Abstract
The aim of this experimental study was to investigate the possible protective effect of dexmedetomidine (DEX) on traumatic spinal cord injury (SCI). Twenty-two New Zealand rabbits were divided into three groups: sham (no drug or operation, n = 6), Control [SCI + single dose of 1 mL saline intraperitoneally (i.p), after trauma; n = 8] and DEX (SCI + 1 μg/kg dexmedetomidine in 1 mL, i.p, after trauma, n = 8). Laminectomy was performed at T10 and balloon angioplasty catheter was applied extradurally. Four and 24 h after surgery, rabbits were evaluated by an independent observer according to the Tarlov scoring system. Blood, cerebrospinal fluid (CSF), tissue samples from spinal cord were taken for biochemical and histopathological evaluations. After 4 h of SCI, all animals in control or DEX treated groups became paraparesic. On the other hand, 24 h after SCI, partial improvements were observed in both control and DEX treated groups. Traumatic SCI leads to increase in the lipid peroxidation and decreases enzymatic or nonenzymatic endogenous antioxidative defense systems. Again, SCI leads to apoptosis in spinal cord. DEX treatment slightly prevented lipid peroxidation and augmented endogenous antioxidative defense systems in CSF or spinal cord tissue, but failed to prevent apoptosis or neurodeficit after traumatic SCI. Therefore, it could be suggested that treatment with dexmedetomidine does not produce beneficial results in SCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic spinal cord injury (SCI) is still a major clinical problem with a permanent neurological deficit and a broad range of secondary complications. The pathophysiology of acute SCI is highly complex and not fully understood. The initial mechanical damage (contusion and compression) causing immediate cell death in the spinal cord is known as primary injury and inevitable. After primary injury, further pathophysiological processes such as hypoxia, edema and inflammation, altered blood flow and changes in microvascular permeability are triggered; thus, lesions greatly enlarge and worsen by secondary injury [33]. Several biochemical events appear to mediate significant secondary developments, resulting in demyelination and further cell death by necrotic and apoptotic pathways [2]. Excessive release of neurotransmitters and inflammatory mediators, increase in lipid peroxidation and reactive oxygen species (ROS) generation are some of them. Previous reports stated that one of the most important factors precipitating posttraumatic degeneration in the spinal cord is oxygen free radical-induced lipid peroxidation [2, 14]. Furthermore, catecholamines are believed to play an important role in the pathogenesis of secondary injury. Norepinephrine release after SCI plays an important role in the increase of neuronal metabolism, impairment in the neuronal cell membrane by induction of lipid peroxidation and in the formation of vasogenic edema [17, 21, 37]. Correlation has been shown between plasma catecholamine levels during ischemia and neurologic outcome [17].
Nowadays, much attention has been focused on the prevention of secondary injury in spinal cord trauma. Previous clinical trials showed that minocycline or early intravenous administration of methylprednisolone significantly improves motor and sensory functions after SCI. [11, 36]. Several pharmacological agents are used against secondary injury after experimental spinal cord trauma. Beneficial effects of melatonin [31], resveratrol [3], etomidate [11], magnesium sulfate [27] and sodium channel blockers mexiletine, phenytoin and riluzole [4] have been shown in traumatic SCI in rodents.
Dexmedetomidine ((+)-4-(S)-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole monohydrochloride) is a short acting, potent and highly selective α2-adrenergic receptor agonist, and used as a sedative, anxiolytic, analgesic and sympatholytic drug. It induces sedation by acting on the α2-adrenergic receptors in the locus ceruleus [9]. It also produces analgesia by stimulating α2-adrenergic receptors in the spinal cord [35]. Furthermore, dexmedetomidine decreases the circulating plasma catecholamines and attenuates central sympathetic activity [35]. The neuroprotective effects of dexmedetomidine in the hippocampus of rabbits after subarachnoid hemorrhage [17], on perinatal excitotoxic brain injury [30], in cell culture or neonatal asphyxia [23] have been demonstrated in a variety of in vivo and in vitro experimental studies. The effect of dexmedetomidine in SCI has not yet been studied. Thus, the aim of current study was to investigate the potential neuroprotective effect of dexmedetomidine against secondary injury after experimental spinal cord trauma in rabbit.
