Abstract
The over-expression of excitotoxic neurotransmitter, such as glutamate, is an important mechanism of secondary injury after spinal cord injury. The authors examined the neuroprotective effect of pregabalin (GP) which is known as to reduce glutamate secretion, in a rat model of spinal cord injury. Thirty-two male Sprague–Dawley rats were randomly allocated to four groups; the control group (contusion injury only), the methylprednisolone treated group, the minocycline treated group and the GP treated group. Spinal cord injury was produced by contusion using the New York University impactor (25 g-cm, at the 9th–10th thoracic). Functional evaluations were done using the inclined plane test and a motor rating scale. Anti-apoptotic and anti-inflammatory effects were evaluated by in situ nick-end labeling staining technique (TUNEL) and immunofluorescence staining of cord tissues obtained at 7 days post-injury. Pregabalin treated animals showed significantly better functional recovery, and anti-apoptotic and anti-inflammatory effects. Mean numbers of TUNEL positive cells in the respective groups were 63.5 ± 7.4, 53.6 ± 4.0, 44.2 ± 3.9 and 36.5 ± 3.6. Double staining (TUNEL and anti-CC1) for oligodendrocyte apoptosis, was used to calculate oligodendrocyte apoptotic indexes (AI), using the following formula AI = (No. of doubly stained cells/No. of anti-CC1 positive cells) × 100. Mean group AIs were 88.6, 46.7, 82.1 and 70.3%, respectively. Mean numbers of activated microglia (anti-OX-42 positive cells) in high power fields were 29.8 ± 3.9, 22.7 ± 4.1, 21.0 ± 3.9 and 17.8 ± 4.3, respectively. This experiment demonstrates that GP can act as a neuroprotector after SCI in rats, and its anti-apoptotic and anti-inflammatory effects are related to its neuroprotective effect. Further studies are needed to unveil the specific mechanism involved at the receptor level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurologic deficits that develop after spinal cord injury (SCI) are the result of a primary mechanical insult and subsequent, multifaceted secondary responses, which involve excitotoxicity, ionic dysfunction, the generation of toxic free radicals and ischemia-reperfusion [5, 7]. Oligodendrocyte apoptosis is a prominent feature of these secondary mechanisms, and is related to axonal functional loss [12, 19].
Excitotoxicity, is one of these secondary mechanisms, and describes the pathological states caused by excessive or prolonged exposure to excitatory neurotransmitters, such as, glutamate, which results in neuronal cell death [20]. Initially, the term excitotoxicity was applied to neurons, but further studies revealed that glial damage is also involved [12, 16, 17]. In particular, in cases of traumatic SCI, excitotoxicity has been highlighted as an important secondary mechanism. The influences of excitotoxicity on white matter, especially on oligodendrocytes and axons, have been demonstrated [13, 17, 19, 20, 31], and several possible mechanisms have been presented. Receptors in membrane of cell body and myelin are related and their aberrant activations alter ionic channels and induce apoptosis and the destruction of myelin and axons. Excessive accumulation of glutamate alters the functions of the ionic channels of neurons and glia, especially of Na+ channel, and this is followed by the induction of apoptosis [16, 17]. Non-N-methyl-d-aspartate (non-NMDA) receptor is known to be involved in this mechanism. As these mechanisms related to excitotoxicity have been unveiled, several experimental trials have been performed, which include the application of NBQX (2, 3-dihydroxy-6-nitro-7sulfamoyl-benzo(f)quinoxaline), a non-NMDA receptor antagonist and anti-excitotoxic agent [29, 30]. Results appear promising.
Pregabalin (GP) is used to control neurogenic pain in various clinical conditions, such as, diabetic neuropathy, neuralgia, and complex regional pain syndrome [25]. Its chemical structure is similar to that of GABA. However, it neither acts like GABA, nor binds to GABA receptor. Pregabalin is known to bind at the α2-δ subunit of voltage-controlled calcium channel. Moreover, its potent binding at this site reduces Ca2+ influx at presynaptic nerve endings, and therefore, reduces the release of several neurotransmitters like glutamate and noradrenalin [11, 21, 25]. Interventions targeting excitotoxicity are considered novel in terms of intervening in the process that follows SCI. The present study was designed to confirm whether the pharmacological mechanism of GP alleviates the secondary injuries, associated with excitotoxicity after SCI. To elucidate its neuroprotective effect, we also incorporated two other agents, i.e., methylprednisolone (MP), that is the only proven clinically used agent, and minocycline (MC), that is a derivative of tetracycline and has been shown to mediate neuroprotection in various experimental models of CNS diseases. Furthermore, MC has been shown to have anti-apoptotic and anti-inflammatory effects [15, 23, 24, 28].
