Abstract
Pirfenidone is known to slow the decline in vital capacity and increase survival in idiopathic pulmonary fibrosis (IPF). Besides, administration of glucocorticoids, e.g., prednisolone has been the conventional strategy to the treatment of patients with this disease, although their efficacy is under debate. Since multiple coactivated pathways are involved in the pathogenesis of IPF, combination therapy is a foundation strategy to cover many more synergetic mechanisms and increase response. The aim of the present study was to compare the therapeutic efficacy of prednisolone plus pirfenidone with pirfenidone alone in PQ-induced lung fibrosis. After development of PQ-induced lung fibrosis, pirfenidone, prednisolone, and their combination were administered for 14 consecutive days. Lung pathological lesions, along with increased hydroxyproline were determined in the paraquat group. Paraquat also caused oxidative stress and increasing the proinflammatory and profibrotic gene expression. Pirfenidone attenuated the PQ-induced pulmonary fibrosis from the analysis of antioxidant enzymes but prednisolone had no such effect. Co-treatment with pirfenidone and prednisolone suppressed lung hydroxyproline content, TGF-β1, and TNF-α; however, prednisolone alone could not suppress pulmonary fibrosis which was significantly suppressed only by pirfenidone. Pirfenidone also suppressed the increase in MMP-2 and TIMP-1 induced by PQ. All of these effects were exaggerated when pirfenidone coadministered with prednisolone. These findings suggest that pirfenidone exerts its antifibrotic effect through regulation of hydroxyproline content, oxidative stress and proinflammatory and profibrotic gene expression during the development of PQ-induced pulmonary fibrosis in rats and combination therapy with prednisolone can represent more potent therapeutic effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Combination therapy is one of the rationale strategies is proposed to cover many more synergetic mechanisms and increase response. In interstitial lung diseases, such as idiopathic pulmonary fibrosis (IPF) combination therapy is concluded to be attractive in principle, both to deal with diagnostic uncertainty and to suppress proinflammatory and profibrotic pathways [1]. Administration of glucocorticoids or immunosuppressive agents has been the conventional strategy to the treatment of patients with this disease, although their efficacy is under debate. It is found that when prednisolone combines with cyclophosphamide or with azathioprine, its potency exaggerates and a significant advantage in survival achieve [2, 3]. In this regard, we tried to compare the therapeutic efficacy of prednisolone plus pirfenidone with pirfenidone and prednisolone alone in PQ-induced pulmonary fibrosis. This protocol of treatment is guided by the pathogenesis of fibrosis and documented preclinical data which proved that inflammation plays a major role in the pathogenesis of PQ toxicity. The present study evaluates the therapeutic efficacy of PF plus PR combination and compares with each one lonely in PQ-induced lung fibrosis as an IPF model. This comparison was performed by evaluating histopathologic changes, hydroxyproline content, tissue oxidative stress parameters, inflammatory and fibrotic genes expression including TGF-β1, TNF-α, TIMP-1, and MMP-2 genes in the lung tissues.
MATERIALS AND METHODS
Chemicals
Paraquat, pirfenidone, prednisolone, hydroxyproline, 4-dimethyl aminobenzaldehyde, chloramine T, and malondialdehyde (MDA) were purchased from Sigma-Aldrich Chemical Co. (USA). TRIzol® RNA isolation reagent and HyperScript™ first-strand cDNA synthesis kit were purchased from Invitrogen (Germany). SYBR Green Master Mix was obtained from Takara (Japan). All other chemicals were bought from Merck (Germany).
Animals and Treatments
Male Sprague-Dawley rats of 8–12 weeks old weighing 200–220 g were housed in a room with a 12-h light/dark cycle and allowed to ad libitum food and water. Pulmonary fibrosis was induced by intraperitoneal (i.p.) administration of a single dose of 20 mg/kg PQ [4, 5] and developed in 2 weeks (day 14), confirmed by morphological changes in the lungs and an accumulation of excessive interstitial collagen [6]. Then, experiment was performed in rats were equally and randomly divided into five groups, namely PQ-induced fibrosis rats (PQ group), PF treatment, PR treatment, PF plus PR treatment, and normal animals receiving vehicle (water) as the negative control. PF (200 mg/kg/day body weight) and PR (3 mg/kg/day body weight) administered by gavage. Selecting the dose of PF and PR was according to clinical trial in patients with idiopathic pulmonary fibrosis and experimental researches [7, 8].