Materials and methods
The investigation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996) and approval has been received from our institutional Animal Ethics Committee.
Animals
Twenty-two New Zealand male and female rabbits, weighing between 2.4 and 3.0 kg were divided into three groups: sham (no drug or operation, n = 6), control (SCI + single dose of 1 mL saline intraperitoneally (i.p), after trauma; n = 8) and DEX (SCI + 1 μg/kg dexmedetomidine (Precedex, Abbott, Turkey) in 1 mL, i.p, after trauma, n = 8). The animals were allowed access to water and food ad libitum, presurgery and postsurgery period. The animals kept at the Animal Care Facility of Afyon Kocatepe University Experimental Research Centre.
Surgical procedures
All rabbits, in control and DEX groups, were anesthetized via intramuscular injection of xylazine (Bayer, Istanbul, Turkey) 5 mg/kg and ketamine hydrochloride (Parke Davis, Istanbul, Turkey) 50 mg/kg; breathing was continued spontaneously with room air. Rabbits were positioned prone on operating table. Under a sterile technique, a midline dorsal incision was done. The laminae and transverse processes of T6 to L2 were exposed by gentle blunt dissection of paravertebral muscles. A self-retaining retractor was placed in operation area, and then laminectomy was performed at T10. A balloon angioplasty catheter (Medtronic-146671, 2.0 mm × 20 mm, USA) was placed extradurally and sublaminary on thoracic spinal cord, upwards below T9. Inflation, slowly until 2 atm pressure was achieved and then was waited for 5 min in 2 atm pressure. After careful removing of balloon catheter, paravertebral fascia and skin were sutured with silk stitches. Just after trauma, animals in control group were given 1 mL of saline, in DEX group were given 1 μg/kg dexmedetomidine. A complete closure of surgical wound was achieved. The aim of use of baloon compression model was to form partial spinal cord lesion.
Neurological evaluation
Four and 24 h after surgery, rabbits were evaluated by an independent observer according to the Tarlov scoring system as described in Table 1 [29]. After last neurological evaluation, the rabbits in all groups were anaesthetized with ketamine (50 mg/kg) and cerebrospinal fluid (CSF), tissue samples from spinal cord, blood were taken. At the end of these procedures, all rabbits were sacrificed under deep anaesthesia.
Chemicals
Hydrogen peroxide, GSH, thiobarbituric acid, phosphate buffer, butylated hydroxytoluene, trichloroacetic acid, EDTA, [5,5-dithiobis-(2-nitrobenzoic acid)], disodium hydrogen phosphate, phenylendiamine, sodium azide, 2,4-dinitrophenylhydrazine, ethanol, hexane, sodium nitrite, sodium nitrate, sulfanilamide, N-(1-Naphthyl) ethylenediamine dihydrochloride, and vanadium (III) chloride were purchased from Sigma Chemical Co (Germany). All other chemicals and reagents used in this study were of analytical grade. In addition, SOD and GPx commercial kits (Randox, UK) were used.
Biochemical analysis
Whole blood was collected into heparinized tubes, and MDA and GSH levels were studied on the same day of admission. Blood was also collected into a polystyrene microtube, and after clotting, centrifuged at 1,000×g for 10 min at +4°C, and the serum was removed using EDTA-washed Pasteur pipettes. The red blood cells that remained after the removal of plasma were washed with isotonic saline (0.89% NaCl), and the buffy coat was removed. The red blood cells were washed again with isotonic saline and further processed for the preparation of haemolysate. The studied tissues were homogenized in tenfold volume of physiological saline solution by using a homogenizer (Ultra-Turrax T25, IKA; Werke 24,000 rpm; Germany). The homogenate was centrifuged at 10,000×g for 1 h to remove debris. Clear upper supernatant was taken, and tissue analyses were carried out in this fraction. The serum, erythrocyte, and tissue samples were stored in polystyrene plastic tubes at −70°C until the time of analysis. malondialdehyde (MDA), reduced glutathione (GSH), nitrate, nitrite, ascorbic acid, retinol, β-carotene and erythrocyte superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) activities were studied by spectrophotometer (Jenway 6305 UV/VIS).
