Abstract
Purpose
To compare the clinical outcomes, radiographic results and fusion rate of ACDF between empty PEEK cages and PEEK cages packed with β-tricalcium phosphate.
Methods
Forty-five patients were prospectively enrolled with cervical degenerative disc disease who requiring ACDF with a PEEK cage. 23 patients were randomised to the study group (empty cages) and 22 patients were in the control group (cages filled with β-tricalcium phosphate). Both patient groups were fixed with a cervical locking plate. A CT scan was performed 12 months postoperatively and 24 months if not confirmed fused at 12 months to evaluate the status of fusion. Clinical status was evaluated using the Japanese Orthopaedic Association (JOA) score, the Oswestry Disability Index (ODI) and the Visual Analogue Scale (VAS).
Results
46 levels (97.88%) in the study group and 44 levels (97.77%) in the control group were confirmed as fused at 24 months. There was no significant difference between the fusion rates observed in the study and control groups (p = 0.82). There was no significant difference in JOA, ODI, or VAS scores at 24 months follow-up. The results showed that the members of the non-fusion group tended to be older than the individuals in the fusion group at 12 months, but was not significant in statistics.
Conclusions
Similar fusion rates and clinical outcomes were achieved when using ACDF with PEEK cages and instrumentation, regardless of whether the cage was filled with bone substitute at 24 months follow-up. Fusion rates improved over time and are comparable between both groups.
Graphical abstract
These slides can be retrieved under Electronic Supplementary material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cervical discectomy and fusion (ACDF) has been widely used in the treatment of cervical spinal disorders since the 1950s [1]. Various materials have been used in fusion such as autograft, allograft, and artificial materials. Fusion rates with these materials have been reported with satisfactory results [1,2,3,4,5,6]. Interbody fusion using cages filled with different materials, such as surgical-site bone, autograft, calcium sulfate, biphasic calcium phosphate, and β-tricalcium phosphate (β-TCP), and bone morphogenic protein (BMP) has been thoroughly investigated with satisfactory results [4, 5, 7,8,9]. Whereas autograft can involve issues related to donor site mobility, the use of a surgical-site bone graft can involve problems related to an insufficient supply. The excessive cost of using additional filling materials, such as calcium phosphate and BMP, is prohibitive.
Many different types of cages have been explored, ranging from the titanium cage and polymethylmethacrylate (PMMA) cage used previously until the recent development of bioabsorbable cages, carbon-fibre cages, polyetheretherketone (PEEK) cages, and silicon nitride spacer, etc [3, 10,11,12,13,14,15,16]. The advantages of the PEEK cage include increased radiolucency, biocompatibility and decreased stiffness [17].
In our experience, a bridging callus is found not only within the cage, but also anterior or posterior to the cage in patients with ACDF. Similar observations have been made by other authors [12]. It is unknown whether the biomaterial packed into the cage plays an important role or whether fusion occurs spontaneously even in the absence of any packing materials. The fusion rate achieved when using an empty PEEK cage in ACDF remains unclear. The purpose of this study is to compare the clinical outcomes, radiographic results and fusion rate of ACDF between empty PEEK cages and PEEK cages packed with β-tricalcium phosphate alone.
Methods
This study included patients with cervical degenerative disc disease causing myelopathy or radiculopathy requiring anterior cervical discectomy and fusion with a PEEK cage. Patients requiring posterior cervical surgery, anterior cervical corpectomy, or revision surgery were excluded. Patients who were chronic smokers or steroid users were also excluded.
Patients were numbered consecutively in the order they were admitted to our hospital and randomised to either the study group or the control group using computer-generated random numbers.
Patients in the study group underwent fusion with an empty PEEK cage, and patients in the control group underwent fusion with a PEEK cage packed with β-tricalcium phosphate alone. Prior to randomisation, the patients were informed about the details of the surgical procedures involved in the two different groups. Randomisation was performed using a statistical program. The study was approved by the Institutional Review Board.
Surgical procedure
Anterior cervical discectomy was performed via a left-sided anterior approach, as described by Smith and Robinson [1]. The cervical disc was excised completely, and spurs were removed using a Kerrison rongeur and curettes. The posterior longitudinal ligament was removed to the greatest extent possible. The subchondral cartilage was curetted to expose the bony endplate prior to implantation of the PEEK cage (Fidji cervical cage, Zimmer spine, Bordeaux, France). In the study group, each individual was implanted with an empty PEEK cage. In the control group, the PEEK cage was packed with β-tricalcium phosphate (ChronOS, Synthes, USA) alone without bone graft (Fig. 1). Both patient groups were fixed with an appropriately sized cervical locking plate (CSLP, Synthes, USA) to stabilise the excision at all levels. Postoperative care was the same in both groups. A semirigid neck collar was used for 2 months, and nonsteroidal anti-inflammatory drugs (NSAID) were avoided for 3 months.