Materials and methods
Subjects
A total of 32 adult male Sprague-Dawley rats (body weights 300–350 g) were used in this study. Animals were kept under standardized conditions (four rats per cage, 20–24°C, 45–65% relative humidity, under a 12-h light/dark cycle) and given food and water ad lib throughout the study. These rats were randomly assigned to one of the following four groups before operations; the control group (n = 8, spinal cord contusion only), the MP treated group (n = 8, spinal cord contusion followed by intraperitoneal (i.p.) injections of MP, as described below), the MC treated group (n = 8, spinal cord contusion followed by i.p. injections of MC), the GP treated group (n = 8, spinal cord contusion followed by i.p. injections of GP).
Operation technique
The rats were anesthetized with ketamine (50 mg/kg) and rompun (2 mg/kg, i.p.). Their backs were shaved and sterilized with antiseptic betadine and lamincetomy was performed at the T9 level after exposing paravertebral muscles through T8–10. Spinal contusion was induced using the New York University (NYU) impactor (10 g rod dropped from a height of 2.5 cm). The NYU impactor, which utilizes an electronically released plunger, produces reproducible injuries in test animals. The 25 g-cm lesion was chosen to evaluate the neuroprotective effect of experimental trials in a severe SCI in which the rats would have insufficient motor recovery to ambulate over several weeks. Postoperatively, 5 mg gentamycin was administrated intramuscularly. Postoperative care procedures involved providing rats with drinking water, softened rat chow and regular pellets in their cages. Bladders were emptied manually twice a day during the experiment. All of surgical interventions and pre surgical and post surgical animal care were provided in accordance with the requirements of the Laboratory Animal Welfare Act and the Guidelines and Policies for Rodent Survival Surgery stipulated by the Animal Studies Committee of The Catholic University of Korea.
Pharmacological administration
In the MP group, 30 mg/kg MP was administered i.p. at 30 min, 12, and 24 h after SCI. In the MC group, 30 mg/kg minocycline (Sigma, St Louis, MO) was administered i.p. at 30 min, 12, 24, 36 and 48 h after contusion, and in the GP group, 30 mg/kg GP (Pfizer Pharm, New York, NY) was administered i.p. at 30 min and every 12 h for postoperative 2 days postoperatively. Minocycline (400 mg) was dissolved in 9.6 ml of sterile distilled water and then sonicated, because it was previously proven to be hardly soluble.
Behavioral assessments
Motor function scores
The motor function scale described by Gale et al. [10] was used to evaluate motor function (Table 1). The animals were allowed to move freely, and an observer, unaware of the treatment administered, observed the rats for at least 1 min on a daily basis and recorded hip, knee and ankle joint movements.
Inclined plane test
All rats were also assessed using a second behavioral task, the inclined plane test, which assesses the animal’s ability to maintain its position on a board, which is raised in increments of 5°. The test is thus used as an index of hindlimb strength. The maximum angle, at which a rat was able to maintain its position for at least 5 s, was defined as the inclined plane score. Rats were subjected to this test at 7 days postoperatively before sacrifice.
Tissue preparation
On the seventh postoperative day, rats were sacrificed under deep anesthesia by administering ketamine and rompun i.p. Transcardiac perfusion was performed with 100 ml of buffered saline followed by 500 ml of 4% paraformaldehyde (pH 7.4) and at least 1.5-cm segment of spinal cord containing the contusion site was excised. Specimens were fixed in cold 4% paraformaldehyde for overnight, incubated 30% sucrose solution at room temperature for 4 h, and stored at −70°C.