MDA assay
MDA (as an important indicator of lipid peroxidation) levels were measured according to a method of Jain et al. [16]. The principle of the method was based on the spectrophotometric measurement of the color that occurred during the reaction of thiobarbituric acid with MDA. The concentration of thiobarbituric acid reactive substances (TBARS) was calculated by the absorbance coefficient of malondialdehyde–thiobarbituric acid complex and is expressed in nmol/ml.
GSH assay
Estimation of the reduced glutathione was measured by the method of Beutler et al. by a spectrophotometric method [7]. After lysing whole blood and the removal of precipitate, disodium hydrogen phosphate and DTNB solution were added and the color formed was read at 412 nm. The results were expressed in mg/dl.
Ascorbic acid, retinol and β-carotene analyses
Serum vitamin C (ascorbic acid) level was determined after derivatization with 2,4-dinitrophenylhydrazine [26]. The levels of β-carotene at 425 nm and vitamin A (retinol) at 325 nm were detected after the reaction of serum: ethanol: hexane at the ratio of 1: 1: 3, respectively [32].
Nitrate and nitrite analyses
The concentrations of nitrate and nitrite were detected by the methods of Miranda et al. [24]. Nitrite and nitrate calibration standards were prepared by diluting sodium nitrite and sodium nitrate in pure water. After loading the plate with samples (100 μl), the addition of vanadium (III) chloride (100 μl) to each well was rapidly followed by the addition of the Griess reagents, sulfanilamide (50 μl) and N-(1-naphthyl) ethylenediamine dihydrochloride (50 μl). The Griess solutions may also be premixed immediately prior to application to the plate. Nitrite mixed with Griess reagents forms a chromophore from the diazotization of sulfanilamide by acidic nitrite, followed by coupling with bicyclic amines, such as N-(1-naphthyl) ethylenediamine. Blank sample values were obtained by substituting a diluting medium for Griess reagent. Nitrite was measured in a similar manner, except that samples and nitrite standards were only exposed to Griess reagents. The absorbance at 540 nm was read to assess the total plasma level of nitrite and nitrate in all samples [24].
CAT, SOD and GPx analyses
CAT activity was measured according to the method of Aebi [1]. The principle of the assay is based on the determination of the rate constant [k (s − 1)] of hydrogen peroxide decomposition by catalase enzyme. The rate constant was calculated from following formula: k = (2.3/Δt)(a/b) log(A1/A2). In this formula, A1 and A2 are the absorbance values of hydrogen peroxide at t1 (0th s) and t2 (15th s) times, “a” is the dilution factor, and “b” is the hemoglobin content of erythrocytes. Erythrocyte SOD and GPx activities were studied on hemolysates by using commercial kits (Randox Laboratories, UK) [12, 28].
