Abstract
Purpose
Current surgical approaches for treatment of lumbar canal stenosis are often associated with relatively high rates of reoperation and recurrent stenosis. We have developed a new approach for treatment of this condition: sublaminar-trimming laminoplasty. To describe the surgical approach of sublaminar-trimming laminoplasty and to assess associated outcomes.
Methods
Patients with extensive lumbar canal stenosis who received sublaminar-trimming laminoplasty from 2006 to 2008 were considered for inclusion in the study. The surgery comprised aspects of laminotomy and laminectomy. The following were assessed before surgery and 3 years after surgery: leg and back pain by visual analog scale (VAS), extent of disability by Oswestry Disability Index (ODI), severity of back pain by Japanese Orthopedic Association Score for Back Pain (JOA), walking tolerance, and leg numbness. Complications were noted.
Results
A total of 49 patients were included in the study (mean age 65.6 ± 10.6 years). VAS leg and back pain, ODI, and JOA scores significantly changed from before surgery to 3 years after surgery (P < 0.001). Mean changes (95 % confidence interval) were −6.2 (−6.7, −5.7), −4.3 (−4.8, −3.8), −21.4 (−23.4, −19.5), and 13.4 (12.1, 14.7) for leg pain, back pain, ODI, and JOA scores, respectively. Patients experienced significant improvements in walking tolerance and leg numbness (P < 0.001). There were no instances of recurrent stenosis or postoperative spinal instability. Complications included intraoperative dural tear (n = 2), postoperative urinary tract infection (n = 2), and inadequate decompression and junctional stenosis during follow-up (both n = 1).
Conclusion
Sublaminar-trimming laminoplasty shows promise as an effective treatment for extensive lumbar canal stenosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extensive lumbar canal stenosis is defined as combined central and peripheral stenosis of the spinal canal and nerve root canals at multiple levels of the lumbar spinal region. This condition is particularly common in the aged or those with degenerative spine disease [1]. Spinal stenosis was reported to be the most frequent cause of the need for lumbar spinal surgery among the elderly population in the United States [2, 3]. Typical symptoms of lumbar canal stenosis include lower back pain, sciatica, numbness, and intermittent claudication [4, 5] all of which may lead to marked functional deficits [6]. Treatment options for lumbar canal stenosis may be surgical or non-surgical. Non-surgical treatments, such as physical and pharmacological therapy [7], were reported to be effective for promoting symptom relief in 15–43 % of patients [8]; however, surgery is indicated for patients who do not respond to conservative treatment. Indeed, most patients with extensive lumbar canal stenosis undergo surgery to alleviate symptoms and improve function [7].
Various surgical procedures were described for the treatment of extensive lumbar canal stenosis, including laminectomy, laminotomy, and laminoplasty [9–15]. Laminectomy is the standard option for surgical decompression of spinal stenosis. However, traditional laminectomy involves extensive removal of the posterior elements including the lamina, spinous processes, superspinous ligaments, interspinous ligaments, and even facet joints, which may result in iatrogenic spinal instability [16, 17]. Foraminotomy has become increasingly popular for treatment of patients with lumbar stenosis. However, the quality of reported studies was variable and the effectiveness of this approach remains uncertain [18]. Unfortunately, current surgical approaches for the treatment of lumbar canal stenosis are, in many cases, associated with relatively high rates of reoperation and indeed recurrent stenosis [5, 9]. Clearly, there is room for further optimization of surgical approaches in the treatment of lumbar canal stenosis.
To relieve the symptoms of extensive lumbar canal stenosis and promote normal lumbar function, we have developed and extensively used a new surgical approach: sublaminar-trimming laminoplasty. This approach comprises aspects of laminotomy and laminectomy, and aims to remove tissue around the thecal sac and nerve root in order to widen the spinal canal, while preserving structures that stabilize the vertebra, such as the facet joint, interspinous ligament, and supraspinous ligament. We have found this to be a safe approach that involves minimal destruction of the lamina and surrounding lumbar spine tissue. The purpose of this retrospective study was to: (a) describe the surgical approach of sublaminar-trimming laminoplasty; and (b) assess outcomes associated with this surgical approach in the treatment of patients with extensive lumbar canal stenosis.
