Abstract
Introduction
There is no comparative study regarding surgical outcomes between microsurgical extraforaminal decompression (MeFD) and posterior lumbar interbody fusion (PLIF) for the treatment of lumbar foraminal stenosis (LFS). Therefore, the purpose of this study was to compare the surgical outcomes of LFS using two different techniques: MeFD alone or PLIF.
Methods
For the purposes of this study, a prospectively collected observational cohort study was conducted. Fifty-five patients diagnosed with LFS who were scheduled to undergo spinal surgery were included in this study. According to the chosen surgical technique, patients were assigned to either the MeFD group (n = 25) or the PLIF group (n = 30). The primary outcome was Oswestry Disability Index (ODI) score at 1 year after surgery.
Results
The baseline patient characteristics and preoperative ODI score, visual analog scale (VAS) scores for back and leg pain, and Short Form-36 score were not significantly different between the two groups. At 12 months postoperative, the mean ODI score in the MeFD and PLIF groups was 25.68 ± 14.49 and 27.20 ± 12.56, respectively, and the 95 % confidence interval (−9.76–6.73) was within the predetermined margin of equivalence. The overall ODI score and VAS scores for back and leg pain did not differ significantly over the follow-up assessment time between the two groups. However, the ODI score and VAS scores for back and leg pain improved significantly over time after surgery in both groups. In the MeFD group, revision surgery was required in three patients (12 %).
Conclusions
This study demonstrated that MeFD alone and PLIF have equivalent outcomes regarding improvement in disability at 1 year after surgery. However, the higher rate of revision surgery in the MeFD group should emphasize the technically optimal amount of decompression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For surgical treatment of lumbar foraminal stenosis (LFS), recent studies have demonstrated the efficacy of microsurgical extraforaminal decompression (MeFD) [1–6]. This procedure involves unroofing of the stenotic foramen. Previous studies have shown very promising surgical outcomes, not only in the short term, but also in long-term follow-up assessments [3–6]. However, most of these studies were clinical case series, and the surgical outcomes of this decompression technique can worsen over time owing to the progression of degenerative changes in corresponding segments. Although this technique is minimally invasive, minimally invasive decompression does not arrest the natural history of the degenerative process already underway in the segment. For this reason, posterior lumbar interbody fusion (PLIF) also has been advocated for direct or indirect decompression and for preventing restenosis and further degeneration of the operated segment [7–9].
Nonetheless, there is no comparative study regarding the surgical outcomes of LFS between MeFD alone and PLIF. Compared with minimally invasive MeFD, fusion surgery has the advantage of preventing restenosis of the index segment, but the disadvantage of requiring more extensive exposure. Therefore, the purpose of this study was to compare the surgical outcomes of LFS using these two different techniques.
Methods
Study design and patients
This study was approved by the hospital’s institutional review board. For the purposes of this study, a prospectively collected observational cohort study was conducted. All prospective data were obtained as part of routine patient care and were included in the medical records. Because all processes were considered part of routine care, no written informed consent was obtained from the patients. Inclusion criteria included patients aged 40–80 years, diagnosed with LFS in the mid and exit zones of the foramen, and scheduled to undergo spinal surgery. Diagnosis of LFS required one or more of the following symptoms: leg pain, numbness, or motor deficits in the lower extremities and buttocks [10, 11], with confirmation of a stenotic lesion in the mid (under the pedicle) and exit zones of the foramen of the lumbar spine on magnetic resonance imaging (MRI) [7]. Patients were excluded if they had spinal instability, including spondylolysis, foraminal stenosis caused by only extruded disc, stenosis severe central stenosis more than grade C by Schizas’s classification [12], and stenosis with an element of entry zone foraminal stenosis [7]. On flexion–extension lateral radiographs of the lumbar spine, anterior translation of the index segment >4.5 mm in the sagittal plane or sagittal plane motion >15 from L1–L2 to L3–L4, >20 at L4–L5 and >25 at L5–S1 from extension to flexion were considered spinal instability [13]. Furthermore, such patients were excluded that they had a history of peripheral vascular disease, any concurrent serious medical condition causing disability, or general health status including sepsis or cancer. The study was performed at the spinal center of a tertiary care teaching institution from June 2012 to March 2013. Patients were assigned to the MeFD alone group or the PLIF group. In both groups, surgical indication was identically lumbar foraminal stenosis at a single level.
