Abstract
Background
Ganglioneuromas are rare, benign, well-differentiated tumors arising from neural crest cells that commonly occur in the posterior mediastinum, retroperitoneum, cervical spine, and adrenal gland. We report an unusual case of an extensive spinal extradural ganglioneuroma, circumferentially and longitudinally affecting the extradural space of the lumbar spine and continuously invading bilateral psoas muscles.
Case description
A 32-year-old man presented with a 1-week history of abdominal pain and diarrhea. Radiographs revealed scalloping of the posterior surfaces of the L2 and L3 vertebral bodies and widening of L2–3 and L3–4 bilateral intervertebral foramina. Computed tomography scans and magnetic resonance imaging showed a well-defined lobulated extradural mass from L1 to L4 and a continuously forming mass in the psoas muscles through L2–3 bilateral neural foramina. The mass demonstrated homogeneously isointense signal on T1-weighted images, inhomogeneously hyperintense-to-isointense signal on T2-weighted images, and inhomogeneous enhancement on fat-suppressed contrast-enhanced T1-weighted images. Radiologic diagnosis included spinal epidural lymphoma. Percutaneous biopsy with sonographic guidance was performed, and the mass was diagnosed on pathological examination as a ganglioneuroma.
Conclusions
This is the first known reported case in the literature of a spinal extradural ganglioneuroma with circumferentially and longitudinally extensive involvement of the extradural space and a large psoas mass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ganglioneuromas are rare, slow-growing, benign tumors arising from neural crest cells. They are a subgroup of neuroblastic tumor with full differentiation—a mature form of neuroblastic tumors. They arise from ganglion cells of the sympathetic nervous system, extending from the skull base to the pelvis and adrenal medulla. Therefore, they are most commonly found in the posterior mediastinum, followed by the retroperitoneum, cervical spine, and adrenal gland [1,2,3,4]. Epicenters of ganglioneuromas of the sympathetic chain originate in the paraspinal space. Therefore, they usually present as paravertebral masses and may infrequently extend into the ipsilateral spinal canal through the neural foramina, forming dumbbell-shaped tumors [5, 6]. To the best of our knowledge, there has been no report of a circumferentially and longitudinally extensive spinal extradural ganglioneuroma with a continuously formed, large psoas mass, mimicking spinal epidural lymphoma. Here, we report an unusual case of a 32-year-old man with an extensive spinal extradural ganglioneuroma circumferentially and longitudinally affecting the extradural space of the lumbar spine and continuously invading bilateral psoas muscles, with adjacent bony erosions and widening of bilateral neural foramina due to chronic pressure remodeling by the mass.
Case report
A 32-year-old man was referred to our hospital after being incidentally found with a mass involving the lumbar extradural space and psoas muscle on contrast-enhanced abdomen–pelvis computed tomography (CT) scans for evaluation of a 1-week history of abdominal pain and diarrhea. He had no back pain or discomfort but a tingling sensation in the right buttock region. Physical examination revealed no tenderness or neurologic compromise, and the results of laboratory tests were within normal limits. The patient’s past medical, surgical, and family histories were unremarkable.
