Abstract
Paraquat-induced pulmonary fibrosis is a progressive and fatal interstitial lung disease, a condition for that, there is no effective treatment and its prognosis is appalling. Since multiple coactivated pathways are involved, targeting these pathways using combination regimens is plausible for successful therapy. So, the aim of the present study was to evaluate the therapeutic efficacy of pirfenidone–vitamin E combination therapy in (paraquat) PQ-induced lung fibrosis model. After the development of PQ-induced lung fibrosis, pirfenidone, vitamin E, pirfenidone plus vitamin E, and water (as a vehicle) were orally administered for 14 consecutive days (From day 14 to day 28). The comparison of efficacies was performed by evaluating histopathology changes, hydroxyproline content, and oxidative stress. Either pirfenidone or vitamin E solely could recover the pathological changes of paraquat, decreased lipid peroxidation, and restored the antioxidant enzymes towards normal values. Hydroxyproline content was significantly reduced by both pirfenidone and vitamin E administration. Concurrent treatment with pirfenidone and vitamin E intensified all of these therapeutics effects indicating more potent antifibrotic and anti-inflammatory impacts. Therefore, pirfenidone plus vitamin E offers potential as a combination therapy for the treatment of paraquat-induced pulmonary fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary fibrosis is a progressive, fatal interstitial lung disease of known or unknown etiology, characterized by the destruction of functional lung parenchyma and altered cellular composition of the alveolar tissue with excessive collagen deposition and aberrant scar tissue (Kar et al. 2016).
Paraquat (PQ), a widely used heterocyclic herbicide, has caused many deaths due to pulmonary failure, a condition for that, there is no effective treatment and its prognosis is appalling (Elenga et al. 2018; Gawarammana and Buckley 2011). There is growing evidence that oxidative stress plays a significant role in PQ-induced pulmonary fibrosis. Oxidative stress is defined as an imbalance between oxidant generation and antioxidant defense, resulting in potential cell death (Veith et al. 2019). So, prevention of oxidative stress by antioxidant therapy has been proposed as an effective treatment for pulmonary fibrosis (Fois et al. 2018a). Recently, arrays of treatments such as anti-inflammatory therapy with corticosteroids or immunosuppressants have been tested in many clinical trials, although the majority of them faced to failure. Besides, other pharmacotherapies such as etanercept, nintedanib, Gleevec, warfarin, and bosentan have limitations due to their serious adverse effects (Kandhare et al. 2016). Therefore, new therapeutic strategies for pulmonary fibrosis have been evolved to focus on antifibrotic and antioxidants. Considering the oxidative stress and profibrotic pathways in the pathogenesis of PQ-induced lung fibrosis, here a combination of pirfenidone (PF) plus vitamin E (VE) was examined as a new oral combination therapy.
VE is known to possess potent antioxidant activity and the ability to modulate protein function and gene expression (Devaraj and Jialal 2005). The antifibrotic effects of VE have been proved against Bleomycin and radiation-induced pulmonary cytotoxicity and fibrosis (Bese et al. 2007; Card et al. 2003; Deger et al. 2007; Futamura 1996) and also non-pulmonary fibrosis (Kachel et al. 1990; Ruch et al. 1991), whereas other antioxidant treatments were ineffective (Card et al. 2003).
PF is the only treatment of pulmonary fibrosis that exhibits a significant effect in slowing down the inevitable declining lung performance and has elicited potent antifibrotic and also anti-inflammatory effects in a variety of animal and cell-based models (Iyer et al. 2000; Oku et al. 2002, 2008; Schaefer et al. 2011). In light of above knowledge, the present study evaluates PF plus VE combination therapy and compares its therapeutic efficacy with each one used alone in PQ-induced lung fibrosis model in rats. This comparison was performed by evaluation of histopathology changes, determination of hydroxyproline content, and estimation of tissue oxidative stress parameters.
