Abstract
Purpose
An incidental durotomy is a common complication of spinal surgery. Its treatment remains challenging, especially in endoscopic procedures. The objective of this study is to describe a technique for endoscopic dural closure which is safe and effective.
Methods
From a prospective database all endoscopic spinal procedures with incidental durotomy were identified. Retrospectively, video recordings were analysed with a special reference to the applied technique of dural closure. Additionally 1, 6 and 12 week follow-up examinations were evaluated for clinical outcome and associated complications.
Results
Out of 212 consecutive patients, an intraoperative dural tear was observed in nine patients (4.2%). A dural tear occurred in 1.1% of cases of lumbar disc herniation, in 7.9% of cases with lumbar spinal stenosis, in 37.5% of cases with a synovial cyst. An autologous muscle sample was harvested within the operative field and grafted at the dural defect in several layers. Fixation of the transplantation and watertight closure were achieved by the application of fibrin sealant with gelfoam. The mean time for dural closure was 209 s (range 47–420 s). Postoperatively no CSF fistula, no new deficits nor worsening of a pre-existing neurological deficit occurred. None of the patients had problems with wound healing, or discomfort which could be related to the CSF leak.
Conclusions
Dural closure with an autologous muscle graft in combination with fibrin sealant patch is a fast, safe and alternative technique for the management of dural tear in microendoscopic surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoscopic spinal surgery is an accepted technique for the treatment of degenerative spinal disorders [1].
An incidental dural tear is a common complication in spinal surgery. It’s incidence is affected by several factors such as patient’s age, diagnosis, surgeon’s experience, presence of synovial cyst and type of surgical procedure. It may range from less than 1 up to 17%. A non-treated dural tear is a complication which might cause a variety of symptoms such as postural headache, nausea, vomiting and/or pseudomeningocele formation. More serious complications such as meningitis, intracranial haemorrhage, nerve root herniation with consecutive radicular symptoms, persistent leakage of cerebrospinal fluid (CSF) with delayed wound healing and wound infection have also been described [2].
Several reports on perioperative management and different surgical techniques for the treatment of dural tears have been published [3, 4]. In contrast there are very few reports on the management of dural tears in the field of minimally invasive—and especially endoscopic—spine surgery [5,6,7]. The incidence of a dural tear in endoscopic techniques is low [8,9,10]. In case of a dural tear, the standard technique of suturing the tear is almost always impossible under endoscopic view because access to the dural tear and handling of the surgical instruments are limited. However, suturing the dura through a tubular retractor is possible if special instruments are available and if the surgeon is used to them [6, 11, 12]. If dural repair by suture is not possible, the surgeon might be forced to terminate the endoscopic or tubular-assisted procedure and change to open surgery [7]. Recently, it has been reported that 79.4% spine surgeons perform dural repair without direct suturing using fibrin sealant patch or another products in conventional spine surgery [13]. Even though placing a patch on the dural surface has been used for decades it has never been assessed if this technique ensures clinical success when applied via endoscopic tube systems followed by early postoperative patient mobilization.
To the best of our knowledge, this is the first detailed report about endoscopic dural tears and a technique for endoscopic dural closure with an autologous muscle graft and fibrin sealant patch that demonstrated the effectiveness of this technique.
Materials and methods
All patients who underwent endoscopic spinal surgery from January 2011 to August 2016 were identified from a prospectively collected database. All procedures were performed with the EasyGO® System (Karl Storz Company, Tuttlingen, Germany). Under endoscopic visualization, cervical, thoracic and lumbar spine procedures have been performed in standard bimanual microsurgical technique. Patient demographics, preoperative and postoperative patient records, and surgical records including the intraoperative video documentation were reviewed. Special focus was given to intraoperative complications and postoperative course. The surgical technique of posterior cervical foraminotomy and lumbar spine surgery has been described in detail elsewhere [14, 15].
Results
Patient collective
Over a 5 year and 8 month period, 212 consecutive patients underwent endoscopic cervical, thoracic and lumbar spine surgery. In nine patients (four men and five women) with a mean age of 57 years (range 39–74) a dural tear occurred (4.2%).