All animals were treated humanely according to the guidelines on ethics standard for investigation of experimental pain in animals and approved by the Animal Experimentation Ethics Committee of Kerman Neuroscience Research Center (EC/KNRC/90).
Sample Collection and Analytical Procedures
At the end of the treatment period (28 days), the rats were anesthetized by intraperitoneally injection with 100 mg/kg ketamine and 10 mg/kg xylazine. Lung promptly removed and divided into two halves. The right lung was stored at − 80 °C for analysis of oxidative stress, hydroxyproline content, and gene expression. The left lung was immersed in 10% buffered formalin for histological examination.
Histopathological Analysis
After embedding the fixed lung tissues into liquid paraffin, 5-μm-thick sections were prepared. The sections were stained with hematoxylin and eosin (H & E) for histopathological examination and Masson’s trichrome staining for evaluation of collagen deposition. All histopathologic sections were blindly evaluated by the two experienced pathologists. For fibrotic/reparative changes, lung sections were assessed qualitatively from normal (absent) to severe according to the scale defined by Ashcroft et al. [6] as follows: normal lung; mild: minimal fibrous thickening of alveolar or bronchiolar vessels; moderate: moderate thickening of walls without obvious damage to lung architecture; severe: increased fibrosis with definite damage to lung structure and formation of fibrous bands or large fibrous areas. A simpler grading system (absent, present, focal) was also used to evaluate the other pathologic lesions [9, 10].
Determination of Oxidative Stress Parameters
Preliminary preparation of lung tissue samples for biochemical evaluation was done with 0.1 M Tris-HCl buffer (pH 7.4) at 4 °C using a tissue homogenizer. The resulting tissue homogenates were used for biochemical measurements. The malondialdehyde in the lung tissue was measured using thiobarbituric acid at 532 nm. Different concentrations of tetrabutylammonium prepared as a standard solution. The activities of SOD was measured according to the method of Beyer and Fridovich [11] and CAT activity was determined using the method of Aebi [12].
Measurement of Collagen
Hydroxyproline, as a measure of collagen deposition in the lung tissue and fibrosis, was assessed using the method of Reddy and Enwemeka [13] with minor modification [14]. Briefly, hydroxyproline in the homogenated lung tissue was hydrolyzed with 1 M acetate buffer and oxidized with 1.4% chloramine T, and was formed the reddish purple complex with 1 M Ehrlich’s reagent (4-dimethylaminobenzaldehyde) and the chromophore was developed at 65 °C for 20 min and measured at 550 nm.
Determination of Fibrotic Genes Expression by Real-Time RT-PCR
Total RNA was extracted from pulmonary tissues using TRIzol® reagent according to the manufacturer’s protocol. Samples (2 μg RNA) were reverse-transcribed using a HyperScript™ first-strand cDNA synthesis kit. Synthesized cDNA was used in real-time RT-PCR (lightcycler® 96 Roche, Germany) experiments using SYBR GREEN Supermix and analyzed with lightcycler® 96 Software. The sequences of primes were as follows: All the real-time PCR primers used in this study are listed: rat TGF-β1 (forward: 5′-GCT CGC TTT GTA CAA CAG CA-3′ and reverse: 5′-GAG TTC TAC GTG TTG CTC CA-3′), rat TNF-α (forward: 5′-CCC ACG TCG TAG CAA ACC ACC AA-3′ and reverse: 5′-ACG TAG TCG GGG CAG CCT TGT-3′), rat MMP-2 (forward: 5′-CTG GGC AAC AAG TAT GAG AG-3′ and reverse: 5’-GTG TAG GTG TAG ATA GGG GC-3′), rat TIMP-1 (forward: 5′-GCC TCT GGC ATC CTC TTG-3′ and reverse: 5′-TGC GGT TCT GGG ACT TGT-3′). Specificity was confirmed by electrophoretic analysis of the reaction products and by the inclusion of template- or reverse transcriptase-free controls. To normalize the amount of total RNA present in each reaction, β-actin cDNA was used as an internal standard.