Materials and methods
Chemicals
Paraquat, pirfenidone, Vitamine E, hydroxyproline, 4-dimethyl aminobenzaldehyde, chloramine T, and malondialdehyde (MDA) were purchased from Sigma–Aldrich Chemical Co. (USA). All other chemicals were bought from Merck (Germany).
Animals and treatments
A total of 50 male Wistar rats, 8–12 weeks old, weighing 200–220 g, were housed in a room with a 12 h light/dark cycle. Animals had free access to tap water and food ad libitum. Intraperitoneal (i.p.) administration of a single 20 mg/kg dose of PQ developed pulmonary fibrosis in 2 weeks. This was confirmed by morphological changes in the lungs and the accumulation of excessive interstitial collagen (Ashcroft et al. 1988). Animals were randomly divided into five equal groups (each group 10 rats), namely PQ-induced fibrosis group (PQ), PF treatment group, VE treatment group, PF plus VE treatment group, and normal group (receiving water as negative control group). Gavage was used as the route of administration in all groups for 2 weeks (From day 14 to day 28). The dosages of PF and VE were 200 and 15 mg/kg body weight, respectively, according to the optimum dosage of our previous studies and others (Pourgholamhossein et al. 2018; Seifirad et al. 2012).
All animals were treated humanely according to the guidelines on ethics standard for investigation of experimental pain in animals and approved by the Animal Experimentation Ethics Committee of Kerman Neuroscience Research Center (EC/KNRC/90).
Sample collection
At the end of the treatment period (28 days), rats were anesthetized by injecting i.p. ketamine/xylazine (100and 10 mg/kg, respectively). Lungs were promptly removed and divided into two halves. The right lung was stored at − 80 °C for the analysis of oxidative stress and hydroxyproline content. The left lung was immersed in 10% buffered formalin for histological examination.
Histopathological assessment
After embedding the fixed lung tissues into liquid paraffin, 5-μm-thick tissue sections were prepared. The sections were stained with hematoxylin and eosin (H&E) and Masson’s trichrome. The mean lung fibrosis score for each histologic section was graded on the basis of classification of Ashcroft et al. (1988) and Hübner et al. (2008). A total of 50 fields with a × 10 magnification were assessed, and a score 0–8 was given to each field. In every field, the predominant degree of fibrosis was recorded as that occupying more than half of the field area. Complete obliteration with fibrotic masses was considered as score 8 and the fields having the normal structure scored as 0. As shown in Table 2, a qualitative evaluation based on a simpler grading system: absent, present, focal or absent, mild, moderate, severe, indicated in the previous studies was also used for the other specific histopathologic features (Cherniack et al. 1991; Rasooli et al. 2018). All histologic sections were blindly evaluated by an expert pathologist.
Determination of oxidative stress parameters
Preliminary preparation of lung tissue samples for biochemical evaluation was done with 0.1 M Tris-HCl buffer (pH 7.4) at 4 °C using a tissue homogenizer. The homogenated tissues were used for biochemical measurement. The malondialdehyde in the lung tissue was determined using their reaction with thiobarbituric acid and measurement of the products at 532 nm. Different concentrations of tetra butyl ammonium prepared as a standard solution. The activity of SOD was measured according to the method of Beyer and Fridovich (1987) and CAT activity was determined using the method of Aebi (1984).
Measurement of collagen
Hydroxyproline, as a measure of collagen deposited in the lung tissue and fibrosis, was assessed using the method of Reddy and Enwemeka (1996) with minor modification (Ghazi-Khansari et al. 2007). Briefly, hydroxyproline in the lung tissue was hydrolyzed with 1 M acetate buffer and oxidized with 1.4% chloramine T. The oxidized hydroxyproline formed a reddish purple chromophore with 1 M Ehrlich’s reagent (4-dimethylaminobenzaldehyde), which was measured at 550 nm.