In all cases with incidental dural tear a unilateral approach to the lumbar spine was performed. None of the patients had a history of previous spinal surgery.
Two patients were operated via a 15 mm and seven patients via a 19 mm tubular retractor. The mean surgical time was 77 min (range 50–115 min) and the mean time per segment was 60 min (range 29–85 min). The mean time for dural closure was 209 s (range 47–420 s). A compilation of patient demographics, of the performed procedure, of the localization of the dural tear, of the underlying pathology and of the time for dural closure is shown on Table 1.
Dural tears in detail
In lumbar spinal canal stenosis the incidence of dural tear was 7.9% (5 cases). In two cases additionally to the posterior spinal canal stenosis a disc prolapse was present.
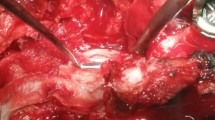
In one case of stenosis with a disc herniation, the tear occurred mediodorsal while using a diamond drill to thin out the base of the spinous process for contralateral decompression. The dural tear was located medial under the base of the spinous process. The contralateral decompression was continued around the dural tear with a Kerrison punch (see Fig. 1). After decompression, dural closure was done as described below.
In the other cases of stenosis with disc herniation, the dural tear occurred using a Kerrison punch for decompression of the shoulder of the nerve root. A subligamentous disc herniation misplaced the dural sac and the nerve root laterally. The decompression was performed for release of the nerve root from the lamina. The dural sac and the nerve root were adherent to the lamina. Once a dural tear occurred, the author used a suction or a dissector to cover and retract the dural tear and decompression was carried out furthermore.
Three dural tears occurred while decompressing the lateral aspect of the dural sac to expose the floor of the spinal canal and the lumbar disc prolapses. After successful decompression a nerve hook was used to release adhesive tissue between disc space and dura. One dural tear was located at the ventral aspects of the dural sac, and the other dura tear was located at the lateral aspect of the dural sac.
In synovial cyst surgery the incidence of dural tear was 37.5% (three cases). This entity is well known for the massive adherence of the cyst tissue to the thecal sac and the high frequency of CSF leakage. Two patients underwent surgery for a left-sided L4–5 synovial cyst and one patient for a right-sided L5–S1 synovial cyst. In two cases, the dural tear occurred at the initial step of the procedure. After exposure of the dura and the cranial part of the synovial cyst, a nerve hook with a blunt tip was used to start peeling the synovial cyst off the dura. While performing this step of the procedure the tip of the nerve hook perforated the dura. To avoid enlarging the dural tear further, the exposure of the synovial cyst was performed from more lateral to caudal. After exposing the entire cyst, again a nerve hook, a Kerrison punch and a grasper were used to peel the cyst of the dura from the caudal side to the cranial side (see Fig. 2). In the other case, the tear occurred using a Kerrison punch to peel remnant pieces of the synovial cyst off the dura.
Mediolateral dural tear. a View onto the dura and the cranial base of the synovial cyst (arrows). b Dissection of the synovial cyst be peeling it off the dura with a nerve hook. c Mediolateral dural tear (stars). d Exposure of the lateral and caudal base of the synovial cyst (arrows), dural tear (stars). e Resection of the synovial cyst using a nerve hook, axilla of the nerve root (arrows). f Dural sac after resection of the synovial cyst, lateral aspect of the dura (arrows), dural tear (stars)
In pure lumbar disc prolapse, the incidence of dural tear was 1.1% (1 case). The dural tear occurred at the initial stage of the procedure while performing a laminotomy; the dura and the lamina were adherent. The underlying pathology was a big lumbar subligamentous disc herniation that displaced the dural sac laterally. Once the CSF leakage was identified, the exposure of the dural tear was complicated by epidural bleeding. The flow of CSF constantly increased while the decompression of the dural sac continued and access to the subligamentous disc herniation was achieved. After the removal of the disc herniation, the pressure on the nerve root decreased rapidly and therefore the size of the dural tear further increased which was followed by high leakage of CSF (see Fig. 3).