Materials and methods
Patients
Sublaminar-trimming laminoplasty has replaced standard laminectomy as the surgical approach of choice at Taiwan Adventist Hospital since 2006. This retrospective study included patients with extensive lumbar canal stenosis (diagnosed by computed tomography or magnetic resonance imaging) who received sublaminar-trimming laminoplasty from 2006 to 2008. Patients with osteoarthritis of the knee or hip who received joint replacement and patients with hip or spinal compression fracture were excluded because we felt that these factors may have confounded preoperative and postoperative assessments of pain and neurological condition. For these reasons and because certain individuals would have been unable to satisfactorily complete the assessment tasks, patients were also excluded from the study if they had heart disease, chronic obstructive pulmonary disease, Parkinsonism, senile dementia, sequela of cerebral accident, uremia, malignancy, a history of long term steroid or anti-depressant use, or a history of previous lumbar spinal surgery.
Surgical procedure
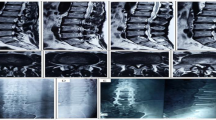
A vertical midline incision was made over the appropriate spinous process, and the lamina from L2 to S1, depending on the involved levels, was explored (Fig. 1). The spinal process, intraspinous ligament, and interspinous ligament were carefully preserved. Foraminotomy was performed by removing the inferior 1/3 of the lamina at the superior level, and the superior 1/3 of the lamina at the inferior level (Fig. 2). The insertion and origin of the ligamenta flava were completely free from the lamina. Facetectomy was performed laterally to the pedicle wall. Half of the facet joint was preserved and the facet capsule was left intact. The ligamenta flava was removed after facetectomy to help prevent spinal cord and nerve root injury.
Schematic illustrating key parts of the sublaminar-trimming laminoplasty procedure. a Surgical procedure: laminotomy, and sublaminar trimming. b Thickness of the lamina before trimming: L3, mean = 9.0 mm (male = 9.5 mm, female = 8.0 mm); L4, mean = 10.0 mm (male = 11.0 mm, female = 9.5 mm); L5, mean = 9.0 mm (male = 9.5 mm, female = 8.5 mm). c Thickness of the lamina after trimming: mean = 3.5 mm
After the laminotomy was complete, foraminotomy was carried out by tracing the nerve root laterally and performing decompression to the foramen. The same procedures were repeated for the neighboring level(s) as necessary. After laminotomy and foraminotomy of the two neighboring levels was complete, a curved Kerrison punch was used for sublaminar trimming in order to enlarge the spinal canal. The nerve root and the spinal cord were carefully protected during the process using gelfoam and neurosponges.
Posterior lumbar fusions (PLF) was performed in cases where there was decompression involving more than three levels, associated disc degeneration, associated grade I degenerative spondylolisthesis (dynamic view), or adult lumbar degenerative scoliosis. PLF or posterior lumbar interbody fusion (PLIF) with instrumentation was performed in cases of spinal canal stenosis with grade II or III degenerative spondylolisthesis.
The wound was closed over a suction drain with suturing of the lumbodorsal fascia directly to the spinous processes using absorbable sutures. After recovery with standard wound care (approximately 1 week), patients were discharged. Post discharge follow-up occurred regularly during the subsequent 3 years.
Outcomes
Outcome assessments were made before surgery and at 3 months, 6 months, 1 year, 2 years, and 3 years after surgery. An experienced physical therapist performed all assessments before and after surgery.
Complete fusion was defined as solid intervertebral trabeculation with no segmental motion in the dynamic view of the lumbar spine. X-ray was used to assess spinal stability, kyphosis, and scoliosis.
Leg and back were assessed by asking patients to place a mark on a visual analog scale (VAS), where 0 indicated no pain and 10 indicated the worst pain imaginable. Patients were also asked to rate their leg numbness (none, mild without discomfort, mild with discomfort, moderate, or severe) and the time they could tolerate walking (<10, 10 to <30, 30 to <60, or ≥60 min).