Immunofluorescence staining
The tissue blocks were cut into 10 μm sections transversely with respect to the long axis of the spinal cord. The sections of epicenter lesion were used for immunofluorescence stain. The sections were mounted on gelatin-coated slides, rinsed for three times 10 min rinses with PBS and blocked with 0.15%Triton X-100 and 5% normal horse serum (NHS) in 0.1 mM PBS for 30 min. They were then exposed to mouse monoclonal antibody CC-1 (Calbiochem, Darmstadt, Germany), a specific oligodendrocyte marker, at a dilution of 1:20 in 0.1 M PBS + 0.15% Triton X-100 + 1% NHS overnight. The following day, the slides were incubated for 2 h with a 1:100 dilution of the secondary antibody, Texas red-conjugated anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA). The slides were then rinsed with 0.1 mM PBS five times, covered, and stored in a freezer. After thawing, slides were photographed using a confocal analysis system (Olympus Microscope Confocal/Image Analysis system, Tokyo, Japan). Cells showing red fluorescence were counted in a 75 × 250 μm area, centered at the epicenter of injured spinal cords. They were also double stained to detect oligodendrocyte apoptosis using the in situ nick-end labeling (TUNEL) staining technique using an apoptosis detection kit (Roche, Mannheim, Germany). Deparaffinized tissue sections were incubated with proteinase K (20 μg/mL). Tissue sections were treated in 3% H2O2 to inhibit endogenous peroxide activity and then incubated in 1× equilibrium buffer at room temperature for 30 min. They were then treated with digoxingenin-labeled deoxynucleotidyl transferase for 1 h at 37°C and the slides were washed in stop/wash buffer for 10 min at room temperature. Tissue sections incubated with anti-digoxigenin-peroxidase antibody at room temperature for 30 min and stained with anti-fluorescein isothiocyanate (FITC) (Jackson ImmunoResearch). They were also stained with monoclonal antibody OX-42 (Serotec, Oxford, UK), which labels complement type 3 receptor on microglia and macrophages, at a dilution of 1:500 in 0.1 M PBS + 0.15% Triton + 1%NHS overnight. The following day, the slides were incubated for 2 h with a 1:100 dilution of secondary antibody; FITC conjugated anti-mouse IgG (Jackson ImmunoResearch).
Quantification of immunofluorescent cells
Morphometric analysis of cells positive for fluorescence was performed under high-power magnification (×400) in a blind fashion. On each slide, six fields were randomly selected and to eliminate possible bias due to hemorrhage and necrosis, positive cells were counted at the peripheries of cystic lesions. To quantitate extents of apoptosis, we recorded numbers of TUNEL positive cells in each group. Finally, overall mean counts for each set of specimens in each group were calculated, and mean group values were compared. Cells doubly stained for TUNEL and anti CC-1 antibody, appeared as yellow bodies in the merged images generated by overlying the two channels. Yellow bodies that represented apoptotic oligodendrocytes were counted at the epicenters of injured spinal cords. The mean numbers were recorded for all specimens and groups were compared.
Statistical analysis
For statistical analysis, all data were assessed using the Kruskal–Wallis test (a nonparametric test) followed by pair-wise multiple comparisons using the Mann–Whitney U test. P values less than 0.05 were considered to indicate statistical significance.
Results
Functional recovery after SCI
Motor function score
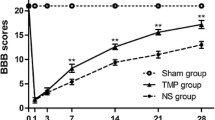
We examined the effect of GP on recovery after SCI and compared this with the effects of MP and MC, which were proved and tried for treatment of acute SCI. Hindlimbs were paralyzed in all rats immediately after injury, and hindlimb movements recovered with a time though degrees of recovery differed in the four groups. The time courses of functional recovery, as determined using Gale rating score, were recorded (Fig. 1). Motor function score results are presented as mean values and SEMs (Table 2). The mean motor function scores of all four groups were significantly different from each other (P = 0.01), by the Kruskal–Wallis test. The mean motor function scores of the control and GP groups were also significantly different (P = 0.01), by the Mann–Whitney U test. The recovery of hindlimbs motor function in the GP group was also superior to those of the MP or MC group (P < 0.05).
Inclined plane test
The results of the inclined plane test are presented as a mean values and SEMs. The mean inclined plane scores of all groups were significantly different (P = 0.01), by the Kruskal–Wallis test. Comparing the inclined plane scores between control and GP group, there was also a statistical significance (P = 0.01), by the Mann–Whitney U test. Results for the GP group were better than those of the MP and MC groups (P < 0.05).
Cystic lesions of spinal cord
Morphometric features at the epicenters stained by H&E stain showed that cystic lesions were usually located in the dorsal column and that cyst sizes were greater in the control group than in the other three groups (Fig. 2). However, no statistical analysis was performed.