Lateral dural tear. a View onto the surgical field after exposure of the ligamentum flavum (stars) and the dura (arrows). b Laminotomy with a Kerrison punch. c Epidural bleeding and CSF flow. d Mobilization of the disc herniation after further exposure. e Disc herniation (arrow) is removed using a grasper followed by high CSF flow. f Dural tear with nerve fascicle herniation (arrows)
Dural repair
Once a dural tear occurred, the primary procedure was completed before the dural tear was treated as described below. A complete exposure to the dural tear is recommended. In case of ventral or ventrolateral dural tears complete exposure might not be feasible. At first a piece of autologous paraspinal muscle about the size of the dural tear was harvested with the Kerrison punch or a grasper. The dural tear was covered with the muscle patch which served as a buttress graft. A dissector or bayonet-shaped forceps was used to place it gently. Next a fibrin sealant patch which consist of a collagen sponge coated with human thrombin and fibrinogen (TachoSil® Takeda Pharmaceutical Company Limited, Ōsaka, Japan) was cut in small squares between 7 and 12 mm in size. A piece in proper size was moistened and then placed over with muscle covering dural tear and gently advanced with forceps or dissector until it adhered to the dura. The flow of CSF is expected to considerably decrease once the surface of the fibrin sealant and the dura became adherent. Additional pieces of fibrin sealant patch were then placed to cover the brim of the initial placed fibrin sealant patch. By then the CSF flow is expected to be stopped. It might not be feasible to cover a ventrolateral dura tear entirely. In this case the authors recommend sealing the entire lateral aspect of the dural tear and furthermore the intact dura cranially and caudally to the dura tear with several pieces. In case of a dural tear at the axilla of the nerve root a piece of collagen protein sponge (Gelita®, Gelita AG, Eberbach, Germany) was used which served as a counter bearing to apply gentle pressure to the fibrin sealant patch. No lumbar or subfascial drains were placed. The tubular retractor was slowly removed, and meticulous hemostasis in paraspinal muscles was achieved if necessary with bipolar coagulation. Fascia was closed with interrupted 2.0 sutures. The subcutaneous tissue and the skin were closed with interrupted 3.0 sutures followed by topical skin adhesive (Dermabond®, Ethicon Inc., Somerville, NJ, USA). An example of endoscopic dural closure after resection of a synovial cyst is shown in Video 1.
Clinical follow-up
All patients were mobilized 6 h after surgery and had a personal examination after 1, 6 and 12 weeks. Preoperatively eight patients had radicular pain, four patients had a motor deficit and two patients had a sensory deficit. At the first postoperative day all patients reported to be pain free or with improved leg pain, four patients reported an improvement in motor strength and one of the two patients with preoperative sensory deficit reported an improvement. No new neurological deficits occurred. No ongoing radicular pain or motor deficit was documented. Postoperatively no CSF fistula occurred. None of the patients had postoperative problems with wound healing or wound infection. Further none of the patients reported any kind of discomfort which could be related to the CSF leak or pseudomeningocele.
One patient who was free of pain developed a sacroiliac joint syndrome 5 months after initial procedure. The patient underwent an injection of steroid and anesthetic mixture into the joint and was free of pain afterwards. In another patient who reported improvement of leg pain after surgery a new disc herniation occurred 4 months after initial procedure. The patient underwent repeat surgery at the adjacent segment.
Three patients underwent postoperative MRI to confirm complete resection of synovial cyst. In all cases there was no evidence of a CSF fistula, no nerve root tethering, no dural sac compression and no pseudomeningocele (see Fig. 4).
Preoperative and postoperative MRI. a, b Preoperative parasagittal and axial MRI of a synovial cyst (white arrow). c, d Postoperative parasagittal and axial MRI after complete resection of the synovial cyst with decompressed dural sac. No signs of and no CSF collection, and no signs of compression of the dural sac
Discussion
At the initial stages of endoscopic spinal surgery, concerns have been raised that endoscopic techniques might cause higher incidence of dural tears than in open surgery because of poor image quality and depth perception [16]. The criticism was refuted by several studies which reported better tissue identification via HD endoscopy a similar rate of dural tear for conventional and endoscopic lumbar spinal surgery [17,18,19,20]. In the present study the rate of dural tear in cases of lumbar disc herniation and lumbar canal stenosis was in line with previously reported numbers in standard open procedure or tubular-assisted procedures [2, 11, 21, 22].
