Abstract
Aims
The aims of the study were to investigate weight loss and glycemic control parameters after different bariatric surgical procedures in type 2 diabetes (T2D) obese patients and identify patients’ factors that predict diabetes remission.
Methods
The study included 105 obese T2D patients (66 women and 39 men) who underwent laparoscopic gastric banding (LAGB, 11 subjects, age 47 ± 10 years, BMI 42.3 ± 8.3 kg/m2), or laparoscopic Roux-en-Y gastric bypass (RYBP, 77 subjects, age 50 ± 8 years, BMI 45.7 ± 6.8 kg/m2), or sleeve gastrectomy (SG, 17 subjects, age 49 ± 11 years, BMI 50.2 ± 8.8 kg/m2) during 2005–2012 period.
Results
The average percentage of weight loss at 12 months after surgery was 26.4 ± 9.8 %, and it was maintained at 24 and 36 months of follow-up. Diabetes remission occurred in 68.6 % of study participants (4/11 of LAGB, 54/77 of RYBP and 14/17 of SG). In multivariate Cox analysis, age, duration of diabetes, surgical procedure and glycated hemoglobin <53 mmol/mol (7 %) resulted significant predictors of diabetes remission (age RR = 0.97, 95 %CI 0.94–1.0, p = 0.05; diabetes duration RR = 0.93, 95 % CI 0.86–0.99, p = 0.036; rif LAGB, RYBP RR = 3.9, 95 % CI 1.31–11.57, p = 0.014; SG RR = 5.6, 95 % CI 1.67–18.64, p = 0.005; glycated hemoglobin RR = 0.54, 95 % CI 0.32–0.92, p = 0.024).
Conclusions
Bariatric surgical procedures that modify the upper gastrointestinal tract anatomy (RYBP and SG) are more successful in producing weight loss and remission of T2D than those that simply restrict stomach capacity (LAGB). Younger age, short duration of diabetes and better glucose control confer higher probability of achieving remission of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is considered a valuable treatment for morbid obesity [1]. It results in a durable weight loss, improvement in quality of life and in obesity-related comorbidity and on survival [2–4]. Obesity, particularly central obesity, is closely associated with insulin resistance, type 2 diabetes and cardiovascular diseases [5, 6]. Recent studies, including five randomized controlled trials [7–11], have shown the remarkable effects of bariatric surgery on improving glycemic control and cardiovascular risk factor modification in type 2 diabetes, emerging as a potential treatment of this disease [12, 13]. As a matter of fact, one of the major goals in the contemporary bariatric surgery is the remission of type 2 diabetes. Nevertheless, question remains regarding the durability of the metabolic benefits of surgery.
The most commonly used bariatric surgery techniques are Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG) and laparoscopic adjustable gastric banding (LAGB). Although type 2 diabetes remission is a goal of the contemporary bariatric surgery, currently, it is difficult to identify the most effective surgical technique based on patient characteristics and comorbidities. Furthermore, little is known about the difference among various weight loss surgical procedures on diabetes remission.
Thus, the aim of this study was to compare the clinical efficacy of laparoscopic adjustable gastric banding (LAGB), Roux-en-Y gastric bypass (RYBP) and sleeve gastrectomy (SG) in terms of type 2 diabetes remission in a clinical setting study.
Subjects and methods
The present prospective monocentric observational study, based on our bariatric surgery cohort, was aimed to evaluate the clinical impact of three different surgical techniques on diabetes remission.
Since 2005 until December 2012, 480 obese patients were operated in the bariatric surgery unit, 105 of these (22 %) were affected by type 2 diabetes (66 women and 39 men, mean age 49.3 ± 8.6 years, mean BMI 46.1 ± 7.5 kg/m2). The diabetic patients were selected for the present analysis. All patients were evaluated by a team of specialist of the Interdisciplinary Group of Bariatric Surgery of Verona: diabetologist, dietician, psychologist and surgeon. All bariatric procedures were carried out by the same surgeon with the same surgical techniques. Furthermore, the same diabetologist evaluated all patients before surgery and during the follow-up.