Determination of apoptosis in spinal cord
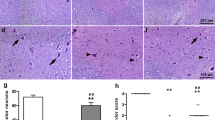
All the specimens were fixed in 10% neutral formalin and 5 μ sections obtained from these specimens were mounted on poly-l-lysine-coated slides. A TUNEL (terminal deoxynucleotidyl-transferase-mediated dUTP nick-end labeling) assay was used to identify double stranded DNA fragmentation, characteristic of DNA degradation by apoptosis. An ApopTag in situ apoptosis detection kit (Oncor, Gaithersburg, MD) was used according to the manufacturer’s directions. Briefly, tissue slides were deparaffinized, treated with proteinase K (20 μg/mL) for 15 min at room temperature, and then quenched in 3% hydrogen peroxide for 5 min. A positive control sample can be prepared from any tissue sample by treating with DNase I (Sigma). After rinsing in phosphate-buffered saline (PBS), pH 7.4, specimens were incubated in 1 × Equilibration Buffer (Oncor) for 10 min. Slides were next incubated with terminal deoxynucleotidyl transferase (Tdt) for 1 hour at 37°C, blocked with Stop/Wash Buffer (Oncor), and then incubated with peroxidase-conjugated antidigoxigenin antibody for 30 minutes at room temperature. Finally, slides were developed using diaminobenzidine (DAB; Sigma, St Louis, MO) and counterstained with methyl green.
On each slide, six fields were randomly selected and positive cells were counted at the healthy tissue which is situation at the peripheries of damaged areas. To quantitate extents of apoptosis, we recorded numbers of TUNEL positive cells in each group. Finally, overall mean counts for each set of specimens in each group were calculated, and mean group values were compared [13].
Statistics
Statistical analysis was performed with the Statistical Package for the Social Sciences for Windows (SPSS version 10.0, Chicago, IL, USA). All values were expressed as mean ± standard deviation. Statistical analysis of data was performed using a one-way analysis of variance (ANOVA) and Tukey’s posttest. A value of P < 0.05 was considered statistically significant.
Result
Neurological outcome:
Animals in sham group had normal neurological outcome (mean Tarlov score was 4). After 4 h of SCI all animals in control or DEX treated groups became paraparesic (mean Tarlov score was 2). On the other hand, 24 h after SCI, partial improvements were observed in both control and DEX treated groups (Table 2).
Biochemical analysis
Effects on whole blood MDA and GSH levels
The levels of MDA and GSH in whole blood of experimental groups were presented in Table 3. We determined that blood MDA levels were minimal in sham and maximal in control groups. MDA levels were very close and there was no significant difference between experimental (traumatized) groups. As for GSH levels in the blood, it was seen that there was an insignificant decrease in GSH levels in experimental groups when compared to those in sham group. GSH levels in experimental groups were very close. Present results show that, after experimental SCI, lipid peroxidation increases in the whole blood and DEX fails to prevent lipid peroxidation.
Effects on serum nitrite, nitrate and vitamins levels
Comparison of nitrate levels in the serum revealed that there was a significant decrease in nitrate level in experimental groups when compared to those in sham group. Nitrate levels in traumatized groups were very close and there was no significant difference between groups. On the other hand, SCI led to decrease in nitrite levels but there was no significant difference when compared to those in sham group. Values of ascorbic acid levels were very close and there was no significant difference between groups. However, SCI slightly increased the retinol and β-carotene levels in rabbits (Table 4).
Effects on antioxidant enzymes levels
Table 5 shows the activities of enzymatic antioxidants (SOD, CAT and GPx) in the erythrocytes of normal and experimental animals in each group. SOD and GPx activities significantly decreased in traumatized rabbits as compared to normal (sham) rabbits. The treatment of traumatized rabbits with DEX failed to prevent decrease in the SOD and GPx activities. On the other hand, CAT activity in sham was lower than that of experimental groups, and DEX treatment led to decrease in CAT activity.
Effects on CSF MDA, GSH nitrite and nitrate levels
Table 6 shows the levels of MDA in the CSF of normal and experimental animals in each group. The MDA levels were greatly increased in nontreatment traumatized rabbits as compared to normal. DEX treatment resulted in a decrease in CSF MDA levels. The GSH level greatly decreased in the CSF of untreated traumatized rabbits as compared to normal animals.
DEX treatment partly prevented the decrease of GSH in CSF. SCI led to increase in nitrite levels but there was no significant difference when compared to those in sham group. Moreover, DEX treatment partly suppressed the increase of nitrite level. Nitrate levels in traumatized groups were very close and there was no significant difference between groups.