Decision of surgical technique
In the present study, the surgical technique was determined through a preference-based, shared-decision making system. The patient was educated about his/her current status of degenerative LFS and the available surgical options. Furthermore, his/her own MRI was shown and explained. Finally, the first author informed the patient about the evidence to support the advantages and disadvantages of each available surgical option based on previous studies [7, 8, 11]. After the patient-education stage, the patient was asked to describe the decision-making process, the pros and cons of the various surgical options, and whether the treatment decision made was congruent with his/her preferences. A surgeon did not recommend any specific surgical option to patients. According to the patient’s preference and shared decision, the patient was allocated to either the MeFD alone group or the PLIF group (Fig. 1). All surgical procedures in both groups were performed by the first author, who has experienced approximately 100 and 600 cases of MeFD and PLIF surgery, respectively, over the past 3 years.
MeFD group
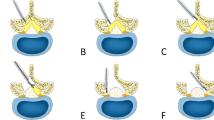
Figure 2 shows a photograph of an actual operative field. First, the lateral portion of the pars interarticularis and the facet joint was exposed by a paramedian approach. Using an operating microscope and high-speed drill, the superolateral portion of the facet joint and the upper and lateral margins of the interarticular part were drilled away. Afterward, the intertransverse ligament was partially excised or released to expose the nerve root lateral to the foramen. Then, the affected nerve root was followed along the intervertebral foramen. Sufficient nerve root decompression was carefully confirmed by moving a small nerve hook from the lateral side through the foramen. If necessary, the disc space was also exposed for complete nerve root decompression.
Schematic drawing and photograph of actual operative field during microsurgical extraforaminal decompression (asterisk indicates left L4 root, star indicates left L4 lamina). a Illustration for lumbar foraminal stenosis (arrow and greyed area is resected using burr). b Illustration after microsurgical extraforaminal decompression. c Photographs of actual operative field (low and high magnifications)
In this technique, an intertransverse interval approach is used via a paraspinal muscle-splitting route, and the superolateral part of the superior articular process of the lower vertebrae, the lateral part of the pars interarticularis, and the superomedial part of the superior articular process are resected, resulting in unroofing of the stenotic foramen. Inferior partial pediculectomy can be performed to achieve adequate decompression [1–6].
PLIF group
PLIF was performed in the usual manner [14, 15]. A single midline incision of approximately 8 cm in length was made, followed by exposure of the spine to the facet joints and the lateral tips of the transverse processes to allow clear identification of the bony landmarks. First, a pedicle screw was inserted using the Weinstein method. Following the decompression procedures, including laminectomy and facetectomy, the compressed nerve root in the extraforaminal area was identified and the final decompression state was confirmed. In the case of unilateral LFS, unilateral decompression and interbody fusion with bilateral fixation was performed. Finally, discectomy was performed and the cage filled with auto bone was inserted.
Surgical outcome assessment
Baseline data, which were collected by a blinded clinical research assistant, included sex, age, height, weight, symptom duration, walking distance in a single trial, preoperative visual analog scale (VAS) scores for back and leg pain, preoperative Oswestry Disability Index (ODI) score, and general health status using the Short Form (SF)-36. The primary outcome was ODI score at 1 year after surgery. The ODI is based on a self-administered questionnaire measuring “back-specific function.” The questionnaire comprises 10 items, each with 6 levels of response. Each item is scored from 0 to 5, and the total summation is converted to a 0–100 scale [16]. Secondary outcome measures included ODI and VAS scores for back and leg pain over all follow-up assessments, and general health status at 1 year after surgery. The VAS for back pain and leg pain comprised a 10-cm line with “none” (0) on one end of the scale and “disabling pain” (10) on the other. Patients were asked to place a mark on the line, which represented their perceived level of back pain, and the measured distance (cm) from the mark to the zero point was considered the score. The ODI score and VAS score for back pain and leg pain were assessed at 3, 6, and 12 months after surgery. The SF-36 score was assessed preoperatively and at 12 months after surgery. The raw scores for the eight subscales (physical function, role physical, bodily pain, general health, vitality, social function, role emotion, and mental health) and two summaries (Physical Component Summary [PCS] and Mental Component Summary [MCS]) of the SF-36 were transformed into norm-based scoring [17]. Rates of revision surgery also were assessed at 12 months after surgery.