Statistical analysis
The quantitative data were presented as mean ± SEM. The Mann–Whitney nonparametric test was used to compare histological scores between the groups. Differences between the means were analyzed using one-way analysis of variance (ANOVA) followed by Tukey HSD post-hoc test. The differences are considered statistically significant when p < 0.05. The data were analyzed using SPSS 18.0.
Results
Lung histopathology
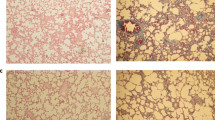
The histopathological changes in the lung tissues were evaluated by H&E and Masson’s trichrome staining. The lungs of the negative control group showed the normal alveolar pattern with bronchioli surrounded by alveolar sacs and alveoli separated by alveolar septa. PQ exposure induced a marked inflammatory response characterized by inflammatory cell aggregation and infiltration in the alveolar spaces and septa, substantially thickening and degrading normal alveolar structure. Masson’s trichrome staining revealed markedly increased collagen deposition, predominately in the thickened alveolar regions and small bronchioles (Fig. 1; Table 1). Both PF and VE treatments attenuated the interstitial thickening, inflammatory responses, and collagen accumulation induced by PQ. These alleviations were more prominent by PF-VE cotreatment. Also, the mean fibrosis score in the combination therapy was significantly improved compared with each treatment alone (P < 0.001) (Table 1). All the histopathologic features are depicted in Fig. 1 and Table 2.
Photomicrographs of hematoxylin and eosin and Masson’s trichrome stained lung tissues. a, a′ (PQ group): Received PQ without treatment shows highest level of fibrosis; b, b′ (PQ + PF group): Received PQ and PF (200 mg/kg) for 14 days as a treatment; c, c′ (PQ + Vit E group): Received PQ and Vit E (15 mg/kg) for 14 days as a treatment; d, d′ (PQ+ PF + Vit E group): Received PQ and PF (200 mg/kg) plus Vit E (15 mg/kg) for 14 days as a treatment (magnification, × 100; scale bar, 50 μm)
Oxidative stress parameters
The levels of lipid peroxidation and the activities of antioxidant enzymes such as SOD and CAT in the lung tissue homogenates are shown in Fig. 2 of the control and experimental rats (Fig. 2). A significant rise in the levels of lipid peroxidation in the lung tissues of PQ-treated animals was observed. This effect was accompanied by a decrease in the enzymatic activities of SOD and CAT. Administration of both PF (p = 0.0007) and VE (p = 0.0001) significantly decreased lipid peroxidation in lung samples and restored the SOD and CAT activity to near normal levels. These effects are exaggerated by coadministration of PF plus VE. Significance levels are separately depicted in Fig. 2.
Lung hydroxyproline content
As shown in Fig. 2, the hydroxyproline content in the lung of the PQ-treated rats significantly increased compared with the control group. Administration of PF (p = 0.004) and VE (p = 0.03) could significantly reduce the content of hydroxyproline in lung tissues. These findings were more typical in combined therapy by PF-VE (p = 0.0002), which all were consistent with the histopathological results.