In situ nick-end labeling staining technique stain and apoptosis of oligodendrocyte
Trauma injury results in the extensive apoptosis of neurons and glia cells in the spinal cord. We investigated whether GP inhibits the neuron and glial cell death after SCI by TUNEL staining. TUNEL positive cells (TPC) were mainly distributed at the periphery of the damaged sites, and extended to the ventral side of cystic lesions. The mean numbers of TPC in each group were 63.5 ± 7.4, 53.6 ± 4.0, 44.2 ± 3.9, and 36.5 ± 3.6, respectively. TPC were significantly numerous in the control group than in the other three groups (P < 0.05) and the number of TPC in the GP group was significantly lower than that in the MP and MC group (P < 0.05) (Fig. 3). In terms of double staining (TUNEL and anti-CC1) for apoptotic oligodendrocytes, apoptotic indexes (AI) were calculated as follows: AI = (No. of double stained cells/No. of anti-CC1 positive cells) × 100. The mean AIs of the list group were 88.6, 46.7, 82.1, and 70.3%, respectively. The apoptotic indexes of oligodendrocyte were significantly lower in the MP and GP groups than in the other two groups (P < 0.05) (Fig. 4).
Representative pictures of In situ nick-end labeling (TUNEL) staining techniqueTUNEL staining in spinal cord sections and the numbers of TUNEL positive cells. Values are expressed as mean ± SEM for each group. Significant differences among the groups were analyzed with the Kruskal–Wallis test (*P < 0.05), and significant differences between the GP group and the MP or MC group were analyzed with the Mann–Witney U test (†P < 0.05, vs the MP and MC group). (N = 8, MP methylprednisolone, MC minocycline, GP pregabalin)
Apoptotic index (AI) of oligodendrocytes; defined as the number of double-stained cells expressed as a percentage of the total number of anti-CC1 stained cells. AIs in the MP and GP groups were significantly lower than in the control and MC groups (Mann–Witney U test, *P < 0.05). (N = 8, Error limits were shown as ±SEM, MP methylprednisolone, MC minocycline, GP pregabalin)
Activation of microglia
Anti-OX-42 positive cells were mainly detected at the peripheries of injured sites (Fig. 5). Mean numbers of anti-OX-42 positive cells in high power fields were 29.8 ± 3.9, 22.7 ± 4.1, 21.0 ± 3.9, and 17.8 ± 4.3 in the name groups, respectively. The higher mean number of anti-OX-42 positive cells in the control group was significant (P = 0.01). The number of anti-OX-42 positive cells in the GP group was significantly lower than that in the MP and MC group (P < 0.05). These findings indicate that GP significantly reduced the activation of microglia and that its effect is comparable to that of MC treatment.
Photomicrographs of cord specimens from each of the four groups. The uptakes of OX-42 positive cells were observed near injured site. Values are expressed as mean ±SEM for each group. Significant difference among the groups were analyzed with the Kruskal–Wallis test (* P < 0.05), and significant differences between the GP and the other treated groups were analyzed with the Mann–Witney U test (†P < 0.05 vs the MP and MC group) (N = 8, MP methylprednisolone, MC minocycline, GP pregabalin)
Discussion
Despite extensive researches, clinical advancements, and improved rehabilitation programs, spinal cord injuries continue to be a major cause of disability and mortality. After initial trauma, injured spinal cords undergo multiple secondary pathological changes and these secondary effects are targets for developmental neuroprotective strategies. Secondary injury mechanisms include, but are not limited to, ischemia-reperfusion, inflammation, the production of toxic free radicals, cytoskeletal degradation, excitotoxicity, and the induction of intrinsic and extrinsic apoptotic pathways [2, 6, 8, 22, 26, 27]. Moreover, these mechanisms are both complicated and interactive. Excitotoxicity refers to the pathological process induced by the over-expression of an excitatory neurotransmitter, and emerging evidence indicates that excitotoxicity plays a key role in both neuronal and glia cell death [13, 16, 19, 20, 31]. A receptor for glutamate, a representative excitatory neurotransmitter, is present on glia cells in white matter of the spinal cord, and their apoptosis, especially that of oligodendrocytes, is well understood. The glutamate receptors comprise the three ionotrophic receptors; N-methyl-d-aspartate (NMDA), (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) and kainite [20]. AMPA–kainate receptor has been shown to be the most important with respect to excitotoxicity, and controls ion permeability, especially, cell membrane Ca2+ permeability, increases in which elevate intracellular Ca2+ concentration and induces apoptosis via mitochondrial dysfunction and the activation of specific enzymes, which trigger the caspase cascade [1]. The expression of glutamatergic AMPA–kainate receptor in oligodendrocyte [1], the protective effect of AMPA–kainate antagonist in experimental SCI, and the vulnerability of oligodendrocytes to excitotoxicity suggest that glutamatergic excitotoxicity is associated with neuronal and oligodendrocyte apoptosis in SCI. Recently, oligodendrocytes have received much attention as potential neuroprotective targets due to their intimate involvements in axonal function.