Patients’ extent of disability was determined using the Oswestry Disability Index (ODI) [19]. The ODI is a ten-item questionnaire that subjectively assesses patients’ pain response to pharmacological therapy, ability to perform daily activities, heavy lifting ability, walking, sitting, standing ability, sleep quality, effect of back pain on sexual activity, effect of pain on social activity, and ability to travel. Each question is rated from 0 (no effect) to 5 (severely affected), with the maximum total score being 50 (higher scores indicate greater disability).
The severity of patients’ back pain was determined using Japanese Orthopedic Association Score for Back Pain (JOA) [20]. The JOA is a 16-item questionnaire that subjectively assesses the severity of back pain, lower limb pain, tolerable walking distance, straight-leg-raise angle at which pain is experienced, mental clarity, muscle strength, bladder control, and difficulty in performing activities of daily living. The maximum total score is 29, with lower scores indicating greater disability.
Complications after surgery and during follow-up were noted.
Statistical analysis
Continuous and categorical variables were compared between the before surgery and 3 years after surgery time points by paired t test and McNemar test, respectively. Ordinal variables were compared by Wilcoxon-signed rank test. All statistical assessments were two-sided and evaluated at the 0.05 level of statistical significance. Statistical analyses were performed using SPSS Version 15.0 statistical software (SPSS Inc, Chicago, IL, USA).
Results
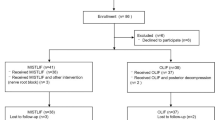
A total of 210 consecutive patients received sublaminar-trimming laminoplasty between 2006 and 2008. Of these patients, 161 were excluded. Reasons for exclusion before follow-up were: hip or knee arthritis, hip fracture, or spinal fracture (n = 27); major medical disease (n = 12); chronic pharmacological treatment (n = 15); and a history of previous spinal surgery (n = 44). Reasons for exclusion during follow-up were: malignancy (n = 10); major medical disease (n = 25); knee or hip surgery (n = 13); and loss to follow-up or death (n = 15). Hence, a total of 49 patients were included in the final evaluation.
The demographic and clinical characteristics of the patients are summarized in Table 1. The patients ranged in age from 47 to 97 years and were mostly (>75 %) female. Most patients (38 of 49; 78 %) had four or five stenoses. Of the 49 patients, 36 received PLF and 8 received PLF or PLIF with instrumentation to increase spinal stability.
The mean operative time was 126.6 min for sublaminar-trimming laminoplasty alone, 213.1 min for sublaminar-trimming laminoplasty with PLF, and 295.7 min for sublaminar-trimming laminoplasty with PLF (or PLIF) and instrumentation. Mean blood loss during surgery was 291.1 mL for sublaminar-trimming laminoplasty alone, 657.7 mL for sublaminar-trimming laminoplasty with PLF, and 1,314.3 mL for sublaminar-trimming laminoplasty with PLF (or PLIF) and instrumentation.
Solid fusion was apparent in 47 of 49 (96 %) patients. Fusion was not apparent in one patient and another patient experience fusion on one side only.
The leg and back VAS, ODI, and JOA scores are summarized in Table 2. For each of these measures, there were significant improvements in scores from before surgery to 3 years after surgery (all P < 0.001). Note: all of the questionnaires administered as part of the study were completed by all patients.
The X-ray diagnosis, walking tolerance, and leg numbness findings are summarized in Table 3. No patient had experienced recurrence of spinal instability 3 years after surgery (P < 0.001). Patients experienced significant improvements in walking tolerance and leg numbness 3 years after surgery (both P < 0.001).
Two patients experienced intraoperative dural tears and two patients experienced postoperative urinary tract infections. The intraoperative dural tears were sutured intraoperatively, while the urinary tract infections were treated with antibiotics. During follow-up, one patient experienced inadequate decompression and one patient experienced adjacent syndrome. Both of these patients underwent a second procedure and recovered without further complication. No patients experienced recurrent stenosis.