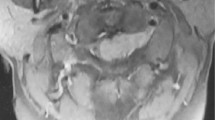
Frontal-view radiographs of the lumbar spine demonstrated that the outline of the psoas muscle showed a bulging contour on the right and a slightly bulging contour on the left. Lateral-view radiographs revealed a scalloping of the posterior surfaces of the L2 and L3 vertebral bodies and widening of L2–3 and L3–4 bilateral intervertebral foramina (Fig. 1). CT scans showed a low-attenuated soft tissue mass with inhomogeneously minimal enhancement circumferentially affecting the extradural space at the L2 to L3 level, with extension into L2–3 and L3–4 bilateral neural foramina and caudally to the posterior epidural space at the L4 level. Adjacent bony erosions of L2 and L3 vertebrae and widening of L2–3 and L3–4 bilateral neural foramina, due to chronic pressure remodeling by the mass were associated. The mass continuously extended into bilateral psoas muscles through widened L2–3 bilateral neural foramina, forming a large mass in the right psoas muscle and a small mass in the left psoas muscle (Fig. 2). Magnetic resonance imaging (MRI) demonstrated an approximately 11.1-cm longitudinally oriented, well-defined lobulated mass in the L1–L4 level extradural space, involving L2–3 and L3–4 bilateral neural foramina. The mass extended continuously into bilateral psoas muscles through L2–3 neural foramina and formed a well-defined lobulated mass. The mass in the psoas muscles was located at the L2–L5 level on the right and the L2–L3 level on the left; these measured approximately 2.8 × 5.1 × 11.8 and 1.2 × 2.0 × 3.7-cm, respectively. The extradural mass encased or indented the dural sac. However, there was no invasion into the intradural space or signal change of the spinal cord and cauda equina. The mass showed homogeneously isointense signal relative to the adjacent muscle on fast spine echo (FSE) T1-weighted images and inhomogeneously hyperintense-to-isointense signal on FSE T2-weighted images. The mass revealed inhomogeneous enhancement on fat-suppressed contrast-enhanced T1-weighted images. Superoanterior displacement of the bilateral exiting second lumbar nerve roots and inferoanterior displacement of the bilateral exiting third lumbar nerve roots due to the mass demonstrated without evidence of invasion of the nerves by the mass. Adjacent bony erosions and widening of bilateral neural foramina were revealed. However, there was no bone marrow signal change (Fig. 3). On the basis of the radiologic findings, the initial differential diagnosis of the mass included spinal epidural lymphoma.
Radiographs of the lumbar spine. On the frontal view (a), the outline of the psoas muscle shows a bulging contour (arrows) on the right and a slightly bulging contour on the left (arrowheads). The lateral view (b) reveals scalloping of the posterior surfaces of the L2 and L3 vertebral bodies (black arrowheads) and widening of L2–3 and L3–4 bilateral intervertebral foramina (long thin arrows)
Computed tomography of ganglioneuroma. On the post-contrast axial (a) and coronal (b) views, the mass (arrows), circumferentially affecting the extradural space and continuously forming in the psoas muscles through L2–3 bilateral neural foramina, demonstrates inhomogeneously minimal enhancement. c The axial view with the bone window setting reveals pressure bony erosions of the L2 vertebra (arrowheads)
Magnetic resonance imaging of ganglioneuroma. a A well-defined lobulated mass is observed in the extradural space. The mass (arrows) continuously extends into bilateral psoas muscles through L2–3 neural foramina. A fast spin echo (FSE) T1-weighted axial image (TR/TE 450/10 ms) of the mass shows homogeneously isointense signal. b An FSE T2-weighted axial image (TR/TE 6300/128 ms) shows that the mass (arrows) has inhomogeneously hyperintense-to-isointense signal. c An FSE fat-suppressed T2-weighted coronal image (TR/TE 3550/121 ms) of the mass, shows inhomogeneously hyperintense-to-isointense signal, involving L2–3 and L3–4 bilateral neural foramina (long thin arrows), and a continuously forming, a well-defined, large mass in the right psoas muscle (arrows). d The mass encases the dural sac. FSE T2-weighted axial imaging shows bony pressure erosions of the L2 vertebra (arrowheads) without bone marrow signal change. The mass (arrows) demonstrates inhomogeneous enhancement on fat-suppressed contrast-enhanced T1-weighted axial (e TR/TE 567/10 ms) and sagittal (f TR/TE 800/8 ms) imaging. The mass (arrows) is revealed in the posterior extradural space at the L1–L4 level (f arrows)
Percutaneous biopsy with sonographic guidance of the right psoas mass was performed without post-procedural complications. The mass showed as well-defined, elongated, and hypoechoic on ultrasonography. Grossly, it was an irregularly-shaped, gray-white soft tissue. Microscopically, clusters of variable-sized mature ganglion cells were deposited in a neuromatous stroma. The nuclei of ganglion cells exhibited mild-to-moderate atypia and bright-pink voluminous cytoplasm. Immunohistochemical staining for S-100 protein showed positive cytoplasmic staining in tumor cells. These findings were compatible with ganglioneuroma (Fig. 4).