Statistical Analysis
The quantitative data were presented as mean ± SEM. Differences between the means were analyzed using one-way analysis of variance (ANOVA) followed by the Tukey HSD post hoc test. The differences are considered statistically significant when p < 0.05. The data were analyzed using SPSS 18.0.
RESULTS
Effect of Pirfenidone, Prednisolone, and Pirfenidone- Prednisolone Coadministration on Histopathological Changes of Lung Tissues
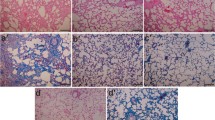
The lungs of the negative control group showed a normal alveolar pattern with broncheoli surrounded by alveolar sacs and alveoli separated by alveolar septa. PQ exposure induced a marked inflammatory response characterized by inflammatory cell aggregation and infiltration in the alveolar space and septum, substantially thickening and degrading normal alveolar structure. Masson’s trichrome staining revealed markedly increased collagen deposition, predominately in the thickened alveolar regions and small bronchioles (Fig. 1). PF and PR treatment attenuated the interstitial thickening, inflammatory responses, and collagen accumulation induced by PQ; however, it seems that their combination more than each of them separately, alleviated the inflammatory and fibrotic lesions. All the lesions are depicted in Fig. 1 and Table 1.
Photomicrographs of hematoxylin and eosin and Masson’s trichrome stained lung tissues. a PQ group: Received PQ without treatment shows the highest level of fibrosis. b PQ + prednisolone group: Received PQ and prednisolone (3 mg/kg) for 14 days as a treatment. c PQ + PF group: Received PQ and PF (200 mg/kg) for 14 days as a treatment. d PQ + PF + prednisolone group: Received PQ and PF (200 mg/kg) plus prednisolone (3 mg/kg) for 14 days as a treatment (magnification × 40).
Effect of Pirfenidone, Prednisolone, and Pirfenidone-Prednisolone Coadministration on the Hydroxyproline Content
As shown in Fig. 2, the hydroxyproline content in the lung of the PQ-treated rats significantly increased compared with the control group. Administration of PF (p < 0.05) and PR could reduce the content of hydroxyproline in lung tissues. These findings were more typical in combined therapy with PF-PR (p < 0.01) which all were consistent with the histopathological results.
Influence of PQ toxicity and therapeutic effects of pirfenidone, prednisolone, and pirfenidone-prednisolone combination on oxidative stress parameters and hydroxyproline content. Data are expressed as the mean ± SEM. Two replicates in each assay, n = 8. *p < 0.05, **p < 0.01, and ***p < 0.001 in comparison with PQ group.
Effect of Pirfenidone, Prednisolone, and Pirfenidone-Prednisolone Coadministration on the Oxidative Stress
A significant rise in the levels of lipid peroxidation in the lung tissue was observed in the PQ-treated animals. This effect was accompanied by a decrease in the enzymatic activities of SOD and CAT. Administration of PF alone or in combination with PR significantly decreased lipid peroxidation in lung samples and restored the SOD and CAT activity to near normal. Significance levels are separately depicted in Fig. 2.
Effect of Pirfenidone, Prednisolone, and Pirfenidone-Prednisolone Coadministration on the Expression of TGF-β1 and TNF-α in PQ-Induced Pulmonary Fibrosis
To quantify the changes of profibrogenic and proinflammatory genes after treatment by PF and PR and comparing to their combination therapy, total RNAs were collected for measurement by quantitative real-time RT-PCR. The results suggested a 5-fold increase of TGF-β1 in lung tissues of PQ-rats, which was gradually reduced to about 2-fold after PF plus PR treatment.
We also analyzed the TNF-α which plays an important role in PQ-induced pulmonary inflammation. As expected, PQ increased lung TNF-α expression, which was significantly inhibited by both PF (p < 0.001) and PR (p < 0.001) treatments (Fig. 3). Similar suppression of TGF-β1, TNF-α gene expression reduced after treatment by PF plus PR, indicating more potent proinflammatory effects of combination therapy.