Discussion
Respiratory or systemic exposure with PQ can lead to extensive lung damage followed by fibrosis in human and animals (Bus and Gibson 1984). PQ-induced lung fibrosis model has been widely used for studying the mechanisms involved in the progression of human pulmonary fibrosis and the impact of various drugs on this progression (Kalantar et al. 2018; Li et al. 2017). PQ primarily accumulates in the lungs through the highly developed polyamine uptake system, leads to the generation of free radicals and NADPH-dependent redox cycling, and results in acute oxidative stress–related damages (Liu et al. 2016). Imbalance of oxidant/antioxidant in the lung is considered to be a key step in the development of many airway diseases. Hence, antioxidant enzymes play critical roles in controlling or preventing pulmonary diseases. Free radical accumulation leads to lipid peroxidation and production of oxidation markers such as MDA, which is a marker of lipid peroxidation (Fois et al. 2018a). In the present study, a significant increase in the levels of lipid peroxidation in the lung tissue was observed in the PQ-treated animals. This effect was accompanied by a decrease in the enzymatic activities of SOD and CAT. Previous studies have demonstrated similar results in PQ-induced lung toxicity (Pourgholamhossein et al. 2016; Rasooli et al. 2018). SOD has a pivotal role in protecting tissues from damage by oxidant stress via superoxide anion scavenging, which prevents the formation of other more potent oxidants such as peroxynitrite and hydroxyl radical (Gao et al. 2008). CAT is another important enzyme that protect cells against oxidative damage, and its activity can indirectly represent the levels of lipid peroxidation (Dou et al. 2015). Here, we suggest that both PF and VE could suppress excessive production of ROS and increase the SOD and CAT activities. Our results are in accordance with other studies that have reported therapeutic effects of PF (Salazar-Montes et al. 2008) and VE (Bese et al. 2007; Card et al. 2003) regarding possessing antioxidant properties. Interestingly, PF plus VE more effectively modified the MDA, SOD, and CAT enzyme activity.
Consistent with the lipid peroxidation and antioxidant findings, a significant rise in the hydroxyproline content, which is a precise criterion for evaluation of fibrotic tissue, showed fibrogenic effects of PQ in lung tissues of intoxicated rats. Since the amino acid hydroxyproline is the precursor for collagen, the estimation of this amino acid is considered as an appropriate biochemical index of collagen content (Verma et al. 2013). Collagen accumulation was also indicated by trichrome staining in fibrotic interstitial tissues. Moreover, lung inflammation and abnormal lung architecture characterized by extensive fibroblast proliferation and collagen-stained matrix deposition were observed. Both PF and VE could significantly decrease hydroxyproline content and alleviate the PQ-induced lesions including inflammatory cell aggregation and infiltration in the alveolar space and septum, substantially thickening and degrading normal alveolar structure and collagen deposition in the thickened alveolar regions and small bronchioles. In this regard, the effect of combination therapy by PF and VE showed much improved histological profile with near normal collagen deposition and a basal level of hydroxyproline content indicating the ameliorative role of combination of PF plus VE against PQ-induced pulmonary fibrosis. The antifibrotic effects of PF have been definitely proved (Seifirad et al. 2012; Selvaggio and Noble 2016). Meanwhile, the protective role of antioxidants including VE in different models of pulmonary fibrosis has been demonstrated (Bese et al. 2007; Day 2008; Hemmati et al. 2008) and suggested that this antioxidant may have nonspecific antifibrotic effects in the lung (Fois et al. 2018b).
In summary, our findings clearly indicate that coadministration of supplementary VE plus PF intensifies the therapeutic effects of PF in PQ-induced pulmonary fibrosis by (1) enhancing antioxidant potency and (2) reducing of oxidative stress. The current study has some limitations; considering the wide range of biologic effects of PF and VE, there may be far more complex mechanisms than we had mentioned in this manuscript. Larger animal studies and clinical investigations are necessary for further. In addition, therapeutic mechanisms of PF plus VE in lung injury treatment should assess in more various doses.