Histological features of ganglioneuroma. a The tumor shows clusters of ganglion cells deposited in a neuromatous stroma, having variable-sized mature ganglion cells (black arrow). The nuclei of ganglion cells exhibit mild-to-moderate atypia and bright-pink voluminous cytoplasm (H&E staining; magnification ×200). b Immunohistochemical staining for S-100 protein shows positive cytoplasmic staining in tumor cells (magnification ×200)
The patient did not receive any surgical treatment because he experienced no significant symptoms. After 3 years, we performed contrast-enhanced abdomen–pelvis CT for evaluation of abdominal pain and a feeling of fullness for several months. The size, character, and extent of the mass had not changed.
Discussion
Ganglioneuromas, ganglioneuroblastomas, and neuroblastomas are subgroups of peripheral neuroblastic tumors arising from cells of the neural crest. These subgroups are classified according to cellular and extracellular differentiation [5, 7, 8]. Ganglioneuromas are a mature form of neuroblastic tumors characterized by rare, slow-growing, benign tumors [5]. They frequently occur before the age of 20 and rarely after the age of 60 [7]. Moreover, both a slight female predominance and no gender difference have been reported [7, 9]. Ganglioneuromas can occur in the sympathetic nervous system from the skull base to the pelvis, but are most commonly found in the paraspinal sympathetic chain ganglia [5]. Therefore, the most common sites are the posterior mediastinum, retroperitoneum, adrenal gland, and neck [8]. Unusual locations such as the spermatic cord, heart, bone, and intestine have also been reported [8].
Ganglioneuromas developing within the spinal canal constitute < 10% of all ganglioneuromas. Most cases involve the paraspinal region with intraspinal extension extradurally through the neural foramen, forming dumbbell-shaped tumors [10,11,12]. Rarely, intramedullary or intradural extramedullary ganglioneuromas have been reported [13]. A few cases of ganglioneuromas occurring in the nerve root have also been reported [5, 12]. Our patient had an uncommon, extensive extradural ganglioneuroma in the lumbar spine that circumferentially and longitudinally affected the extradural space, and continuously extended into the bilateral psoas muscles through neural foramina. To the best of our knowledge, prior to our case, there have only been two case reports of huge extradural ganglioneuromas with scalloping of the posterior surface of bodies of upper lumbar vertebrae and widening of intervertebral foramina, in the literature [10, 11]. One of these was a dumbbell-shaped extradural tumor at the T11–L4 level, with extension through the intervertebral foramina on both sides. There was no psoas mass formation. The mass revealed substantial enhancement on post-contrast MR imaging. However, neither a CT scan nor pre-contrast T1- and T2-weighted images were taken [10]. The other case, unlike ours, was a paraspinal ganglioneuroma with extension into the spinal extradural space, which presented with a dumbbell shape and bony indentation [11]. Spinal extradural ganglioneuromas may originate from dorsal root ganglions or neural crest remnants [6, 14, 15]; therefore, we thought that our tumor had probably originated from a neural crest remnant in the extradural space based on its circumferential and longitudinal extension into the extradural space.
The radiologic findings for spinal ganglioneuromas indicate bony erosion, scalloping of the posterior vertebral body, widening of the spinal canal or neural foramen, and scoliosis due to spinal deformity, according to a tumor’s size and location. On CT scans, they appear as homogeneous, well-circumscribed, low or intermediate attenuated masses with heterogeneous enhancement after injection of intravenous contrast [3]. Discrete punctate calcifications are demonstrated in 42–60% of ganglioneuromas [7, 8].