Effects on spinal cord MDA and GSH levels
Table 7 shows the spinal cord MDA and GSH levels in normal and experimental animals in each group. The spinal cord MDA level slightly increased after injury and increase in DEX treated was lower that that of untreated groups. SCI led to increase in GSH level and there was no significant difference when compared to those in sham group. But the difference between control and DEX groups was significant.
Apoptosis
Apoptosis was seen especially glial cells of spinal cord. The results of our study showed that the number of apoptotic cell significantly increase after SCI (Table 2). Furthermore, DEX treatment could not prevent SCI-induced apoptosis (Fig. 1).
Discussion
Secondary injury in spinal cord trauma is believed to be a result of a several destructive process such as increase in some excitotoxic neuromediators, excessive calcium release, ROS and lipid peroxidation, odema, hemorrhage and ischemia, all of them can cause dysfunction and death in neuronal cells. Thus, after spinal cord trauma, permanent neurological deficits due to the loss of neuronal cells and axons may arise. Although some therapetic agents are used in SCI, but there is still no effective treatment for prevention of secondary injury. Nowadays, a number of studies have been focused on the treatment of secondary injury.
ROS-induced lipid peroxidation is well known that one of the most important component precipitating posttraumatic neuronal degeneration in the SCI. ROS react with lipids and cause peroxidative changes that result in elevated lipid peroxidation. The increase in lipid peroxidation may be related to decrease in enzymatic and nonenzymatic antioxidants of defense mechanisms. Due to large lipid content and high oxygenation, lipid peroxidation related cellular damage in central nervous system might be easily formed by ROS. Moreover, antioxidative defense system of neurons, unlike many other cells, are believed to be insufficient. Thus, susceptibility of the neurons from oxidative stress and permanent tissue damage caused by ROS are more than that of other cells.
In the present study, we tested whether the treatment of dexmedetomidine immediately after experimental SCI has protective effect on biochemical, histopathological and behavioral recovery. Various methods are used to show the level of lipid peroxidation in the organisms. MDA is an important and the most commonly used indicator of lipid peroxidation and its level increases in tissues when they are exposed to oxidative stress. Numerous studies have postulated that MDA is significantly increased in animals exposed to traumatic SCI [3, 4, 8]. The results presented in this study have also indicated that lipid peroxidation increases in all blood, CSF, spinal cord tissues of the rabbits. Thus, prevention of lipid peroxidation is important for attenuation of secondary damage. Some neuroprotective agents with antioxidant activity have been investigated in traumatic SCI and some of them have been found useful [3, 22]. Due to neuroprotective [10, 23] property, we used the dexmedetomidine in SCI and found that it only and slightly reduced lipid peroxidation in CSF and spinal cord tissue of the rabbits.
GSH, a ubiquitous thiol-containing tripeptide and an important cellular antioxidant, has various biological functions, one of which is scavenging of ROS, free radicals, and reactive metabolites. Its level is often increased in the blood and tissues as an adaptive response against oxidative stress. It also acts as a substrate for antioxidant and detoxifying enzyme GPx [25]. In the current study, GSH level in spinal cord clearly increased after SCI, and treatment with dexmedetomidine prevented this increase. But increase in the GSH level could not observed in whole blood, CSF. On the other hand, dexmedetomidine treatment partly leads to increase in the GSH level in CSF. It seems that increase in GSH level after SCI is not sufficient to prevent oxidative damage in spinal cord.
In the present study, values of serum ascorbic acid levels were very close and there was no significant difference between groups. Again, retinol and β-carotene levels were very close, but SCI minimally increased their levels. The cause of increase in the retinol and β-carotene levels of serum in SCI groups might be due to adaptive response against SCI-induced oxidative stress.