Radiologic evaluation
Plain radiographs of the cervical spine (anterior–posterior view, lateral view, flexion–extension view) were taken at 1, 3, 6, 12, and 24 months postoperatively. A computerised tomography (CT) scan was taken 12 months after the operation to evaluate the status of fusion. If fusion is not confirmed at 12 months, another CT scan will be arranged at 24 months postoperatively. Fusion status was assessed in the window at a setting of 420/40, 120 kV, 60–200 mA (Toshiba, Aquilion, Tokyo, Japan) to optimise the trabecular bone detail.
The fusion was defined as follows: (1) rotation < 4° and < 1.25 mm translation [16] with the absence of motion adjacent to interspinous processes (> 3 mm) in the flexion–extension view [18] and (2) the presence of continuous trabecular bone bridging was revealed by CT scan in at least one of the following locations: anterior, within, or posterior to the PEEK cage. A radiologist and a senior spine surgeon evaluated the fusion status independently without any preconceptions regarding patients’ clinical outcomes. A fused status was recorded only when both reviewers agree.
Outcome assessment
Clinical status was evaluated using the Japanese Orthopaedic Association (JOA) score, the Oswestry Disability Index (ODI) and the Visual Analogue Scale (VAS). Each patient’s clinical status was evaluated preoperatively, 1 month postoperatively, 12 months and 24 months postoperatively. Evaluations were performed by an independent researcher who was not aware of whether the patient was assigned to the study group or the control group.
Statistical analysis
Patient demographics and fusion rates were analysed using Fisher’s exact test. Clinical outcomes were analysed using a nonparametric Mann–Whitney test. Statistical significance was defined as p < 0.05. Interobserver reliability was evaluated using kappa coefficients (strength of agreement defined as < 0 poor, 0.01–0.2 slight, 0.21–0.4 fair, 0.41–0.6 moderate, 0.61–0.8 substantial, and 0.81–1 almost perfect).
Results
From May 2010 to March 2011, a total of 45 patients fulfilling the inclusion criteria underwent anterior cervical discectomy and fusion with a PEEK cage. In total, 23 patients (10 male, 13 female) were assigned to the study group, and 22 patients (16 male, 6 female) were assigned to the control group. In the study group, the mean age was 64.3 years (range 33–88 years). One-level ACDF was performed in five patients, whereas two-level ACDF was performed in 12 patients, and three-level ACDF was performed in six patients. A total of 47 levels were treated, primarily at C4/5 and C5/6. Among these patients, 12 had radiculopathy, five had myelopathy, and six had radiculomyelopathy. In the control group, the mean age was 57.8 years (range 27–84 years). One-level ACDF was performed in four patients, two-level ACDF was performed in 13 patients, and three-level ACDF was performed in five patients. A total of 45 levels were treated, primarily at C4/5 and C5/6. Of these patients, eight had radiculopathy, seven had myelopathy, and seven had radiculomyelopathy (Table 1).
Radiographic evaluation
At postoperative 12 months follow-up, 39 levels (82.98%) in the study group and 37 levels (82.22%) in the control group were confirmed as fused. Eight levels (17.02%) in eight patients in the study group and eight levels (17.78%) in seven patients in the control group were not fused.
At postoperative 24 months follow-up, 46 levels (97.88%) in the study group and 44 levels (97.77%) in the control group were confirmed as fused (Table 2). There was one level in each group considered not fused.
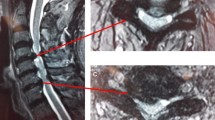
No subsidence, collapse, extrusion or other cage-related complications were observed in either group. Trabecular bony bridging could be observed anteriorly, within and posteriorly to the cages. There was no significant difference between the fusion rates observed in the study and control groups at postoperative 1 year (p = 1.00) or 2 years (p = 1.00) (Table 2).
Clinical outcomes
Patients in both groups showed improvements in VAS, JOA and ODI scores during the 24-month follow-up. Patients in the control group had better improvement at both 12 and 24 months, although there was no significant difference. All outcome measures are illustrated in Table 2.