Influence of therapeutic effects of pirfenidone, prednisolone, and pirfenidone-prednisolone combination on the expression of fibrotic genes in PQ-induced lung fibrosis. The rats treated for 14 days after development of fibrosis. The expressions of TGF-β, TNF-α, TIMP-1, and MMP-2 mRNA were determined in the lung of the rats by real-time RT-PCR. The data are means ± SE (two replicates in each assay) for eight rats. *p < 0.05, ***p < 0.001, and **p < 0.01 in comparison with the PQ group.
Effect of Pirfenidone, Prednisolone, and Pirfenidone-Prednisolone Coadministration on the Expression of TIMP-1 and MMP-2 in PQ-Induced Lung Fibrosis
The mRNA expression of TIMP-1 and MMP-2 genes was increased in the PQ group. PF and PR solely decreased the expression of the TIMP-1 and MMP-2 genes. As a combination therapy by PF-PR, rats obviously decreased the mRNA expression of the TIMP-1 and MMP-2 (p < 0.01) which suggested its more ability to inhibit the activation of ECM deposition and the progression of fibrosis.
DISCUSSION
Intoxication with PQ as a quaternary ammonium herbicide can lead to severe fibrotic lung damage [15]. The pulmonary response to stimulation of PQ is manifested by excessive scarring and architectural restructuring [16]. The pathologic characteristics of PQ-induced pulmonary fibrosis in primary stage are alveolitis, pulmonary edema, and infiltration of inflammatory cells. Finally, the changes are followed by stimulating fibroblast proliferation and collagen deposition. First of all, a fibrogenic effect of PQ in lung tissue of the rats was proved by a significant increasing in hydroxyproline content after PQ intoxication. The results showed that prednisolone alone could not effectively decrease the hydroxyproline content. The effect of pirfenidone in reducing hydroxyproline level was more than prednisolone. Besides, a combination of pirfenidone and prednisolone was more effective than each agent alone. This effect could be explained with possible mechanisms including inhibition of free oxygen radical production and subsequent inflammatory cell accumulation which is generated by PQ, and thus inhibition of fibroblast proliferation. Consistent with hydroxyproline, the results suggested that the PQ poisoning model was also successful histologically. In general, the histopathological lesions for IPF are those of usual interstitial pneumonia (UIP) [17, 18]. A combination of fibrosis, scarring, and honeycombing prominently in the subpleural and paraseptal regions of parenchyma were also observed in the present study.
The highly developed polyamine uptake system following initial accumulating PQ in the lungs leads to overproduction of free radicals and dysregulation of redox signaling as well as a reduction in the antioxidant capability of the cell that ultimately results in acute tissue oxidative damage [19]. Free radicals activate the lipid peroxidation process in the cellular level. An increase in the free radicals gives rise to overproduction of MDA that is one of the final products of polyunsaturated fatty acid peroxidation in the cells [20]. PQ exposure caused a significant reduction in the enzymatic activities of SOD and CAT along with an increase in the tissue levels of LPO compared to control rats. Our results are in accordance with other studies in PQ-induced lung toxicity [5, 21]. In the present study, the effect of pirfenidone alone in reducing SOD and CAT activity was similar to the combination therapy with prednisolone. Also, there was no significant difference in the enzymatic activity of SOD and CAT or LPO between the groups receiving prednisolone versus that receiving PQ. Based on the previous studies, this effect can be attributed to the antioxidant property of pirfenidone and lack of this trait in prednisolone [22]. In this regards, the efficacy of pirfenidone could be partially related to its antioxidant properties.
It has been documented that proinflammatory and profibrogenic cytokines play an important role in both initiation and progression of PQ-induced pulmonary fibrosis [23]. Alveolar macrophages and other cell types in the early inflammatory and late fibrotic phases of lung injury produce TNF-α. TNF-α, in turn, causes proliferation of fibroblasts, expression of cellular matrix metalloproteinases (MMPs), and the release of other proinflammatory cytokines [24].