Statistical analysis
Preoperative ODI score, VAS scores for back and leg pain, MCS and PCS scores of the SF-36, and demographic data were compared between the two groups using an independent t test. The outcome analysis was performed by comparisons between the MeFD alone and PLIF groups. All data were evaluated using intention-to-treat analyses. The ODI scores (mean and 95 % confidence interval [CI]) at 1 year after surgery were compared between the MeFD and PLIF groups using an independent t test. If the CIs of the differences between the two groups were within ± the predetermined margin of equivalence (minimal important change of 10 for the ODI score) [18], the two surgical methods were considered equivalent. Analysis of variance for repeated measures was performed to examine the surgical outcome measures (VAS scores for back and leg pain and ODI score) between the two groups over the follow-up assessment period. In addition, general health status (PCS and MCS scores) at 12 months after surgery was examined using an independent t test. Furthermore, in each group, any changes in general health status, such as PCS and MCS scores, from study enrollment to 12 months after surgery were compared with a paired t test. All statistical analyses were performed with the SPSS 20.0.0 statistics package (IBM Corporation, Armonk, NY), with an alpha level of significance set at 0.05.
Results
Between June 2012 and March 2013, 75 patients were assessed for study eligibility. Fifty-five participants met the inclusion criteria. According to the chosen surgical technique, patients were assigned to either the MeFD group (n = 25) or the PLIF group (n = 30). Figure 1 shows the number of patients involved in the present study, from eligibility assessment through 12 months of follow-up. At the 12 month assessment after surgery, complete data were available for 22 and 27 patients in the MeFD and PLIF groups, respectively. The baseline characteristics of the patients were similar between the two groups (Table 1). All patients had a single-level lumbar stenotic lesion. The mean age of the two groups was 73.12 ± 6.75 years and 70.00 ± 5.62 years in the MeFD and PLIF groups, respectively. Preoperative ODI score, VAS scores for back and leg pain, and PCS/MCS scores of the SF-36 were not significantly different between the two groups (Table 1).
According to surgical technique, the ODI scores (primary endpoint) at 12 months after surgery were equivalent between groups (Fig. 3). At the 12 month assessment, the mean ODI score in the MeFD and PLIF groups was 25.68 ± 14.49 and 27.20 ± 12.56, respectively, and the 95 % CI (−9.76–6.73) was within the ± predetermined margin of equivalence (ODI score, 10).
There were no significant differences in any of the secondary endpoint variables, including ODI score and VAS scores for back and leg pain, over the follow-up assessments for 1 year between the two groups (Fig. 3). The overall changes in ODI and VAS scores for back and leg pain did not differ significantly over a 12 month period between the two surgical techniques (effect of surgical technique on overall changes in ODI and VAS scores for back and leg pain for follow-up periods, P = 0.978, 0.626, and 0.909, respectively). The patterns of changes in the ODI and VAS scores for back pain and leg pain during the follow-up period were not significantly different between the both cohorts (effect of the interaction between surgical technique and postoperative follow-up assessment time on ODI and VAS scores for back and leg pain, P = 0.381, 0.246, 0.364, respectively). However, the ODI score and VAS scores for back and leg pain improved significantly with time after surgery in both groups (effect of postoperative follow-up assessment time on ODI and VAS scores for back and leg pain, P < 0.001 for all variables). General health status, represented by PCS and MCS scores of the SF-36, at 1 year after surgery did not differ significantly between the two groups (P = 0.643 and 0.818, respectively) (Fig. 4). However, compared with the initial values, both the MeFD and PLIF groups showed significant increases in the PCS score at 12 months (P = 0.010 and 0.021, respectively), whereas the MCS scores at 12 months were not different from the initial values in both groups (Fig. 4).
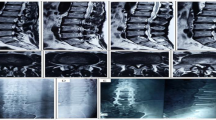
In the MeFD group, revision surgery was required in 3 of 25 patients (12 %). Two patients underwent revision surgery (PLIF) at 2 and 3 months, respectively, after the first MeFD due to foraminal stenosis at the level of first decompression surgery, and one patient underwent revision surgery (PLIF) due to iatrogenic pars and pedicle fractures at the level of first decompression surgery at 3 months postoperatively (Fig. 5).