However, the incidence of dural tear in the present study was considerably higher (i.e. 37.5%) compared to cases of lumbar disc herniation or lumbar stenosis. One reason for this high dural tear rate might be the limited instrument workability of the endoscopic tubular-assisted procedure. A greater outer diameter of the tubular retractor might be useful in the dissection of a very adhesive synovial cyst and to reduce the rate of dural tears.
Once a serious complication occurs, the surgical procedure may be no longer minimally invasive. Clinical outcome in a long-term seems to be worse in cases with dural tear compared to those without the occurrence of dural injury [21]. Some authors believe that it is inevitable to repair the dural tear to prevent the occurrence of a pseudomeningocele or even spinal fluid leak that other surgeons do not feel that to repair a dural tear in tubular-assisted surgery is generally necessary because of the minimal dead space [3, 23]. Till today there is no clear evidence for that kind of arguing and even in percutaneous and tubular-assisted surgery with very minimal death space, an unrecognized dural tear might be followed by repeat surgery and repair of a pseudomeningocele [8, 24]. Therefore the authors recommend dural closure even in procedures via muscle splitting approach.
In minimally invasive spine surgery, direct repair via suturing is not always feasible due to inaccessible location of dural tear and limited working space. Operating through a small working tube may not allow the manipulation of instruments as accustomed in conventional surgery which could then force the surgeon to convert the procedure to open surgery. A case report of primary dural repair through an 18 mm tubular retractor using pituitary instruments has been first described by Chou et al. in 2009 [6].
In 2011, Ruban et al. reported good result of dural repair via suturing which was followed with the application of fibrin glue and patients were kept in bed rest overnight. However, the outer diameter of the tubular retractor in this series has been reported to be 22–26 mm with an option to expand it up to 40 mm [11]. To the best of the authors’ knowledge there is no series of patients with primary suturing through a tubular retractor with 18 mm or less in outer diameter.
In the present series the outer diameter was considerably smaller (15–19 mm) and the procedure was performed under endoscopic visualization. It remains unclear if direct dural repair via suturing could be performed under endoscopic or microscopic visualization view through a 15–19 mm tubular retractor at the same time as dural closure was performed with the described technique. Dural repair without direct suturing is a well-known technique in conventional spine surgery and about 80% of spine surgeons reported good experience with this technique [13].
The U-clip was originally designed to tie abdominal vessels and fascia endoscopically. Spine surgeons used this self-closing device in combination with fibrin glue for dural repair in tubular-assisted spinal surgery [25]. However, the disadvantage of the U-clip is that the needle has to be passed with an instrument similar to suturing before the surgeon is confronted with the unfamiliar release mechanism. Dural closure without suturing via augmentation (i.e. fat graft, collagen matrix, fibrin glue, U-clip etc.) is commonly performed in tubular-assisted surgery. The authors believe that the decreased dead space is an important benefit of the muscle splitting approach because it reduces the space for CSF accumulation and formation of a pseudomeningocele.
Postoperative immobilization due to bed rest is associated with a prolonged hospital stay, the risk of postoperative complications such as deep venous thrombosis, infection and additional costs. The goal therefore should be to close the dural tear in a way that allows early mobilization. To the best of the authors’ knowledge there are a few reports that described the closure of a dural tear through a tubular retractor without suturing in detail. In those reports the patients were put in bed rest and the presented technique of dural repair was not consistent [7]. Shibayama et al. reported about seven patients with incidental dural tear. In all cases dural repair was successfully performed without suturing by applying a polyglactin sheet that was soaked in fibrinogen and patients were mobilized at the second postoperative day [5].