Diabetes was diagnosed by ADA criteria [14]. Twenty-one subjects (20 %) received a diagnosis of diabetes concurrently to bariatric surgery, 43 patients (41 %) had diabetes with duration between 0 and 4 years, 23 patients (22 %) had diabetes with duration between 5 and 9 years, and finally, 18 patients (17 %) had diabetes with duration of more than 10 years. Treatment of diabetes was by insulin in 22 patients, by oral antidiabetic agents in 66 patients and by diet alone in 17 patients. All participants completed research assessments prior to surgery and at 3, 6, 12 months, and then annually after surgery for 3 years.
Diabetes remission was defined as glycated hemoglobin (HbA1c) <42 mmol/mol Hb (6 %) and fasting blood glucose (FBG) <100 mg/dl off diabetic medications, according to ADA 2009 criteria [15].
The study was approved by the Ethical Committee of Verona, and all participants gave written informed consent to it.
Clinical and laboratory data
Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Blood pressure was measured with a standard mercury manometer. Venous blood was drawn in the morning after an overnight fast in all patients. Serum creatinine, lipids and other biochemical blood measurements were determined by standard laboratory procedures (DAX 96; Bayer Diagnostics, Milan, Italy). Hemoglobin A1c (HbA1C) was measured according to the standard operating procedure of the IFCC reference, by an automated high-performance liquid chromatography analyzer (Bio-Rad Diamat, Milan, Italy). Patients were considered to have arterial hypertension if their blood pressure values were >140/90 mmHg or they were taking any antihypertensive drugs.
Statistical analysis
The remission of diabetes was the endpoint of the study. Continuous variables with normal distribution are presented as mean ± SD. Categorical variables are expressed as absolute frequencies and percentage (%). Differences between groups were evaluated using parametric or nonparametric tests as appropriate, the Chi-square test was used for categorical variables. The impact of different surgical procedures on diabetes remission was estimated in univariate analysis using Kaplan–Meier (log-rank test) and in multivariate using Cox proportional hazards methods adjusting for age, sex, BMI, duration of diabetes and metabolic control (glycated hemoglobin >53 vs ≤ 53 mmol/mol Hb). In the attempt to characterize an ideal patient with a higher probability of remission of diabetes, subjects were divided in tertiles of age (I tertile age ≤45 years; II tertile age 46–53 years; III tertile age ≥54 years) and duration of diabetes (I tertile 0–2 years; II tertile 3–5 years; III tertile ≥6 years), and these variables were introduced in the Cox proportional model.
Results
Surgical procedures included LAGB (11 subjects, age 47 ± 10 years, BMI 42.3 ± 8.3 kg/m2), RYBP (77 subjects, age 50 ± 8 years, BMI 45.7 ± 6.8 kg/m2) and SG (17 subjects, age 49 ± 11 years, BMI 50.2 ± 8.8 kg/m2). Baseline characteristics of diabetic patients are reported in Table 1: age, sex distribution, duration of diabetes, glycated hemoglobin, fasting plasma glucose, treatment of diabetes, hypertension and hypercholesterolemia were not different among the groups, while BMI was significantly higher in patients who underwent SG.
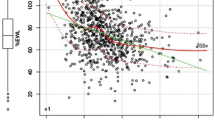
A significant weight loss (p < 0.01) was obtained after each single surgical procedure as shown in Fig. 1. The percentage of weight lost reached its nadir by 12 months after surgery (in whole cohort Δkg % −26.4 ± 9.8), and it was maintained at 36 months of follow-up. In the whole group, 72 patients (68.6 %) achieved diabetes remission (four patients of LAGB group, 54 patients of RYBP group and 14 patients of SG group, respectively). The average time to diabetes remission was 7.9 ± 6.6 months after surgery, without any differences among surgery procedures (8.5 ± 8.6 months for LAGB; 8.0 ± 7.0 months for RYBP; 7.3 ± 4.8 months for SG; p = 0.92).