Typical MRI findings for ganglioneuromas reveal homogeneously low signal intensity on T1-weighted images, heterogeneously high signal intensity on T2-weighted images, and heterogeneous enhancement on contrast-enhanced T1-weighted images, with gradually increasing enhancement on dynamic imaging [16]. According to Zhang et al. [17], tumor signal intensity on T2-weighted images depends on the proportion of myxoid stroma to cellular components and collagen fibers, and tumors are classified as having either markedly high or intermediate-to-high signal intensity. Intermediate-to-high signal intensity on T2-weighted images, as in our case, is caused by an abundance of cellular components and collagen fibers with relatively scarce myxoid stroma in the tumor [17]. Ganglioneuromas display different degrees of contrast enhancement: no enhancement, or inhomogeneous moderate or marked enhancement. These different levels of enhancement are due to variations in myxoid stroma volume and amount of cellular components and collagen fibers [17]. On dynamic MR images, enhancement patterns are determined by the tissue vascularity and capillary permeability. Early enhancement is seen with increased capillary permeability in tumors with hypervascularity. Abundant myxoid stroma and fibrous capsules result in late enhancement [7].
In our case, the differential diagnosis could have included spinal epidural lymphoma and neurogenic tumors such as schwannomas or neurofibromas. However, the most likely differential diagnosis was spinal epidural lymphoma, because of the tumor’s circumferentially and longitudinally extensive extradural space involvement associated with bony pressure erosions of adjacent bones, encasement of the thecal sac without invasion into the intradural space, and displacement of the exiting lumbar nerve roots without invasion of the nerves. Within the spinal canal, spinal epidural lymphoma is usually located dorsally rather than ventrally [18]. Its mean longitudinal extension is 2.6 vertebral segments [19]. MRI findings of spinal epidural lymphoma demonstrate homogeneously isointense signal on T1-weighted images and isointense-to-hyperintense signal on T2-weighted images, with homogeneous contrast enhancement [19, 20]. Moreover, this lymphoma can induce vertebral body changes due to pressure remodeling by the mass and spinal cord compression due to an epidural mass, and can extend into the paraspinal space through the neural foramen [20, 21]. Therefore, in our case, it was initially difficult to consider the possibility of ganglioneuroma or neurogenic tumor. Other differential diagnoses of spinal extradural tumors include metastasis, meningioma, hemangioma, malignant peripheral nerve sheath tumors, Ewing’s sarcoma, epidural abscess, epidural hematoma, tuberculosis, parasitosis, and herniated disk [19].
Total surgical excision remains the treatment of choice in symptomatic patients. The long-term prognosis is excellent regardless of tumor location when total tumor excision is achieved. Adjuvant chemotherapy and radiotherapy play limited therapeutic roles because of ganglioneuromas’ benign biological nature [7, 13]. In our case, the patient did not receive any treatment because he had no significant symptoms. After 3 years, the size, character, and extent of the mass had not changed, and contrast-enhanced abdomen–pelvis CT revealed no evidence of tumor progression.
In conclusion, although a ganglioneuroma is usually located in the paraspinal region, it sometimes exhibits intraspinal extension extradurally through the ipsilateral neural foramen. However, it may manifest as an extensive extradural mass circumferentially and longitudinally affecting the extradural space with bone erosions, as in this case. Thus, ganglioneuroma should be included in the differential diagnosis of a benign spinal extradural mass.