It is well known that nitric oxide (NO) possesses both antioxidant and pro-oxidant properties [6, 34]. An antioxidative property of NO has been shown by some investigators [15, 18]. NO is an effective chain-breaking antioxidant in free radical-mediated LPO, and reacts rapidly with peroxyl radicals as a sacrificial chain-terminating antioxidant. In the present study, we also found that LPO was increased while the serum levels of nitrate and nitrite were decreased in the SCI-applicated rabbits. On the other hand, unlike serum, the levels of nitrate and nitrite in CSF increased after SCI in rabbits, and dexmedetomidine treatment partly prevented this increase. Effect of SCI on NO pathway, may be mediated either by an activation or inhibition of NO synthase. Thus, it may be suggested that the effect of dexmedetomidine against SCI-induced oxidative stress in CSF, at least in part, may be related to inhibition of nitrosative stress.
Apart from the nonenzymatic antioxidants, enzymatic antioxidants such SOD, CAT and GPx play an important role in preventing the cells from oxidative damage. SOD is an enzymatic antioxidant which catalyzes the conversion of superoxide radical to hydrogen peroxide and molecular oxygen. Other enzymatic antioxidant CAT catalyses the reduction of hydrogen peroxides and protects the tissues against to reactive hydroxyl radicals. GPx, a selenoprotein, oxidizes GSH to glutathione disulfide (GSSG) which is then reduced to GSH by glutathione reductase, and reduces the hydroperoxides. Decreased activities of enzymatic antioxidants SOD and GPx have been well documented in SCI [19]. The present study revealed that SCI lead to significant decrease in the SOD and GPx activities when compared to those in sham group (P < 0.05). Moreover, there was no significant change in SOD and GPx activities in dexmedetomidine administered group when compared to those in control group. The decreased activity of SOD and GPx in SCI, as reported previous which could be due to increased consumption for free radicals’ detoxification. In previous study, increased CAT activity in SCI has been shown [5, 20]. We determined that CAT activity was insignificantly elevated as a result of SCI and this elevation was prevented by the treatment with dexmedetomidine. The results obtained in this study revealed that, dexmedetomidine may partly modulate the SCI-induced changes in the activity of CAT and counteracts the imbalance produced by the generation of excessive quantities of ROS.
Used trauma method in the present study for SCI led to significant neurological deficite. Some neurological deficits after traumatic SCI may arise from the first hours on, up to the first week and neurological recovery is seen after a long time. Tarlov’s score is frequently used behavioral tests for the revealing of functional recovery after SCI in animals. Results obtained from this study showed that DEX treatment failed to prevent traumatic SCI related neurodeficite. In spite of some better biochemical results in the dexmedetomidine treated group, administration of dexmedetomidine was insufficient to prevent spinal cord changes by neuronal and glial apoptosis, and all SCI subjected groups revealed similar histopathological results.