We sub-divided the patients into a fusion group and a non-fusion group at 12 months follow-up. If the patient exhibited at least one level without fusion, then the patient was placed in the non-fusion group. The clinical outcomes were analysed accordingly. The results show that the members of the non-fusion group at 12 months tended to be older than the individuals in the fusion group, but this trend was not significant in statistics. No significant difference was observed for any other clinical outcome (Table 3).
Discussion
Previous studies have shown that the fusion rate for cervical spine ACDF using strut autografts ranges from 56 to 100% with an average of 77% [15, 19, 20]. The wide range of fusion rates observed for ACDF when performed with an autograft may be related to the investigation of multiple levels and whether a plate was used. Some studies have shown that union rate was higher if plating was used at two or three levels [19, 20]. The fusion rate ranged from 39 to 100% when an artificial spacer was used, regardless of the material (e.g., hydroxyapatite, PEEK, titanium or PMMA) [15]. Recent studies showed ACDF performed with titanium cages and PEEK cages could achieve a fusion rate ranging from 87 to 100% [4,5,6, 12, 21,22,23,24,25,26,27,28]. This wide range of fusion rates achieved with artificial materials might be due to the different evaluation methods used by various authors and the mode of assessment.
It remains unclear whether packing filling materials into the cage can affect the fusion rate. We were able to find some studies that discussed the fusion rate after the implantation of an empty cage [12, 25, 29]. Pechlivanis reported 52 patients with 60 levels affected who had undergone ACDF using an empty PEEK cage (AMT, Nonnweiler, Germany). Bony fusion was present at 43 levels (71.7%). In the study, another 29 patients with ACDF treated with various types of empty PEEK cages (Pina, Signus, Germany) were retrospectively evaluated. Fusion was present at 30 levels (71.4%). Statistical analysis revealed no significant difference between the two groups that were treated with different types of empty PEEK cages. Zevgaridis compared titanium cages containing iliac crest autografts with empty titanium cages, and the results showed that the fusion rates of treated levels were 91 and 87%, respectively. There was no significant difference between the two groups (p = 1.00). Shiban et al. also reported very good fusion rate with stand-alone empty peek cage in one- and two-level ACDF in his studies with a minimum follow-up of 12 months [29]. In his study, fusion was achieved in 85, 95, and 94% of segments in one-, two-, and three-level surgeries, respectively.
The present study is a prospective randomised controlled study comparing individuals implanted with empty PEEK cages or PEEK cages filled with β-TCP alone. β-tricalcium phosphate is the only bone substitute could be used in our hospital and is covered by the national health insurance system. The results showed a fusion rate of 82.98% in the study group vs. 82.22% in the control group at 12 months, and 97.88% in the study group vs. 97.77% in the control group at 24 months. The relatively lower fusion rate at 1 year may have several explanations. First, our patients typically exhibited lesions at multiple levels (79.3% in the study group and 81.8% in the control group). Second, we used a CT scan as the primary method of assessment, whereas most studies used X-rays as the mode of assessment. Notably, the use of a CT scan to assess cervical fusion seems to be the most accurate approach with the best interobserver reliability [18].
The cervical spine has very good fusion potential. Even with no fusion technique performed after discectomy, fusion rate of 64–70% was achieved [30, 31]. A bridging callus formed not only within, but also around the cage. We assume that this is because in order to perform adequate nerve root decompression, part of the joint of Luschka was removed, similarly to the decortications necessary for the fusion procedure. Decortications of the joint of Luschka and the posterior margin of the vertebral body may have been the main cause of the high fusion rate in ACDF. Even the high occurrence rate of heterotopic ossification or spontaneous fusion after cervical artificial disc arthroplasty may be due to the same reason [32].
Cho et al. compared ACDF using PEEK cages containing iliac autografts (66 treated levels) with autogenous iliac crest autografts (58 treated levels). The fusion rates of the two groups were not significantly different (100 vs. 93.1%, p = 0.18) [26]. In another study, Cho et al. compared the results of using a PEEK cage containing a biphasic calcium ceramic with the use of a PEEK cage containing autogenous iliac bone graft. For both, the 6-month fusion rate was 100% [5]. These two studies demonstrate that the cage-filling materials may not influence the fusion rates of the ACDF. As mentioned before, Zevgaridis’s study showed that the fusion rate was similar for titanium cages containing iliac crest autograft and empty titanium cages. These studies suggest that the fusion potential of the cervical spine is higher than we had thought and that the wide range of fusion rates observed for different ACDF techniques may be due to various authors’ evaluation methods and modes of assessment.