Cases requiring revision surgery after microsurgical extraforaminal decompression (MeFD). a Case 1: foraminal stenosis was noted even after MeFD. (arrow head indicates foraminal stenois). b Case 2: pedicle and pars fractures were noted. (arrow head indicates pars fractures after extraforaminal decompression)
Discussion
This study demonstrates that MeFD alone for LFS can provide equivalent results regarding improvement in ODI score at 1 year after surgery, compared with PLIF. Furthermore, there were no differences in improvement in disability, back and leg pain, and general health status (PCS and MCS scores of the SF-36) between the two groups across 1 year of follow-up assessments after surgery. However, it should be acknowledged that revision surgery was required in 3 of 25 patients (12 %) within 3 months after the initial surgery in the MeFD group.
These two surgical methods can be considered equivalent regarding improvement in ODI score at 1 year after surgery. Furthermore, the overall improvements in ODI score and VAS scores for back and leg pain with time after surgery did not differ between the two groups. These results agree with those of previous studies [8, 19]. They reported no obvious additional benefit by combining decompression with instrumented fusion in degenerative LSS, compared with decompression alone; thereby advocating decompression alone [8, 19]. Furthermore, the exclusion of patients with spinal instability would contribute to this result. The present study also suggests that MeFD can yield not only equivalent improvement in disability at 1 year after surgery, compared with PLIF, but also overall improvement in disability, back pain, and leg pain across 1 year postoperatively were not different between the two groups.
Notably, in the MeFD group, three patients required revision surgery due to recurrence of similar leg pain, whereas no patient required revision surgery in the PLIF group. All revision surgeries were PLIFs, which were performed within 3 months after the initial operation. Two patients underwent revision surgery due to foraminal stenosis at the level of first decompression surgery, and one patient due to iatrogenic pars and pedicle fractures at the level of first decompression surgery. The former two cases are not considered as degenerative restenosis at the decompression level, but as inadequate decompression in the first MeFD, because the patient’s symptoms, such as leg pain, arose again within only 2 and 3 months, respectively. The latter case is considered as over decompression. Excessive decompression can cause the operated segment to be more vulnerable to external moments or load, which can result in pars and pedicle fractures at the same level within 2 months after the initial MeFD surgery. In summation, these findings indicate that MeFD is more technically demanding regarding the optimal amount of decompression. In our study, the two inadequate decompression cases could have been treated successfully with additional partial pediculectomy, which has been suggested in previous studies regarding MeFD [2, 3, 6]. However, the one case of pedicle fracture has also raised worries regarding over decompression. Therefore, an ideal amount of decompression optimized for each case is necessary for successful outcomes of MeFD.
The current study has both strengths and weaknesses. Most of all, the homogenous diagnosis in both groups is a strength. All patients in both groups had single-level LFS. Regarding limitations of this study, first, the present study was not a randomized controlled trial, but a prospective observational cohort study. Even though there were no differences in the demographic data between the two groups (Table 1), patient allocation was decided by a preference under the shared-decision-making system. The individual preference was associated with psychosocial factors and socioeconomic status [20], which could have influenced the results. Therefore, it should be acknowledged that the present results might have unrevealed bias due to the study design. Second, patients in the present study were followed up for 1 year postoperatively. In the long term, patients who underwent MeFD alone could develop restenosis at the operated level due to progression of degenerative changes [5], while patients in the PLIF group have higher risk of adjacent segment degeneration. Therefore, a long-term follow-up study would provide more comprehensive understanding regarding the effectiveness of both surgical methods. Nonetheless, the present results after 1 year of follow-up show a higher rate of revision surgery after MeFD alone, which emphasizes the technically optimal amount of decompression. Third, authors did not use a more specific questionnaire for lumbar stenosis, such as the Zurich claudication questionnaire because there was not a language-validated version. In a future study of outcome analysis for spinal stenosis, this should be included for a more verified comparative outcome analysis.
In conclusion, we demonstrated that MeFD alone and PLIF have equivalent outcomes regarding improvement in disability at 1 year after surgery. However, the higher rate of revision surgery in the MeFD group should emphasize the technically optimal amount of decompression.