Table 2 shows that patients who achieved diabetes remission (n = 72) were younger (48.1 ± 8.1 vs 52.1 ± 7.7 years, p = 0.026), with a shorter duration of diabetes (3.5 ± 3.6 vs 8.8 ± 7.2 years, p = 0.001) and a lower fasting plasma glucose (149 ± 48 vs 179 ± 54 mg/dl, p = 0.005) in comparison with patients who failed (n = 33). They were more frequently on diet treatment only and less frequently treated by drugs either insulin or oral antidiabetic agents (Table 2). There were no differences in antihypertensive treatment, while the use of statins was significantly higher in patients who did not reach remission of diabetes.
The Kaplan–Meiers analysis (Fig. 2) shows that subjects that underwent RYBP and SG tended to reach diabetes remission in a higher percentage than patients of LAGB group (p = 0.07). When SG and RYBP surgical procedures were combined, the differences in the probability of diabetes remission resulted significant (p = 0.036) (data not shown).
To study the predictors of diabetes remission, a multivariate Cox proportional analysis was modelled. As shown in Table 3, the significant predictors of diabetes remission were age (RR = 0.97, 95 % CI 0.94–1.0, p = 0.05), diabetes duration (RR = 0.93, 95 % CI 0.86–0.99, p = 0.036), surgical procedures (rif LAGB, RYBP RR = 3.9, 95 % CI 1.31–11.57, p = 0.014; SG RR = 5.6, 95 % CI 1.67–18.64, p = 0.005) and glycated hemoglobin <53 mmol/mol (7 %) (RR = 0.54, 95 % CI 0.32–0.92, p = 0.024). In order to characterize an ideal patient with a higher probability of remission of diabetes, we modelled a multivariate Cox model using tertiles of age and duration of diabetes. According to the results of this analysis, the ideal patient should be younger than 45 years, with a duration of diabetes <2 years and in good metabolic control (HbA1c ≤53 mmol/mol = 7 %).
The use of statins (yes/no) was not significant when included in the multivariate Cox model (data not shown).
Discussion
Our Study once again confirms that bariatric surgery is a safe surgical procedure to reach a durable reduction in body weight and the remission of type 2 diabetes. Moreover, we found that surrogate markers of β-cell function, such as duration of diabetes and glycemic control, may have a role in selecting patients with a higher probability of achieving remission of diabetes [16, 17]. Our results also suggest that surgical procedures such as RYBP and SG may be a better choice if remission of diabetes is also a goal of therapy.
An increasing body of evidence emphasizes the bariatric surgery benefits for treatment of the type 2 diabetes in obese patients. Short-term diabetes remission occurs after bariatric surgery in 60–90 % of patients [2, 7–11, 18]. To improve the duration of remission, Pok and Lee [19] proposed to select patients with higher BMI and better β-cell function. Accordingly, a recent meta-analysis by Wang et al. [20] observed that type 2 diabetes remission was negatively correlated with age, diabetes duration, insulin use and HbA1c levels that may be considered as clinical surrogate markers of β-cell function [21]. Furthermore, both higher BMI and C-peptide levels before surgery were shown to be associated with a better chance of diabetes remission rate in Asian patients [22].
The choice of surgical procedure to be used is a further important issue; many variations in selection of the surgical technique are observed among obesity care centers. Campos et al. [23], in a two-cohort pair-matched study, showed that RYGB versus LAGB presented a greater weight loss, a greater resolution of diabetes and better improved quality of life. In a recent systematic review [24], gastric bypass had better outcome than gastric band procedures for long-term weight loss, type 2 diabetes control and remission, and [25] in a retrospective study, the resolution rate of diabetes 1 year after surgery was significantly higher after SG than LAGB, but not significantly different between SG and RYBP.