Discussion
Creating sufficient space for the spinal canal is the key to improving symptoms of spinal stenosis and is thus the main purpose of any surgical treatment. Herein, we have described our experience using a new surgical approach, sublaminar-trimming laminoplasty, for the treatment of extensive lumber canal stenosis. This approach, which involves adequate decompression of the spinal canal, led to obvious improvement in pain, neurological symptoms, and function 3 years after surgery. These findings are similar to those reported after laminectomy [21] and appear to be better than those associated with minimally invasive foraminotomy [11, 12, 18]. In our experience, sublaminar-trimming laminoplasty allows for sufficient widening of the spinal canal to resolve the symptoms of spinal stenosis. Importantly, none of the patients treated in this manner experienced recurrent stenosis, which is a relatively common problem with other surgical approaches [5, 9]. We routinely treat patients with degenerative spondylolisthesis, adult lumbar scoliosis, or spinal stenosis at more than three levels with PLF or PLIF. This may help prevent recurrent stenosis and instability, as suggested by the findings reported by Postacchini and Cinotti [22], who found that patients with spinal stenosis and degenerative spondylolisthesis who received spinal fusion in addition to laminectomy experienced significantly better outcomes than patients who received laminectomy alone.
A key feature of sublaminar-trimming laminoplasty is preservation of the facet joint and supraspinal and interspinal ligament. We suggest that this may help prevent spinal instability, which can occur after traditional laminectomy [9]. Indeed, Tai et al. [17] have suggested that maintained integrity of the posterior complex helps stabilize the decompressed spine. In addition to preserving the facet joint and supraspinal ligament, PLF or PLIF with or without instrumentation was performed for patients with degenerative spondylolisthesis, adult lumbar scoliosis, or multilevel stenosis. It is noteworthy that none of the patients treated with sublaminar-trimming laminoplasty in our study have experienced spinal instability to date, thus emphasizing the effectiveness of the surgical approach for maintaining spinal stability.
A procedure similar to sublaminar-trimming laminoplasty known as the “Windows technique” was previously reported by Fu et al. [14]. As with sublaminar-trimming laminoplasty, a key feature of the Windows technique is preservation of the facet joint and supraspinous ligament. Different to our approach, is the thickness of retained lamina (3.5 mm with our approach vs inferior 1/3 removed with the approach of Fu et al.) and the use of spinal fusion (PLF or PLIF with our approach vs not mentioned with the approach of Fu et al.). Similar to our study, Fu et al. found that the Windows technique was associated with significant improvements in VAS pain, ODI, leg numbness, and walking tolerance, and no instances of spinal instability. Notably, these improvements were significantly more pronounced when compared with traditional laminectomy. Taken together, the findings from our study and that by Fu et al. highlight the importance of maintaining the posterior spinal stability in the surgical treatment of lumbar canal stenosis.
A potential drawback of the sublaminar-trimming laminoplasty surgical approach is the limited exposure afforded by the laminoplasty for adequate decompression of the canal and nerve root. This limitation may be expected to increase operation time compared with conventional laminectomy. Indeed, we found this to be the case when we first started using this approach; however, with experience and technique refinement, including ensuring less ligamentous and bony disruption, there was a dramatic decrease in operation times. Of note, we now perform multiple level laminoplasty within the same or less time than that required for conventional laminectomy at equivalent levels.
Our study has a number of limitations that warrant mention. First, our study did not include any comparator groups. Further studies are warranted to directly compare sublaminar-trimming laminoplasty with other surgical approaches for the treatment of lumbar canal stenosis. Secondly, our patients were followed-up for a maximum of 3 years. Continued monitoring is warranted to determine if the efficacy of this approach is maintained beyond 3 years. Finally, a relatively large number of patients (15 in total) were lost to follow-up due to death, which may have biased our findings to some extent. This loss to follow-up due to death likely reflects the fact that our cohort of patients was relatively old and therefore more susceptible to being affected by life-threatening conditions such as heart attack and cerebral stroke.
Conclusion
In summary, we have described a new surgical approach, sublaminar-trimming laminoplasty, for the treatment of extensive lumbar canal stenosis. As detailed in this manuscript, we have found this approach to be effective as indicated by significant improvements in function and resolution of symptoms. Notably, no patients experienced recurrence of spinal instability, and the few complications reported were satisfactorily resolved. Our findings lead us to suggest that sublaminar-trimming laminoplasty shows promise as an effective treatment, with favorable safety, for extensive lumbar canal stenosis.