Pregabalin was devised to control neurogenic pain in different clinical situations, e.g., diabetic neuropathy, neuralgia and complex regional pain syndrome. Its chemical structure is similar to GABA, but it does not act like GABA, and in particular, does not bind to GABA receptor. Moreover, GP is known to potently bind to the α2-δ subunit of voltage-controlled calcium channels, and its binding at this site reduces Ca2+ influx at presynaptic nerve endings and therefore, reduces the release of several neurotransmitters, which included glutamate and noradrenalin [3, 9, 11, 21]. Given this theoretical background, we wondered whether GP might act as a neuroprotector after SCI, and thus we examined the possibility that GP acts as has a neuroprotective agents. This study demonstrates, for the first time, that GP induces substantial functional recovery after SCI, and in particular, that it attenuates cellular apoptosis (decreased TPCs), especially apoptosis of oligodendrocytes (decreased AIs), and the activation of microglia. Moreover, the neuroprotective effects of GP were found to be superior to those of MP and MC. Although further studies on the detail pharmacological mechanisms are clearly required, the actions of GP on neuropeptide release could be the result of a direct action on the peptide containing neuronal cells or an indirect action on excitatory or inhibitory neuro-modulator release. Two results of the present study are difficult to reconcile, namely, that the MP group had the lowest oligodendrocyte AI and a relatively high TPC count and the other concerns the observed anti-inflammatory effect of GP. In general, it is considered that steroid therapy can enhance neurologic recovery after SCI augmenting behavior recovery and it is in fact the most widely used therapy in clinical conditions [2, 32]. Nevertheless, exact mechanism of action is not fully understood. The most reliable proposed mechanisms are the inhibition of lipid peroxidation and an anti-inflammatory effect [2, 14]. However, although a few studies have been conducted on the effects of steroid on neuronal and glial apoptosis, results are controversial. Yu et al. [32] reported that early treatment with MP after SCI reduces inducible nitric oxide synthetase expression, and concluded that MP acts as a neuroprotector by inhibiting neuronal apoptosis. On the other hand, Li et al. [18] concluded that MP treatment after SCI does not reduce the expression of caspase-3, a key enzyme of apoptosis, after rat spinal cord transection, and Diem et al. [4] reported that steroid treatment has a negative effect on neuronal apoptosis in the autoimmune disease of the optic nerve. According to our results, the MP group had the lowest oligodendrocyte AI, and a high TPC count. We presumed that MP, even though it has an anti-apoptotic effect of oligodendrocyte, did not show anti-apoptotic effect on neuron. To our knowledge, it has not been previously reported that MP has different effects on neuronal and glial cell apoptosis. Further studies on these effects of MP are warranted to clarify the positive or negative effect of that. In terms of the anti-inflammatory effect of GP, confirmatory research and additional study on it effects on microglia should do much to clarify the mechanism underlying its anti-inflammatory effect. Further studies on the effect of GP are mandatory to clarify the protective effect on neuron and the anti-inflammatory effect in regard of the quantitative analysis of RNA and protein levels.
Our data suggest that GP has a novel neuroprotective effect, and that this may be attributable to its anti-apoptotic and anti-inflammatory effects. Thus, an understanding of the mode of action of GP will undoubtedly assist in the development of more potent and effective analogs for the treatment of SCI.