References
Papavero L, Kothe R (2013) Microsurgical extraforaminal decompression of lumbar root canal stenosis. Operative Orthopadie und Traumatologie 25(1):16–30. doi:10.1007/s00064-012-0194-3
Yoshimoto M, Takebayashi T, Kawaguchi S, Tsuda H, Ida K, Wada T, Suzuki D, Yamashita T (2011) Minimally invasive technique for decompression of lumbar foraminal stenosis using a spinal microendoscope: technical note. Minim Invasive Neurosurg 54(3):142–146. doi:10.1055/s-0031-1279716
Yamada K, Matsuda H, Nabeta M, Habunaga H, Suzuki A, Nakamura H (2011) Clinical outcomes of microscopic decompression for degenerative lumbar foraminal stenosis: a comparison between patients with and without degenerative lumbar scoliosis. Eur Spine J 20(6):947–953. doi:10.1007/s00586-010-1597-1
Chang HS, Zidan I, Fujisawa N, Matsui T (2011) Microsurgical posterolateral transmuscular approach for lumbar foraminal stenosis. J Spinal Disorders Tech 24(5):302–307
Matsumoto M, Watanabe K, Ishii K, Tsuji T, Takaishi H, Nakamura M, Toyama Y, Chiba K (2010) Posterior decompression surgery for extraforaminal entrapment of the fifth lumbar spinal nerve at the lumbosacral junction. J Neurosurg Spine 12(1):72–81. doi:10.3171/2009.7.SPINE09344
Ozeki N, Aota Y, Uesugi M, Kaneko K, Mihara H, Niimura T, Saito T (2008) Clinical results of intrapedicular partial pediculectomy for lumbar foraminal stenosis. J Spinal Disorders Tech 21(5):324–327
Jenis LG, An HS (2000) Spine update. Lumbar foraminal stenosis. Spine (Phila Pa 1976) 25(3):389–394
Hallett A, Huntley JS, Gibson JN (2007) Foraminal stenosis and single-level degenerative disc disease: a randomized controlled trial comparing decompression with decompression and instrumented fusion. Spine (Philadelphia, Pa 1976) 32(13):1375–1380
Infusa A, An HS, Glover JM, McGrady L, Lim TH, Riley LH 3rd (1996) The ideal amount of lumbar foraminal distraction for pedicle screw instrumentation. Spine (Phila Pa 1976) 21(19):2218–2223
Katz JN, Harris MB (2008) Clinical practice. Lumbar spinal stenosis. N Engl J Med 358(8):818–825
Watters WC 3rd, Baisden J, Gilbert TJ, Kreiner S, Resnick DK, Bono CM, Ghiselli G, Heggeness MH, Mazanec DJ, O’Neill C, Reitman CA, Shaffer WO, Summers JT, Toton JF, North American Spine S (2008) Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J 8(2):305–310. doi:10.1016/j.spinee.2007.10.033
Schizas C, Theumann N, Burn A, Tansey R, Wardlaw D, Smith FW, Kulik G (2010) Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Philadelphia, Pa 1976) 35(21):1919–1924
Bernhardt M, White A, Panjabi M (1992) Biomechanical considerations of spinal stability. The spine. WB Saunders, Philadelphia
Wang JC, Mummaneni PV, Haid RW (2005) Current treatment strategies for the painful lumbar motion segment: posterolateral fusion versus interbody fusion. Spine (Phila Pa 1976) 30(16 Suppl):S33–S43
Enker P, Steffee AD (1994) Interbody fusion and instrumentation. Clin Orthop Relat Res 300:90–101
Fairbank JC, Pynsent PB (2000) The Oswestry disability index. Spine (Philadelphia, Pa 1976) 25(22):2940–2952
Ware J, Snow K, Kosinski M, Gandek B (1993) SF-36 health survey manual and interpretation guide. New England Medical Center, The Health Institute, Boston
Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, Bouter LM, de Vet HC (2008) Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 33(1):90–94. doi:10.1097/BRS.0b013e31815e3a10
Katz JN, Lipson SJ, Lew RA, Grobler LJ, Weinstein JN, Brick GW, Fossel AH, Liang MH (1997) Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis. Patient selection, costs, and surgical outcomes. Spine (Phila Pa 1976) 22(10):1123–1131
Katz JN (2001) Patient preferences and health disparities. JAMA J Am Med Assoc 286(12):1506–1509
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, HJ., Jeong, JH., Cho, HG. et al. Comparative observational study of surgical outcomes of lumbar foraminal stenosis using minimally invasive microsurgical extraforaminal decompression alone versus posterior lumbar interbody fusion: a prospective cohort study. Eur Spine J 24, 388–395 (2015). https://doi.org/10.1007/s00586-014-3592-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3592-4