In conclusion, traumatic SCI leads to increase in the lipid peroxidation and decreases enzymatic or nonenzymatic endogenous antioxidative defense systems. Again, SCI leads to apoptosis in spinal cord. DEX treatment slightly prevented lipid peroxidation and augmented endogenous antioxidative defense systems in CSF or spinal cord tissue, but failed to prevent apoptosis or neurodeficite after traumatic SCI. Thus, it may be said that dexmedetomidine does not produce beneficial results in SCI in rabbits. But, further detailed experimental studies are needed to clarify the effects of dexmedetomidine in SCI.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Anderson DK, Hall ED (1993) Pathophysiology of spinal cord trauma. Ann Emerg Med 22:987–992. doi:10.1016/S0196-0644(05)82739-8
Ates O, Cayli SR, Altinoz E, Gurses I, Yucel N, Kocak A, Yologlu S, Turkoz Y (2006) Effects of resveratrol and methylprednisolone on biochemical, neurobehavioral and histopathological recovery after experimental spinal cord injury. Acta Pharmacol Sin 27:1317–1325. doi:10.1111/j.1745-7254.2006.00416.x
Ates O, Cayli SR, Gurses I, Turkoz Y, Tarim O, Cakir CO, Kocak A (2007) Comparative neuroprotective effect of sodium channel blockers after experimental spinal cord injury. J Clin Neurosci 14:658–665. doi:10.1016/j.jocn.2006.03.023
Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE (1997) Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res 765:283–290. doi:10.1016/S0006-8993(97)00573-8
Blanchard B, Pompon D, Ducrocq C (2000) Nitrosation of melatonin by nitric oxide and peroxynitrite. J Pineal Res 29:184–192. doi:10.1034/j.1600-079X.2000.290308.x
Beutler E, Dubon O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 6:882–888
Cayli SR, Ates O, Karadag N, Altinoz E, Yucel N, Yologlu S, Kocak A, Cakir CO (2006) Neuroprotective effect of etomidate on functional recovery in experimental spinal cord injury. Int J Dev Neurosci 24:233–239. doi:10.1016/j.ijdevneu.2006.04.003
Cormack JR, Orme RM, Costello TG (2005) The role of a2-agonists in neurosurgery. J Clin Neurosci 12:375–378. doi:10.1016/j.jocn.2004.06.008
Cosar M, Eser O, Fidan H, Sahin O, Buyukbas S, Ela Y, Yagmurca M, Ozen OA (2009) The neuroprotective effect of dexmedetomidine in the hippocampus of rabbits after subarachnoid hemorrhage. Surg Neurol 71:54–59
Fehlings MG, Baptiste DC (2005) Current status of clinical trials for acute spinal cord injury. Injury 36:113–122. doi:10.1016/j.injury.2005.06.022
Flohe L, Otting F (1984) Superoxide dismutase assays. Methods Enzymol 105:93–104. doi:10.1016/S0076-6879(84)05013-8
Ha KY, Kim YH, Rhyu KW, Kwon SE (2008) Pregabalin as a neuroprotector after spinal cord injury in rats. Eur Spine J 17:864–872. doi:10.1007/s00586-008-0653-6
Hall ED (1993) Lipid peroxidants in acute central nervous system injury. Ann Emerg Med 22:1022–1027. doi:10.1016/S0196-0644(05)82745-3
Hayashi K, Noguchi N, Niki E (1995) Action of nitric oxide as an antioxidant against oxidation of soybean phosphatidylcholine liposomal membranes. FEBS Lett 370:37–40. doi:10.1016/0014-5793(95)00786-9
Jain SK, McVie R, Duett J, Herbst JJ (1989) Erythrocyte membrane lipid peroxidase and glycolylated hemoglobin in diabetes. Diabetes 38:1539–1543. doi:10.2337/diabetes.38.12.1539
Janke EL, Sarma S (2006) Dexmedetomidine and neuroprotection. Seminars in Anesthesia. Perioper Med Pain 25:71–76. doi:10.1053/j.sane.2006.02.002
Jessup W, Mohr D, Gieseg SP, Dean RT, Stocker R (1992) The participation of nitric oxide in cell free and its restriction of macrophage-mediated oxidation of low-density lipoprotein. Biochim Biophys Acta 1180:73–82
Kanter M, Coskun O, Kalayci M, Buyukbas S, Cagavi F (2006) Neuroprotective effects of Nigella sativa on experimental spinal cord injury in rats. Hum Exp Toxicol 25:127–133. doi:10.1191/0960327106ht608oa