References
Bhand AA (2005) Ganglioneuroma of the cervical spine. J Coll Phys Surg Pak 15(2):114–116
Marchevsky AM (1999) Mediastinal tumors of peripheral nervous system origin. Semin Diagn Pathol 16(1):65–78
Radin R, David CL, Goldfarb H, Francis IR (1997) Adrenal and extra-adrenal retroperitoneal ganglioneuroma: imaging findings in 13 adults. Radiology 202(3):703–707
Cerullo G, Marrelli D, Rampone B, Miracco C, Caruso S, Di Martino M, Mazzei MA, Roviello F (2007) Presacral ganglioneuroma: a case report and review of literature. World J Gastroenterol 13(14):2129–2131
Kyoshima K, Sakai K, Kanaji M, Oikawa S, Kobayashi S, Sato A, Nakayama J (2004) Symmetric dumbbell ganglioneuromas of bilateral C2 and C3 roots with intradural extension associated with von Recklinghausen’s disease: case report. Surg Neurol 61(5):468–473
Ugarriza LF, Cabezudo JM, Ramirez JM, Lorenzana LM, Porras LF (2001) Bilateral and symmetric C1–C2 dumbbell ganglioneuromas producing severe spinal cord compression. Surg Neurol 55(4):228–231
Mounasamy V, Thacker MM, Humble S, Azouz ME, Pitcher JD, Scully SP, Temple HT, Eismont F (2006) Ganglioneuromas of the sacrum—a report of two cases with radiologic–pathologic correlation. Skelet Radiol 35(2):117–121
Lonergan GJ, Schwab CM, Suarez ES, Carlson CL (2002) Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic–pathologic correlation. Radiographics 22(4):911–934
Geoerger B, Hero B, Harms D, Grebe J, Scheidhauer K, Berthold F (2001) Metabolic activity and clinical features of primary ganglioneuromas. Cancer 91(10):1905–1913
Sun WS, Jung YT, Kim SC, Sim JH (2002) A dumbbell-shaped thoraco-lumbar extradural ganglioneuroma: case report. J Korean Neurosurg Soc 32:481–484
Tsai FJ, Kuo KL, Tzou RD, Cheng YH, Hwang SL, Lieu AS (2014) A huge extradural ganglioneuroma of the lumbar spine. Formos J Surg 47(4):160–165
Shephard RH, Sutton D (1958) Dumb-bell ganglioneuromata of the spine with a report of four cases. Br J Surg 45(192):305–317
Pang BC, Tchoyoson Lim CC, Tan KK (2005) Giant spinal ganglioneuroma. J Clin Neurosci 12(8):967–972
Suetake K, Niwa J, Okuyama T, Hirai H, Shimoyama N, Ishidate T (1993) Ganglioneuroma in the cervical ganglion with neurofibromatosis-2: a case report. No Shinkei Geka 21(7):629–632
Maggi G, Dorato P, Trischitta V, Varone A, Civetta F (1995) Cervical dumbbell ganglioneuroma in an eighteen month old child. A case report. J Neurosurg Sci 39(4):257–260
Ichikawa T, Ohtomo K, Araki T, Fujimoto H, Nemoto K, Nanbu A, Onoue M, Aoki K (1996) Ganglioneuroma: computed tomography and magnetic resonance features. Br J Radiol 69(818):114–121
Zhang Y, Nishimura H, Kato S, Fujimoto K, Ohkuma K, Kojima K, Uchida M, Hayabuchi N (2001) MRI of ganglioneuroma: histologic correlation study. J Comput Assist Tomogr 25(4):617–623
Boukobza M, Mazel C, Touboul E (1996) Primary vertebral and spinal epidural non-Hodgkin’s lymphoma with spinal cord compression. Neuroradiology 38(4):333–337
Mascalchi M, Torselli P, Falaschi F, Dal Pozzo G (1995) MRI of spinal epidural lymphoma. Neuroradiology 37(4):303–307
Cugati G, Singh M, Pande A, Ramamurthi R, Balasubramanyam M, Sethi SK, Singh AK (2011) Primary spinal epidural lymphomas. J Craniovertebr Junction Spine 2(1):3–11
Jeong M, Lee S, Joo KB, Jang K-S, Bae J (2013) Ganglioneuroma of lumbar nerve root: a case report. J Korean Soc Radiol 68(2):153–156
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kang, S.H., Lee, S.M., Ha, D.H. et al. Extensive spinal extradural ganglioneuroma of the lumbar spine: mimicking lymphoma. Eur Spine J 27 (Suppl 3), 520–525 (2018). https://doi.org/10.1007/s00586-018-5568-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-018-5568-2