Our results are basically in agreement with those previously reported [23, 25, 26] as significant differences were observed in diabetes remission rate depending on the surgical method used: RYBP and SG, that modify upper gastrointestinal tract anatomy, showed a greater diabetes remission rate in comparison with LAGB.
It is important to note that the lack of standardization of the criteria of remission of diabetes among studies introduces a limit to the comparison. In published studies, resolution of T2D was defined and reported in a variety of ways (HbA1c levels of <5–6, 5.7, 6.5 and 7 %) without glucose-lowering therapy. In our study, we used ADA 2009 criteria (HbA1c <6 %, 42 mmol/mol, and fasting blood glucose <100 mg/dl off diabetic medications).
In our study, diabetes remission occurred in 68.6 % of our patients, but also patients who failed diabetes remission showed a better glycemic control after surgery [HbA1c at baseline: 8.1 ± 1.4 % (65 ± 13 mmol/mol); 12 months follow-up: 6.8 ± 0.9 % (51 ± 7.5 mmol/mol); 24 months follow-up: 7.0 ± 0.9 % (53 ± 7.5 mmol/mol); 36 months follow-up: 7.0 ± 0.8 % (53 ± 6.4 mmol/mol)] and a reduction in diabetic medications. Furthermore, in our study, no diabetes relapse was observed in patients with diabetes remission during the follow-up.
The use of statins has been shown to increase the risk of developing diabetes [27]. In our study the use of statins was more frequent in patients who did not reach remission of diabetes, apparently suggesting a possible negative effect of statins on remission of diabetes. However, this effect was lost in the multivariate analysis. Maybe a study should be designed specifically to investigate this issue.
Strengths of our study are: (1) all patients were evaluated by the same team of specialist; therefore, our data are homogeneous; (2) the completeness of the data; (3) the follow-up was regularly executed. The major limitation of our study is its observational design and therefore the absence of randomization. A further limitation may be the number of patients in the LAGB group which was mainly composed of female. However, when only females were included in the multivariate analysis, the results did not change. Nevertheless, patients were comparable for the major clinical characteristics (Table 1) before surgery. Therefore, we believe that our result can truly represent the clinical setting to select patients and offer the best surgical procedure if the remission of diabetes is a further goal of treatment. According to the results of our multivariate model, the ideal type 2 diabetic obese patients should have a duration of diabetes of <2 years, be in good glycemic control (HbA1c <53 mmol/mol = 7 %) and with an age below 45 years. This ideal patient should be considered for sleeve or bypass surgical procedures to improve its chance to achieve remission of diabetes.
To conclude, the results of our study suggest that among the three surgical procedures available for the management of obese patients with type 2 diabetes, RYBP and SG might be more efficient than LAGB to induce diabetes remission. Furthermore, in our obese T2DM patients, age, history of diabetes and glycemic control can be assumed to be predictors for diabetes remission.