This manuscript was not meant to persuade other spine surgeons that the endoscopic visualization is the golden standard. The authors are very experienced in endoscopic spinal surgery and favour the endoscopic HD visualization over the microscopic visualization because in their experience under endoscopic visualization the surgical field does not get obstructed by the shaft of the surgical instrument. Even though the authors have never performed this technique of dural closure under microscopic visualization or under magnification via loupe, the authors are convinced that this technique offers the same result as long as a tubular retractor and muscle splitting approach is used for procedure. The authors are of the opinion that dural closure through a tubular retractor should be safe, fast and easy to learn. The angled 30° endoscopic optic offers ideal visualization of the surgical field. The position of the endoscope is adjustable and therefore allows for inspection of remote corner of the surgical field which is very helpful. In the present study the average time for dural closure was 209 s and no intraoperative complication occurred while dural repair. All patients were mobilized 6 h postoperatively and no cutaneous CSF fistula or symptoms that could be related to CSF fistula were noted. Further, all postoperative MRI showed no evidence of CSF fistula or nerve root tethering in patients.
Conclusions
Dural closure with a combination of an autologous muscle graft and a fibrin sealant patch is may be a fast, safe and alternative technique for the management of dural tear in endoscopic tubular-assisted spinal surgery.
References
He J, Xiao S, Wu Z, Yuan Z (2016) Microendoscopic discectomy versus open discectomy for lumbar disc herniation: a meta-analysis. Eur Spine J 25(5):1373–1381. doi:10.1007/s00586-016-4523-3
Stromqvist F, Jonsson B, Stromqvist B, Swedish Society of Spinal S (2010) Dural lesions in lumbar disc herniation surgery: incidence, risk factors, and outcome. Eur Spine J 19(3):439–442. doi:10.1007/s00586-009-1236-x
Guerin P, El Fegoun AB, Obeid I, Gille O, Lelong L, Luc S, Bourghli A, Cursolle JC, Pointillart V, Vital JM (2012) Incidental durotomy during spine surgery: incidence, management and complications. A retrospective review. Injury 43(4):397–401. doi:10.1016/j.injury.2010.12.014
Tafazal SI, Sell PJ (2005) Incidental durotomy in lumbar spine surgery: incidence and management. Eur Spine J 14(3):287–290. doi:10.1007/s00586-004-0821-2
Shibayama M, Mizutani J, Takahashi I, Nagao S, Ohta H, Otsuka T (2008) Patch technique for repair of a dural tear in microendoscopic spinal surgery. J Bone Jt Surg Brit 90(8):1066–1067. doi:10.1302/0301-620X.90B8.20938
Chou D, Wang VY, Khan AS (2009) Primary dural repair during minimally invasive microdiscectomy using standard operating room instruments. Neurosurgery 64(5 Suppl 2):356–358. doi:10.1227/01.NEU.0000338942.11337.DA (discussion 358–359)
Than KD, Wang AC, Etame AB, La Marca F, Park P (2008) Postoperative management of incidental durotomy in minimally invasive lumbar spinal surgery. Minim Invas Neurosurg 51(5):263–266. doi:10.1055/s-0028-1082313
Ahn Y, Lee HY, Lee SH, Lee JH (2011) Dural tears in percutaneous endoscopic lumbar discectomy. Eur Spine J 20(1):58–64. doi:10.1007/s00586-010-1493-8
Wu X, Zhuang S, Mao Z, Chen H (2006) Microendoscopic discectomy for lumbar disc herniation: surgical technique and outcome in 873 consecutive cases. Spine 31(23):2689–2694. doi:10.1097/01.brs.0000244615.43199.07