Previous studies have shown that plating has no effect on single-level lesion [33]. However, instrumentation appears to be helpful for ACDF involving two or more levels [19, 20]. To achieve consistent external environments for every level, our study used plate immobilisation for even single-level ACDF. Our study shows high fusion rates at 24 months with no significant difference between the two groups (Figs. 2, 3). This might also indicate that the presence or absence of fusion materials is not as important as stability. However, further comparative studies on autologous bone grafting, which remains the gold standard for fusion, are needed in the future studies due to the relative low fusion rate in this study.
Another factor affecting fusion could be the duration of the follow-up interval. Many studies show that longer follow-up periods were associated with higher rates of fusion [5, 10, 13]. Theoretically, the fusion rate of the present study may have been higher if follow-up had continued for more than 1 year. Thus, larger comparative studies with longer follow-up are needed to assess these results.
The present study showed that similar fusion rates and clinical outcomes were achieved when using ACDF with PEEK cages and instrumentation, regardless of whether the cage was filled with bone substitute. However, the study also demonstrated that the patients with solid fusion or non-union had similar functional results, which was similar to the results reported by previous studies [25, 30, 31].
References
Smith GW, Robinson RA (1958) The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J B Jt Surg Am 40-A(3):607–624
Yue WM, Brodner W, Highland TR (2005) Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year radiologic and clinical follow-up study. Spine (Phila Pa 1976) 30(19):2138–2144
Majd ME, Vadhva M, Holt RT (1999) Anterior cervical reconstruction using titanium cages with anterior plating. Spine (Phila Pa 1976) 24(15):1604–1610
Boakye M, Mummaneni PV, Garrett M, Rodts G, Haid R (2005) Anterior cervical discectomy and fusion involving a polyetheretherketone spacer and bone morphogenetic protein. J Neurosurg Spine 2(5):521–525
Cho DY, Lee WY, Sheu PC, Chen CC (2005) Cage containing a biphasic calcium phosphate ceramic (Triosite) for the treatment of cervical spondylosis. Surg Neurol 63(6):497–503 (discussion-4)
Chiang CJ, Kuo YJ, Chiang YF, Rau G, Tsuang YH (2008) Anterior cervical fusion using a polyetheretherketone cage containing a bovine xenograft: 3 to 5-year follow-up. Spine (Phila Pa 1976) 33(23):2524–2528
Tumialan LM, Pan J, Rodts GE, Mummaneni PV (2008) The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenetic protein-2: a review of 200 patients. J Neurosurg Spine 8(6):529–535
Hwang SL, Lin CL, Lieu AS et al (2004) Three-level and four-level anterior cervical discectomies and titanium cage-augmented fusion with and without plate fixation. J Neurosurg Spine 1(2):160–167
Dai LY, Jiang LS (2008) Anterior cervical fusion with interbody cage containing beta-tricalcium phosphate augmented with plate fixation: a prospective randomized study with 2-year follow-up. Eur Spine J 17(5):698–705
Chen JF, Wu CT, Lee SC, Lee ST (2005) Use of a polymethylmethacrylate cervical cage in the treatment of single-level cervical disc disease. J Neurosurg Spine 3(1):24–28
Barlocher CB, Barth A, Krauss JK, Binggeli R, Seiler RW (2002) Comparative evaluation of microdiscectomy only, autograft fusion, polymethylmethacrylate interposition, and threaded titanium cage fusion for treatment of single-level cervical disc disease: a prospective randomized study in 125 patients. Neurosurg Focus 12(1):E4
Zevgaridis D, Thome C, Krauss JK (2002) Prospective controlled study of rectangular titanium cage fusion compared with iliac crest autograft fusion in anterior cervical discectomy. Neurosurg Focus 12(1):E2
Agrillo U, Mastronardi L, Puzzilli F (2002) Anterior cervical fusion with carbon fiber cage containing coralline hydroxyapatite: preliminary observations in 45 consecutive cases of soft-disc herniation. J Neurosurg 96(3 Suppl):273–276
Vavruch L, Hedlund R, Javid D, Leszniewski W, Shalabi A (2002) A prospective randomized comparison between the Cloward procedure and a carbon fiber cage in the cervical spine: a clinical and radiologic study. Spine (Phila Pa 1976) 27(16):1694–1701