In mammals, three different forms of TGF-β exist of which TGF-β1 is considered as a principal profibrotic agent upregulated in areas of regeneration and remodeling foci in lung fibrosis. Some of its activities include synthesis promotion and deposition of collagen, extracellular matrix production, differentiation of fibroblasts to myofibroblasts, and inhibition of fibroblast autophagy [25,26,27]. In contrast to a study conducted by a Japanese group [8], in our study after PQ intoxication, both pirfenidone and prednisolone attenuated the increases in TGF-β1 levels in the lung tissue, suggesting that the antifibrotic effects could be partly explained as a result of the suppression of TGF-β1 signalling. Besides, consistent with other studies, the authors found that pirfenidone has a preventive effect on TGF-β1 elevation, and inhibits the progression of fibrosis [8, 28].
In some reports, TNF-α expression in the early inflammatory and late fibrotic phases of lung injury has been documented [29]. In the present study, PQ caused a significant elevation of TNF-α in lung tissue level compared with that in the control group. Treatment of rats with both pirfenidone and prednisolone significantly reduced the lung TNF-α level compared with the PQ group. Following combination therapy, the elevated level of TNF-α induced by PQ arrived at the same production level of this cytokine in the control group. The anti-inflammatory property of both drugs could also be considered for the effect on TNF-α level.
Inflammatory responses and the fibroblast to myofibroblast conversion are intensified with releasing the inflammatory cytokines, chemokines, or growth factors, e.g., TNF-α, TGF-β1, MMP 2, and TIMP-1 by alveolar macrophages along with epithelial and endothelial cells of the lung [30]. The proteases such as MMP2 and TIMP-1 partially regulate the renewing of the extracellular matrix. The MMP family and tissue inhibitors of MMPS certainly have a key role in pathological conditions by degrading matrix proteins and connective tissues. The precise pathophysiological mechanisms of the pathologic wound healing in PQ-induced lung fibrosis are not clear. However, a large collection of knowledge strongly suggests that imbalance expression/activity of MMPs and TIMPs play a fundamental role in this process [31,32,33,34]. Consistent with previous studies, in the present study, overexpression of MMP2 and TIMP1 was seen in rats poisoned by PQ. Pirfenidone in the regulation of the MMP2 and TIMP1 expression showed a more potent therapeutic effect in comparison to prednisolone.
The present study indicates that pirfenidone plus prednisolone might be considered as a combination therapeutic regimen for the treatment of pulmonary fibrosis induced by PQ intoxication. At least based on the findings of the present study, we can say that the proven therapeutic effect of pirfenidone on PQ-induced pulmonary fibrosis can be amplified when coadministrated with prednisolone.
Our study should also be evaluated based on its weaknesses. Using many different doses, investigation of molecular mechanism on the in vitro models (cell lines) and also larger clinical trials in animals and humans, evaluation of more genes especially those involved in EMT, and investigation of the protein level using western blotting seem to be considered in future studies.
Change history
15 November 2017
Unfortunately, the original publication of this article contained mistakes, and the authors would like to correct them. The corrected details are given below:
Abbreviations
- CAT:
-
Catalase
- IPF:
-
Idiopathic pulmonary fibrosis
- MDA:
-
Malondialdehyde
- MMP-2:
-
Matrix metalloproteinase-2
- PF:
-
Pirfenidone
- PQ:
-
Paraquat
- PR:
-
Prednisolone
- SOD:
-
Superoxide dismutase
- TGF-β:
-
Transforming growth factor beta
- TIMP-1:
-
Tissue inhibitor of metalloproteinase-1
- TNF-α:
-
Tumor necrosis factor alpha
References
Wuyts, W.A., K.M. Antoniou, K. Borensztajn, U. Costabel, V. Cottin, B. Crestani, J.C. Grutters, T.M. Maher, V. Poletti, and L. Richeldi. 2014. Combination therapy: the future of management for idiopathic pulmonary fibrosis? The Lancet Respiratory Medicine 2: 933–942.
Raghu, G., W.J. Depaso, K. Cain, S.P. Hammar, C.E. Wetzel, D.F. Dreis, J. Hutchinson, N.E. Pardee, and R.H. Winterbauer. 1991. Azathioprine combined with prednisone in the treatment of idiopathic pulmonary fibrosis: a prospective double-blind, randomized, placebo-controlled clinical trial. American Review of Respiratory Disease 144: 291–296.