Abbreviations
- Paraquat:
-
(PubChem CID: 15938)
- Pirfenidone:
-
(PubChem CID: 40632)
- Vitamin E:
-
(PubChem CID: 14985)
- Malondialdehyde:
-
(PubChem CID: 10964)
- Hydroxyproline :
-
(PubChem CID: 5810)
- PF:
-
Pirfenidone
- PQ:
-
Paraquat
- CAT:
-
Catalase
- SOD:
-
Super oxide dismutase
- MDA:
-
Malondialdehyde
- HP:
-
Hydroxyproline
References
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126
Ashcroft T, Simpson JM, Timbrell V (1988) Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 41:467–470
Bese N et al (2007) Vitamin E protects against the development of radiation-induced pulmonary fibrosis in rats. Clin Oncol 19:260–264
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bus JS, Gibson JE (1984) Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect 55:37
Card JW, Racz WJ, Brien JF, Massey TE (2003) Attenuation of amiodarone-induced pulmonary fibrosis by vitamin E is associated with suppression of transforming growth factor-β1 gene expression but not prevention of mitochondrial dysfunction. J Pharmacol Exp Ther 304:277–283
Cherniack RM, Colby TV, Flint A, Thurlbeck WM, Waldron J, Ackerson L, King TE Jr (1991) Quantitative assessment of lung pathology in idiopathic pulmonary fibrosis. The BAL Cooperative Group Steering Committee. Am Rev Respir Dis 144:892–900. https://doi.org/10.1164/ajrccm/144.4.892
Day BJ (2008) Antioxidants as potential therapeutics for lung fibrosis. Antioxid Redox Signal 10:355–370
Deger Y, Yur F, Ertekin A, Mert N, Dede S, Mert H (2007) Protective effect of α-tocopherol on oxidative stress in experimental pulmonary fibrosis in rats. Cell Biochem Funct 25:633–637
Devaraj S, Jialal I (2005) α-Tocopherol decreases tumor necrosis factor-α mRNA and protein from activated human monocytes by inhibition of 5-lipoxygenase. Free Radic Biol Med 38:1212–1220
Dou T et al. (2015) Nrf2/ARE pathway involved in oxidative stress induced by paraquat in human neural progenitor cells. Oxidative medicine and cellular longevity 2016
Elenga N et al (2018) Clinical features and prognosis of paraquat poisoning in French Guiana: a review of 62 cases. Medicine 97
Fois AG et al (2018a) Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: a systematic review. Respir Res 19:51
Fois AG et al (2018b) Antioxidant activity mediates pirfenidone antifibrotic effects in human pulmonary vascular smooth muscle cells exposed to sera of idiopathic pulmonary fibrosis patients. Oxidative Med Cell Longev:2018
Futamura Y (1996) Toxicity of amiodarone on mouse pulmonary endothelial cells cultured with or without alveolar macrophages. J Toxicol Sci 21:253–267
Gao F, Kinnula VL, Myllärniemi M, Oury TD (2008) Extracellular superoxide dismutase in pulmonary fibrosis. Antioxid Redox Signal 10:343–354
Gawarammana IB, Buckley NA (2011) Medical management of paraquat ingestion. Br J Clin Pharmacol 72:745–757. https://doi.org/10.1111/j.1365-2125.2011.04026.x
Ghazi-Khansari M, Mohammadi-Karakani A, Sotoudeh M, Mokhtary P, Pour-Esmaeil E, Maghsoud S (2007) Antifibrotic effect of captopril and enalapril on paraquat-induced lung fibrosis in rats. J Appl Toxicol 27:342–349. https://doi.org/10.1002/jat.1212
Hemmati A, Nazari Z, Ranjbari N, Torfi A (2008) Comparison of the preventive effect of vitamin C and E on hexavalent chromium induced pulmonary fibrosis in rat. Inflammopharmacology 16:195–197
Hubner RH et al (2008) Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 44(507-511):514–507. https://doi.org/10.2144/000112729
Iyer SN, Hyde DM, Giri SN (2000) Anti-inflammatory effect of pirfenidone in the bleomycin-hamster model of lung inflammation. Inflammation 24:477–491
Kachel D, Moyer T, Martin W (1990) Amiodarone-induced injury of human pulmonary artery endothelial cells: protection by alpha-tocopherol. J Pharmacol Exp Ther 254:1107–1112
