR, Duker JS, Fujimoto JG (2017) Ultrahigh speed swept source optical coherence tomography angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy. Retina (Philadelphia, Pa) 37:11–21. https://doi.org/10.1097/iae.0000000000001250
Conti FF, Qin VL, Rodrigues EB, Sharma S, Rachitskaya AV, Ehlers JP, Singh RP (2018) Choriocapillaris and retinal vascular plexus density of diabetic eyes using split-spectrum amplitude decorrelation spectral-domain optical coherence tomography angiography. Br J Ophthalmol 103:452–456. https://doi.org/10.1136/bjophthalmol-2018-311903
Nesper PL, Roberts PK, Onishi AC, Chai H, Liu L, Jampol LM, Fawzi AA (2017) Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 58:Bio307–bio315. https://doi.org/10.1167/iovs.17-21787
Carnevali A, Sacconi R, Corbelli E, Tomasso L, Querques L, Zerbini G, Scorcia V, Bandello F, Querques G (2017) Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol 54:695–702. https://doi.org/10.1007/s00592-017-0996-8
Lim HB, Kim YW, Kim JM, Jo YJ, Kim JY (2018) The importance of signal strength in quantitative assessment of retinal vessel density using optical coherence tomography angiography. Sci Rep 8:12897. https://doi.org/10.1038/s41598-018-31321-9
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Zhang M, Hwang TS, Campbell JP, Bailey ST, Wilson DJ, Huang D, Jia Y (2016) Projection-resolved optical coherence tomographic angiography. Biomed Opt Express 7:816–828. https://doi.org/10.1364/boe.7.000816
Fukushima I, McLeod DS, Lutty GA (1997) Intrachoroidal microvascular abnormality: a previously unrecognized form of choroidal neovascularization. Am J Ophthalmol 124:473–487
Funatsu H, Yamashita H, Ikeda T, Nakanishi Y, Kitano S, Hori S (2002) Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. Am J Ophthalmol 133:537–543
Melancia D, Vicente A, Cunha JP, Abegao Pinto L, Ferreira J (2016) Diabetic choroidopathy: a review of the current literature. Graefes Arch Clin Exp Ophthalmol 254:1453–1461. https://doi.org/10.1007/s00417-016-3360-8
Paine SK, Mondal LK, Borah PK, Bhattacharya CK, Mahanta J (2017) Pro- and antiangiogenic VEGF and its receptor status for the severity of diabetic retinopathy. Mol Vis 23:356–363
Ting DSW, Tan GSW, Agrawal R, Yanagi Y, Sie NM, Wong CW, San Yeo IY, Lee SY, Cheung CMG, Wong TY (2017) Optical coherence tomographic angiography in type 2 diabetes and diabetic retinopathy. JAMA Ophthalmol 135:306–312. https://doi.org/10.1001/jamaophthalmol.2016.5877
Lee DH, Yi HC, Bae SH, Cho JH, Choi SW, Kim H (2018) Risk factors for retinal microvascular impairment in type 2 diabetic patients without diabetic retinopathy. PLoS One 13:e0202103. https://doi.org/10.1371/journal.pone.0202103
Van Belle E, Rivard A, Chen D, Silver M, Bunting S, Ferrara N, Symes JF, Bauters C, Isner JM (1997) Hypercholesterolemia attenuates angiogenesis but does not preclude augmentation by angiogenic cytokines. Circulation 96:2667–2674
Pedersen BK (2017) Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Investig 47:600–611. https://doi.org/10.1111/eci.12781
Ohno T, Takamoto S, Motomura N (2008) Diabetic retinopathy and coronary artery disease from the cardiac surgeon’s perspective. Ann Thorac Surg 85:681–689. https://doi.org/10.1016/j.athoracsur.2007.07.066
Pant S, Deshmukh A, Gurumurthy GS, Pothineni NV, Watts TE, Romeo F, Mehta JL (2014) Inflammation and atherosclerosis--revisited. J Cardiovasc Pharmacol Ther 19:170–178. https://doi.org/10.1177/1074248413504994
Hsieh YT, Tsai MJ, Tu ST, Hsieh MC (2018) Association of abnormal renal profiles and proliferative diabetic retinopathy and diabetic macular edema in an Asian population with type 2 diabetes. JAMA Ophthalmol 136:68–74. https://doi.org/10.1001/jamaophthalmol.2017.5202
Chang YC, Wu WC (2013) Dyslipidemia and diabetic retinopathy. Rev Diabet Stud 10:121–132. https://doi.org/10.1900/rds.2013.10.121
Sas KM, Kayampilly P, Byun J, Nair V, Hinder LM, Hur J, Zhang H, Lin C, Qi NR, Michailidis G, Groop PH, Nelson RG, Darshi M, Sharma K, Schelling JR, Sedor JR, Pop-Busui R, Weinberg JM, Soleimanpour SA, Abcouwer SF, Gardner TW, Burant CF, Feldman EL, Kretzler M, Brosius FC 3rd, Pennathur S (2016) Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 1:e86976. https://doi.org/10.1172/jci.insight.86976
Suzuma I, Hata Y, Clermont A, Pokras F, Rook SL, Suzuma K, Feener EP, Aiello LP (2001) Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2: potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes 50:444–454
Sizmaz S, Kucukerdonmez C, Pinarci EY, Karalezli A, Canan H, Yilmaz G (2013) The effect of smoking on choroidal thickness measured by optical coherence tomography. Br J Ophthalmol 97:601–604. https://doi.org/10.1136/bjophthalmol-2012-302393
Teberik K (2019) The effect of smoking on macular, choroidal, and retina nerve fiber layer thickness. Turk J Ophthalmol 49:20–24. https://doi.org/10.4274/tjo.galenos.2018.80588
Remen T, Pintos J, Abrahamowicz M, Siemiatycki J (2018) Risk of lung cancer in relation to various metrics of smoking history: a case-control study in Montreal. BMC Cancer 18:1275. https://doi.org/10.1186/s12885-018-5144-5
Acknowledgments
Thanks are due to Chenxi Zhang, Minghang Pei, and Shan Wu for collecting data and supporting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Wang, E., Zhao, X. et al. Optical coherence tomography angiography analysis of the choriocapillary layer in treatment-naïve diabetic eyes. Graefes Arch Clin Exp Ophthalmol 257, 1393–1399 (2019). https://doi.org/10.1007/s00417-019-04326-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04326-x