References
Verbiest H (2001) A radicular syndrome from developmental narrowing of the lumbar vertebral canal. 1954. Clin Orthop Relat Res 1:3–9
Deyo RA, Ciol MA, Cherkin DC et al (1993) Lumbar spinal fusion. A cohort study of complications, reoperations, and resource use in the Medicare population. Spine (Phila Pa 1976) 18:1463–1470
Deyo RA, Gray DT, Kreuter W et al (2005) United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 30:1441–1445 (discussion 1446–1447)
Deyo RA, Mirza SK, Martin BI et al (2010) Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 303:1259–1265
Mazanec DJ, Podichetty VK, Hsia A (2002) Lumbar canal stenosis: start with nonsurgical therapy. Cleve Clin J Med 69:909–917
Spivak JM (1998) Degenerative lumbar spinal stenosis. J Bone Joint Surg Am 80:1053–1066
Weinstein JN, Tosteson TD, Lurie JD et al (2010) Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976) 35:1329–1338
Simotas AC (2001) Nonoperative treatment for lumbar spinal stenosis. Clin Orthop Relat Res 384:153–161
Hulen CA (2008) A review of the significance, indications, techniques, and outcomes of revision lumbar laminectomy surgery. Semin Spine Surg 20:270–276
Jakola AS, Sorlie A, Gulati S et al (2010) Clinical outcomes and safety assessment in elderly patients undergoing decompressive laminectomy for lumbar spinal stenosis: a prospective study. BMC Surg 10:34
Ahn Y, Lee SH, Park WM et al (2003) Posterolateral percutaneous endoscopic lumbar foraminotomy for L5–S1 foraminal or lateral exit zone stenosis. Technical note. J Neurosurg 99:320–323
Christie SD, Song JK (2006) Minimally invasive lumbar discectomy and foraminotomy. Neurosurg Clin N Am 17:459–466
Yucesoy K, Ozer E (2002) Inverse laminoplasty for the treatment of lumbar spinal stenosis. Spine (Phila Pa 1976) 27:316–320
Fu YS, Zeng BF, Xu JG (2008) Long-term outcomes of two different decompressive techniques for lumbar spinal stenosis. Spine (Phila Pa 1976) 33:514–518
Englund J (2007) Lumbar spinal stenosis. Curr Sports Med Rep 6:50–55
Weiner BK, Walker M, Brower RS et al (1999) Microdecompression for lumbar spinal canal stenosis. Spine (Phila Pa 1976) 24:2268–2272
Tai CL, Hsieh PH, Chen WP et al (2008) Biomechanical comparison of lumbar spine instability between laminectomy and bilateral laminotomy for spinal stenosis syndrome—an experimental study in porcine model. BMC Musculoskelet Disord 9:84
Nellensteijn J, Ostelo R, Bartels R et al (2010) Transforaminal endoscopic surgery for lumbar stenosis: a systematic review. Eur Spine J 19:879–886
Fairbank JC, Pynsent PB (2000) The Oswestry Disability Index. Spine (Phila Pa 1976) 25:2940–2952 (discussion 2952)
Nakamura M, Miyamoto K, Shimizu K (2009) Difference in evaluation of patients with low back pain using the Japanese Orthopaedic Association Score for Back Pain and the Japanese Version of the Roland-Morris Disability Questionnaire. J Orthop Sci 14:367–373
Javid MJ, Hadar EJ (1998) Long-term follow-up review of patients who underwent laminectomy for lumbar stenosis: a prospective study. J Neurosurg 89:1–7
Postacchini F, Cinotti G (1992) Bone regrowth after surgical decompression for lumbar spinal stenosis. J Bone Joint Surg B 74:862–869
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, WJ., Hong, SW., Liou, DY. et al. Clinical outcomes following sublaminar-trimming laminoplasty for extensive lumbar canal stenosis. Eur Spine J 23, 80–86 (2014). https://doi.org/10.1007/s00586-013-2888-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-013-2888-0