References
Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ (2013) Bariatric surgery versus non surgical treatment for obesity: a systematic review and meta-analysis of randomized controlled trials. BMJ 347:f5934
Sjöström L, Lindroos AK, Peltonen M, Torgerson J et al (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351:2683–2693
Sjöström L, Peltonen M, Jacobson P et al (2012) Bariatric surgery and long-term cardiovascular events. JAMA 307:56–65
Sjöström L, Narbro K, Sjöström CD, Karaso K, Larsson B, Wedel H et al (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357:741–752
Bonora E (2006) The metabolic syndrome and cardiovascular disease. Ann Med 38:64–80
Colagiuri S (2010) Diabesity: therapeutic options. Diabetes Obes Metab 12:463–473
Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M (2008) Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299:316–323
Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwa JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL (2012) Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366:1567–1576
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F (2012) Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366:1577–1585
Ikramuddin S, Korner J, Lee WJ, Connett JE, Inabnet WB, Billington CJ, Thomas AJ, Leslie DB, Chong K, Jeffery RW, Ahmed L, Vella A, Chuang LM, Bessler M, Sarr MG, Swain JM, Laqua P, Jensen MD, Bantle JP (2013) Roux-en-Y gastric bypass versus intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the diabetes surgery study randomized clinical trial. JAMA 309:2240–2249
Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X (2013) Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 101:50–56
Brethauer SA, Aminian A, Romer-Talamas H et al (2013) Can diabetes be surgically cured? Ann Surg 258:628–637
Adams ST, Salhab M, Hussain ZI, Miller GV, Leveson SH (2013) Preoperatively determinable factors predictive of diabetes mellitus remission following Roux-en-Y gastric bypass: a review of the literature. Acta Diabetol 50:475–478
Moghissi ES, Korytkowski MT, DiNardo M et al (2009) American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 32:1119–1131
Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, McLaughlin S, Phillips GL 2nd, Robertson RP, Rubino F, Kahn R, Kirkman MS (2009) How do we define cure of diabetes? Diabetes Care 32:2133–2135
Kim D, Kim KJ, Huh JH, Lee BW, Kang ES, Cha BS, Lee HC (2012) The ratio of glycated albumin to glycated haemoglobin correlates with insulin secretory function. Clin Endocrinol 77:679–683
Kahn SE (2003) The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46:3–19
Buchwald H, Estok R, Fahrbach K et al (2009) Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122:248–256
Pok EH, Lee WJ (2014) Gastrointestinal metabolic surgery for the treatment of type 2 diabetes mellitus. World J Gastroenterol 20:14315–14328
Wang GF, Yan YX, Xu N, Yin D, Hui Y, Zhang JP, Han GJ, Ma N, Wu Y, Xu JZ, Yang T (2015) Predictive factors of type 2 diabetes mellitus remission following bariatric surgery: a meta-analysis. Obes Surg 25:199–208
Li C, Yang H, Tong G, Shen S, Feng W, Bi Y, Zhu D (2014) Correlations between A1c, fasting glucose, 2 h postload glucose, and βcell function in the Chinese population. Acta Diabetol 51:601–608
Funakoshi S, Fujimoto S, Hamasaki A, Fujiwara H, Fujita Y, Ikeda K, Hamamoto Y, Hosokawa M, Seino Y, Inagaki N (2008) Analysis of factors influencing pancreatic beta-cell function in Japanese patients with type 2 diabetes: association with body mass index and duration of diabetic exposure. Diabetes Res Clin Pract 82:353–358
Campos GM, Rabl C, Roll GR, Peeva S, Prado Kris, Smith J, Vittinghoff E (2011) Better weight loss, resolution of diabetes, and quality of life for laparoscopic gastric bypass versus banding results of a two-cohort pair-matched study. Arch Surg 146:149–155
Puzziferri N, Roshek TB 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH (2014) Long-term follow-up after bariatric surgery. Syst Rev JAMA 312:934–942
Pham S, Gancel A, Scotte M, Houivet E, Huet E, Lefebvre H, Khun JM, Prevost G (2014) Comparison of the effectiveness of four bariatric surgery procedures in obese patients with type 2 diabetes: a retrospective study. J Obes. doi:10.1155/2014/638203 Epub 2014 May
Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N (2010) Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass and adjustable gastric banding on type 2 diabetes. Surg Endosc 24:1005–1010
Erqou S, Lee CC, Adler AI (2014) Statins and glycaemic control in individuals with diabetes: a systematic review and meta-analysis. Diabetologia 57:2444–2452
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The study has been reviewed by the Ethic Committee of the University of Verona, and has been performed in accordance with the ethical standards laid down in an appropriate version of the 1964 Declaration of Helsinki.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Massimo Federici.
Rights and permissions
About this article
Cite this article
Zenti, M.G., Rubbo, I., Ceradini, G. et al. Clinical factors that predict remission of diabetes after different bariatric surgical procedures: interdisciplinary group of bariatric surgery of Verona (G.I.C.O.V.). Acta Diabetol 52, 937–942 (2015). https://doi.org/10.1007/s00592-015-0738-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0738-8