Tsutsumimoto T, Yui M, Uehara M, Ohta H, Kosaku H, Misawa H (2014) A prospective study of the incidence and outcomes of incidental dural tears in microendoscopic lumbar decompressive surgery. Bone Jt J 96-B(5):641–645. doi:10.1302/0301-620X.96B5.32957
Ruban D, O’Toole JE (2011) Management of incidental durotomy in minimally invasive spine surgery. Neurosurg Focus 31(4):E15. doi:10.3171/2011.7.FOCUS11122
Tan LA, Takagi I, Straus D, O’Toole JE (2014) Management of intended durotomy in minimally invasive intradural spine surgery: clinical article. J Neurosurg Spine 21(2):279–285. doi:10.3171/2014.3.SPINE13719
Gautschi OP, Stienen MN, Smoll NR, Corniola MV, Tessitore E, Schaller K (2014) Incidental durotomy in lumbar spine surgery—a three-nation survey to evaluate its management. Acta Neurochir (Wien) 156(9):1813–1820. doi:10.1007/s00701-014-2177-7
Oertel JM, Mondorf Y, Gaab MR (2009) A new endoscopic spine system: the first results with “Easy GO”. Acta Neurochir 151(9):1027–1033. doi:10.1007/s00701-009-0454-7
Oertel JM, Philipps M, Burkhardt BW (2016) Endoscopic posterior cervical foraminotomy as a treatment for osseous foraminal stenosis. World Neurosurg 91:50–57. doi:10.1016/j.wneu.2016.02.073
Teli M, Lovi A, Brayda-Bruno M, Zagra A, Corriero A, Giudici F, Minoia L (2010) Higher risk of dural tears and recurrent herniation with lumbar micro-endoscopic discectomy. Eur Spine J 19(3):443–450. doi:10.1007/s00586-010-1290-4
Quigley MR, Bost J, Maroon JC, Elrifai A, Panahandeh M (1998) Outcome after microdiscectomy: results of a prospective single institutional study. Surg Neurol 49(3):263–267 (discussion 267–268)
Daneyemez M, Sali A, Kahraman S, Beduk A, Seber N (1999) Outcome analyses in 1072 surgically treated lumbar disc herniations. Minim Invas Neurosurg 42(2):63–68. doi:10.1055/s-2008-1053372
Nohara Y, Taneichi H, Ueyama K, Kawahara N, Shiba K, Tokuhashi Y, Tani T, Nakahara S, Iida T (2004) Nationwide survey on complications of spine surgery in Japan. J Orthop Sci 9(5):424–433. doi:10.1007/s00776-004-0802-7
Philipps M, Oertel J (2010) High-definition imaging in spinal neuroendoscopy. Minim Invas Neurosurg 53(3):142–146. doi:10.1055/s-0030-1262811
Saxler G, Kramer J, Barden B, Kurt A, Pfortner J, Bernsmann K (2005) The long-term clinical sequelae of incidental durotomy in lumbar disc surgery. Spine 30(20):2298–2302
Khan MH, Rihn J, Steele G, Davis R, Donaldson WF 3rd, Kang JD, Lee JY (2006) Postoperative management protocol for incidental dural tears during degenerative lumbar spine surgery: a review of 3183 consecutive degenerative lumbar cases. Spine 31(22):2609–2613. doi:10.1097/01.brs.0000241066.55849.41
Epstein NE (2013) A review article on the diagnosis and treatment of cerebrospinal fluid fistulas and dural tears occurring during spinal surgery. Surg Neurol Int 4(Suppl 5):S301–S317. doi:10.4103/2152-7806.111427
Palmer S, Davison L (2012) Minimally invasive surgical treatment of lumbar spinal stenosis: two-year follow-up in 54 patients. Surg Neurol Int 3:41. doi:10.4103/2152-7806.94294
Park P, Leveque JC, La Marca F, Sullivan SE (2010) Dural closure using the U-clip in minimally invasive spinal tumor resection. J Spinal Disord Tech 23(7):486–489. doi:10.1097/BSD.0b013e3181c7e901
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Oertel and B. Burkhardt have no conflict of interest.
Funding
None.
Disclosure
J. Oertel is a consultant to the Karl Storz Company.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oertel, J.M., Burkhardt, B.W. Full endoscopic treatment of dural tears in lumbar spine surgery. Eur Spine J 26, 2496–2503 (2017). https://doi.org/10.1007/s00586-017-5105-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-017-5105-8