Wigfield CC, Nelson RJ (2001) Nonautologous interbody fusion materials in cervical spine surgery: how strong is the evidence to justify their use? Spine (Phila Pa 1976) 26(6):687–694
Arts MP, Wolfs JF, Corbin TP (2017) Porous silicon nitride spacers versus PEEK cages for anterior cervical discectomy and fusion: clinical and radiological results of a single-blinded randomized controlled trial. Eur Spine J 26:2372–2379
Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS (2006) Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 27(3):324–334
Buchowski JM, Liu G, Bunmaprasert T, Rose PS, Riew KD (2008) Anterior cervical fusion assessment: surgical exploration versus radiographic evaluation. Spine (Phila Pa 1976) 33(11):1185–1191
Wang JC, McDonough PW, Endow KK, Delamarter RB (2000) Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 25(1):41–45
Wang JC, McDonough PW, Kanim LE, Endow KK, Delamarter RB (2001) Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 26(6):643–646 (discussion 6–7)
Moreland DB, Asch HL, Clabeaux DE et al (2004) Anterior cervical discectomy and fusion with implantable titanium cage: initial impressions, patient outcomes and comparison to fusion with allograft. Spine J 4(2):184–191 (discussion 91)
Hwang SL, Hwang YF, Lieu AS et al (2005) Outcome analyses of interbody titanium cage fusion used in the anterior discectomy for cervical degenerative disc disease. J Spinal Disord Tech 18(4):326–331
Schmieder K, Wolzik-Grossmann M, Pechlivanis I, Engelhardt M, Scholz M, Harders A (2006) Subsidence of the wing titanium cage after anterior cervical interbody fusion: 2-year follow-up study. J Neurosurg Spine 4(6):447–453
Caroli E, Orlando ER, D’Andrea G, Ferrante L (2007) Anterior cervical fusion with interbody titanium cage containing surgical bone site graft: our institution’s experience in 103 consecutive cases of degenerative spondylosis. J Spinal Disord Tech 20(3):216–220
Pechlivanis I, Thuring T, Brenke C et al (2011) Non-fusion rates in anterior cervical discectomy and implantation of empty polyetheretherketone cages. SpineSpine (Phila Pa 1976) 36(1):15–20
Cho DY, Liau WR, Lee WY, Liu JT, Chiu CL, Sheu PC (2002) Preliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease. Neurosurgery 51(6):1343–1349 (discussion 9–50)
Shad A et al (2005) Use of the Solis cage and local autologous bone graft for anterior cervical discectomy and fusion: early technical experience. J Neurosurg Spine 2(2):116–122
Kulkarni AG, Hee HT, Wong HK (2007) Solis cage (PEEK) for anterior cervical fusion: preliminary radiological results with emphasis on fusion and subsidence. Spine J 7(2):205–209
Shiban E, Gapon K, Wostrack M et al (2016) Clinical and radiological outcome after anterior cervical discectomy and fusion with stand-alone empty polyetheretherketone (PEEK) cages. Acta Neurochir (Wien) 158(2):349–355
Abd-Alrahman N, Dokmak AS, Abou-Madawi A (1999) Anterior cervical discectomy (ACD) versus anterior cervical fusion (ACF), clinical and radiological outcome study. Acta Neurochir (Wien) 141(10):1089–1092
Dowd GC, Wirth FP (1999) Anterior cervical discectomy: is fusion necessary? J Neurosurg 90(1 Suppl):8–12
Mehren C, Suchomel P, Grochulla F et al (2006) Heterotopic ossification in total cervical artificial disc replacement. Spine (Phila Pa 1976) 31(24):2802–2806
Samartzis D, Shen FH, Lyon C, Phillips M, Goldberg EJ, An HS (2004) Does rigid instrumentation increase the fusion rate in one-level anterior cervical discectomy and fusion? Spine J 4(6):636–643
Acknowledgements
The study was supported by Division of Spine Surgery, Orthopaedic Department, Taipei Veterans General Hospital. We thank Hsin-Yi Huang from the Biostatistics Task Force, Taipei Veterans General Hospital, for the statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, SW., Chang, MC., Chou, PH. et al. Implantation of an empty polyetheretherketone cage in anterior cervical discectomy and fusion: a prospective randomised controlled study with 2 years follow-up. Eur Spine J 27, 1358–1364 (2018). https://doi.org/10.1007/s00586-017-5450-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-017-5450-7