Kaynar MY, Hanci M, Kuday C, Belce A, Gumustas K, Kokoglu E (1994) Changes in the activity of antioxidant enzymes (SOD, GPX, CAT) after experimental spinal cord injury. Tokushima J Exp Med 41:133–136
Kurihara M (1985) Role of monoamines in experimental spinal cord injury in rats. Relationship between Na + −K + −ATPase and lipid peroxidation. J Neurosurg 62:743–749
Liu JB, Tang TS, Yang HL (2006) Antioxidation of quercetin against spinal cord injury in rats. Chin J Traumatol 9:303–307
Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, Maze M (2004) Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol 11:87–97. doi:10.1016/j.ejphar.2004.08.044
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71. doi:10.1006/niox.2000.0319
Nogues MR, Giralt M, Romeu M, Mulero M, Sanchez-Martos V, Rodriguez E, Acuna-Castroviejo D, Mallol J (2006) Melatonin reduces oxidative stress in erythrocytes and plasma of senescence-accelerated mice. J Pineal Res 41:142–149. doi:10.1111/j.1600-079X.2006.00344.x
Omaye ST, Turnbul JD, Savberlich HE (1979) Ascorbic acid analysis II. Determination after derivatisation with 2.2. dinitrophenylhidrazine. Selected methods for determination of ascorbic acid in animal cells tissues and fluids. In: McCormick DB, Wright LD (eds) Methods in Enzymology, vol 62. Academic Press, New York, pp 7–8
Ozdemir M, Cengiz SL, Gurbilek M, Ogun TC, Ustun ME (2005) Effects of magnesium sulfate on spinal cord tissue lactate and malondialdehyde levels after spinal cord trauma. Magnes Res 18:170–174
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Papakostas JC, Matsagas MI, Toumpoulis IK, Malamou-Mitsi VD, Papa LS, Gkrepi C, Anagnostopoulos CE, Kappas AM (2006) Evolution of spinal cord injury in a porcine model of prolonged aortic occlusion. J Surg Res 133:159–166. doi:10.1016/j.jss.2005.10.007
Paris A, Mantz J, Tonner PH, Hein L, Brede M, Gressens P (2006) The effects of dexmedetomidine on perinatal excitotoxic brain injury are mediated by the alpha2A-adrenoceptor subtype. Anesth Analg 102:456–461. doi:10.1213/01.ane.0000194301.79118.e9
Samantaray S, Sribnick EA, Das A, Knaryan VH, Matzelle DD, Yallapragada AV, Reiter RJ, Ray SK, Banik NL (2007) Melatonin attenuates calpain upregulation, axonal damage and neuronal death in spinal cord injury in rats. J Pineal Res 44:348–357. doi:10.1111/j.1600-079X.2007.00534.x
Suzuki I, Katoh N (1990) A simple and cheap method for measuring serum vitamin A in cattle using spectrophototmeter. Jpn J Vet Sci 52:1281–1283
Tator CH, Fehlings MG (1991) Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 75:15–26
Taysi S, Koc M, Buyukokuroglu ME, Altinkaynak K, Sahin YN (2003) Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J Pineal Res 34:173–177. doi:10.1034/j.1600-079X.2003.00024.x
Tsifansky MD, Schmitt CG, Muñoz RA (2007) Dexmedetomidine: do we know enough? Pediatr Crit Care Med 8:492–493
Tsutsumi S, Ueta T, Shiba K (2006) Effects of the Second National Acute Spinal Cord Injury Study of high-dose methylprednisolone therapy on acute cervical spinal cord injury-results in spinal injuries center. Spine 31:2992–2997. doi:10.1097/01.brs.0000250273.28483.5c
Yoshino S, Yone K (1998) Role of norepinephrine and excitatory amino acids in edema of the spinal cord after experimental compression injury in rats. J Orthop Sci 3:54–59. doi:10.1007/s007760050021
Acknowledgments
This work was supported by Afyon Kocatepe University Scientific Research Fund (Project No: 051.TIP.17) and it was presented as e-poster in Spineweek 2008 (EuroSpine) in Geneva, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aslan, A., Cemek, M., Eser, O. et al. Does dexmedetomidine reduce secondary damage after spinal cord injury? An experimental study. Eur Spine J 18, 336–344 (2009). https://doi.org/10.1007/s00586-008-0872-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-008-0872-x