References
Agrawal SK, Fehlings MG (1996) Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na(+)-K(+)-ATPase, the Na(+)-H+ exchanger, and the Na(+)-Ca2+ exchanger. J Neurosci 16(2):545–552
Bracken MB, Shepard MJ, Holford TR et al (1997) Administration of methyprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 277:1597–1604
Coderre TJ, Kumar N, Lefebvre CD, Yu JS (2005) Evidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem 94:1131–1139
Diem R, Hobom M, Maier K et al (2003) Methylprednisolone increases neuronal apoptosis during autoimmune CNS inflammation by inhibition of an endogenous neuroprotective pathway. J Neurosci 23(18):6993–7000
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24(5):254–264
Dumont RJ, Verma S, Okonkwo DO, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part II: contemporary pharmacotherapy. Clin Neuropharmacol 24(5):265–276
Enomoto M, Wakabayashi Y, Qi ML, Sinomiya K (2004) Present situation and future aspect of spinal cord regeneration. J Orthop Sci 9:108–112
Fehlings MG, Baptiste DC (2005) Current status of clinical trials for acute spinal cord injury. Injury 36(S):B113–122
Fehrenbacher JC, Taylor CP, Vasko MR (2003) Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain 105:133–141
Gale K, Kerasidis H, Wrathall JR (1985) Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol 88(1):123–134
Joshi I, Taylor CP (2006) Pregabalin action at a model synapse: binding to presynaptic calcium channel alpha2-delta subunit reduces neurotransmission in mice. Eur J Pharmcol 553:82–88
Karadottir R, Attwell D (2007) Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience 146:1426–1438
Karadottir R, Cavelier P, Bergersen LH, Attwell D (2005) NMDA receptors are expressed in oligodendrocytes and activated in ischemia. Nature 438:1162–1166
Kiwerski JE (1993) Application of dexamethasone in the treatment of acute spinal cord injury. Injury 24(7):457–460
Lee SM, Yune TY, Kim SJ et al (2004) Minocycline inhibits apoptotic cell death via attenuation of TNF-a expression following iNOS/NO induction by lipopolysaccharide in neuron/glia co-culture. J Neurochem 91:568–578
Li S, Mealing GA, Morley P, Stys PK (1999) Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+dependent glutamate transport. J Neurosci 19(14):RC 16
Li S, Stys PK (2000) Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci 20(3):1190–1198
Li X, Oudega M, Dancausse HA, Levi AD (2000) The effect of methylprednisolone on caspase-3 activation after rat spinal cord transection. Restor Neurol Neurosci 17(4):203–209
Liu XZ, Xu XM, Hu R et al (1997) Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci 17:5395–5406
Park E, Velumian AA, Fehlings MG (2004) The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 21(6):754–774
Shneker BF, McAuley JW (2005) Pregabalin: a new neuromodulator with broad therapeutic indications. Ann Pharmacother 39(12):2029–2037
Shuman SL, Bresnahan JC, Beattie MS (1997) Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res 50:798–808
Stirling DP, Khodarahmi K, Liu J et al (2004) Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci 24(9):2182–2190
Stirling DP, Khodarahmi K, Steeves JD, Tetzlaff W (2005) Minocycline as a neuroprotective agent. Neuroscientist 11(4):308–322
Tassone DM, Boyce E, Guyer J, Nuzum D (2007) Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther 29(1):26–48
Tator CH (2006) Review of treatment trials in human spinal cord injury: Issues, difficulties, and recommendations. Neurosurgery 59(5):957–987
Tator CH, Fehlings MG (1991) Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 75(1):15–26
Wells JE, Hurlbert RJ, Fehling MG, Yong VW (2003) Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain 126:1628–1637
Wrathall JR, Teng YD, Choiniere D (1996) Amelioration of functional deficits from spinal cord trauma with systemically administered NBQX, an antagonist of Non-N-methyl-aspartate receptors. Exp Neurol 137(1):119–126
Wrathall JR, Teng YD, Marriott R (1997) Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Exp Neurol 145:565–573
Xu GY, Hughes MG, Ye Z, Hulsebosch CE, McAdoo DJ (2004) Concentrations of glutamate released following spinal cord injury kill oligodendrocytes in the spinal cord. Exp Neurol 187(2):329–336
Yu Y, Matsuyama Y, Nakashima S, Yanase M, Kiuchi K, Ishiguro N (2004) Effects of MPSS and a potent iNOS inhibitor on traumatic spinal cord injury. Neuroreport 15(13):2103–2107
Acknowledgment
This study was partially supported by IIR (investigator initiated research) program of Pfizer Pharm.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ha, KY., Kim, YH., Rhyu, KW. et al. Pregabalin as a neuroprotector after spinal cord injury in rats. Eur Spine J 17, 864–872 (2008). https://doi.org/10.1007/s00586-008-0653-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-008-0653-6