Kalantar M, Khodayar MJ, Kalantari H, Khorsandi L, Hemmati AA (2018) Therapeutic effect of gallic acid against paraquat-induced lung injury in rat. Jundishapur J Nat Pharm Prod 13:13
Kandhare AD, Mukherjee A, Ghosh P, Bodhankar SL (2016) Efficacy of antioxidant in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. EXCLI J 15:636
Kar S, Biswas S, Banerjee ER (2016) Evaluating the ameliorative potential of plant flavonoids and their nanocomposites in bleomycin induced idiopathic pulmonary fibrosis. Biomed Res Therapy 3:707–722
Li T, Yang X, Xin S, Cao Y, Wang N (2017) Paraquat poisoning induced pulmonary epithelial mesenchymal transition through Notch1 pathway. Sci Rep 7:924
Liu M-W et al (2016) Radix puerariae extracts ameliorate paraquat-induced pulmonary fibrosis by attenuating follistatin-like 1 and nuclear factor erythroid 2p45-related factor-2 signalling pathways through downregulation of miRNA-21 expression. BMC Complement Altern Med 16:1
Oku H, Nakazato H, Horikawa T, Tsuruta Y, Suzuki R (2002) Pirfenidone suppresses tumor necrosis factor-α, enhances interleukin-10 and protects mice from endotoxic shock. Eur J Pharmacol 446:167–176
Oku H et al (2008) Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol 590:400–408
Pourgholamhossein F et al (2016) Thymoquinone effectively alleviates lung fibrosis induced by paraquat herbicide through down-regulation of pro-fibrotic genes and inhibition of oxidative stress. Environ Toxicol Pharmacol 45:340–345. https://doi.org/10.1016/j.etap.2016.06.019
Pourgholamhossein F et al (2018) Pirfenidone protects against paraquat-induced lung injury and fibrosis in mice by modulation of inflammation, oxidative stress, and gene expression. Food Chem Toxicol 112:39–46
Rasooli R, Pourgholamhosein F, Kamali Y, Nabipour F, Mandegary A (2018) Combination therapy with pirfenidone plus prednisolone ameliorates paraquat-induced pulmonary fibrosis. Inflammation 41:134–142. https://doi.org/10.1007/s10753-017-0671-9
Reddy GK, Enwemeka CS (1996) A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem 29:225–229
Ruch RJ, Bandyopadhyay S, Somani P, Klaunig JE (1991) Evaluation of amiodarone free radical toxicity in rat hepatocytes. Toxicol Lett 56:117–126
Salazar-Montes A, Ruiz-Corro L, López-Reyes A, Castrejón-Gómez E, Armendáriz-Borunda J (2008) Potent antioxidant role of pirfenidone in experimental cirrhosis. Eur J Pharmacol 595:69–77
Schaefer C, Ruhrmund D, Pan L, Seiwert S, Kossen K (2011) Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev 20:85–97
Seifirad S, Keshavarz A, Taslimi S, Aran S, Abbasi H, Ghaffari A (2012) Effect of pirfenidone on pulmonary fibrosis due to paraquat poisoning in rats. Clin Toxicol 50:754–758
Selvaggio AS, Noble PW (2016) Pirfenidone Initiates a New Era in the Treatment of Idiopathic Pulmonary Fibrosis. Annu Rev Med 67:487–495
Veith C, Boots AW, Idris M, van Schooten F-J, van der Vliet A (2019) Redox imbalance in idiopathic pulmonary fibrosis: a role for oxidant cross-talk between NADPH oxidase enzymes and mitochondria. Antioxid Redox Signal
Verma R, Kushwah L, Gohel D, Patel M, Marvania T, Balakrishnan S (2013) Evaluating the ameliorative potential of quercetin against the bleomycin-induced pulmonary fibrosis in wistar rats. Pulm Med:2013
Funding
This study was funded by Kerman University of Medical Sciences (grant number 97000369).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Yes
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rasooli, R., Kamali, Y. & Mandegary, A. Effects of pirfenidone, vitamin E, and pirfenidone–vitamin E combination in paraquat-induced pulmonary fibrosis. Comp Clin Pathol 29, 667–673 (2020). https://doi.org/10.1007/s00580-020-03104-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-020-03104-0