Kondoh, Y., H. Taniguchi, T. Yokoi, O. Nishiyama, T. Ohishi, T. Kato, K. Suzuki, and R. Suzuki. 2005. Cyclophosphamide and low-dose prednisolone in idiopathic pulmonary fibrosis and fibrosing nonspecific interstitial pneumonia. European Respiratory Journal 25: 528–533.
Khodayar, M.J., M. Kiani, A.A. Hemmati, A. Rezaie, M.R. Zerafatfard, M. Rashidi Nooshabadi, and M. Goudarzi. 2014. The preventive effect of atorvastatin on paraquat-induced pulmonary fibrosis in the rats. Adv Pharm Bull 4: 345–349.
Pourgholamhossein, F., F. Sharififar, R. Rasooli, L. Pourgholi, F. Nakhaeipour, H. Samare-Fekri, M. Iranpour, and A. Mandegary. 2016. Thymoquinone effectively alleviates lung fibrosis induced by paraquat herbicide through down-regulation of pro-fibrotic genes and inhibition of oxidative stress. Environmental Toxicology and Pharmacology 45: 340–345.
Ashcroft, T., J.M. Simpson, and V. Timbrell. 1988. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. Journal of Clinical Pathology 41: 467–470.
Azuma, A., T. Nukiwa, E. Tsuboi, M. Suga, S. Abe, K. Nakata, Y. Taguchi, S. Nagai, H. Itoh, and M. Ohi. 2005. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine 171: 1040–1047.
Oku, H., T. Shimizu, T. Kawabata, M. Nagira, I. Hikita, A. Ueyama, S. Matsushima, M. Torii, and A. Arimura. 2008. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. European Journal of Pharmacology 590: 400–408.
Sur, S., J.S. Wild, B.K. Choudhury, N. Sur, R. Alam, and D.M. Klinman. 1999. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. Journal of Immunology 162: 6284–6293.
Samareh-Fekri, M., H.R. Poursalehi, A. Mandegary, F. Sharififar, R. Mahmoudi, A. Izadi, M.H. Nematolahi, N. Jamshidi, F. Pourgholamhossein, and M.R. Lashkarizadeh. 2015. The effect of methanol extract of fennel on bleomycin-induced pulmonary fibrosis in rats. Journal of Kerman University of Medical Sciences 22: 470–483.
Beyer, W.F., and I. Fridovich. 1987. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analytical Biochemistry 161: 559–566.
Aebi, H. 1984. [13] Catalase in vitro. Methods in Enzymology 105: 121–126.
Reddy, G.K., and C.S. Enwemeka. 1996. A simplified method for the analysis of hydroxyproline in biological tissues. Clinical Biochemistry 29: 225–229.
Ghazi-Khansari, M., A. Mohammadi-Karakani, M. Sotoudeh, P. Mokhtary, E. Pour-Esmaeil, and S. Maghsoud. 2007. Antifibrotic effect of captopril and enalapril on paraquat-induced lung fibrosis in rats. Journal of Applied Toxicology 27: 342–349.
Tsai, W.-T. 2013. A review on environmental exposure and health risks of herbicide paraquat. Toxicological & Environmental Chemistry 95: 197–206.
Dong, J., X. Yu, D.W. Porter, L.A. Battelli, M.L. Kashon, and Q. Ma. 2016. Common and distinct mechanisms of induced pulmonary fibrosis by particulate and soluble chemical fibrogenic agents. Archives of Toxicology 90: 385–402.
Raghu, G., H.R. Collard, J.J. Egan, F.J. Martinez, J. Behr, K.K. Brown, T.V. Colby, J.-F. Cordier, K.R. Flaherty, and J.A. Lasky. 2011. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American Journal of Respiratory and Critical Care Medicine 183: 788–824.
Rafii, R., M.M. Juarez, T.E. Albertson, and A.L. Chan. 2013. A review of current and novel therapies for idiopathic pulmonary fibrosis. Journal of Thoracic Disease 5: 48–73.
M-w, Liu, R. Liu, H.-y. Wu, Li Y-y, Su M-x, M.-n. Dong, W. Zhang, and C.-y. Qian. 2016. Radix puerariae extracts ameliorate paraquat-induced pulmonary fibrosis by attenuating follistatin-like 1 and nuclear factor erythroid 2p45-related factor-2 signalling pathways through downregulation of miRNA-21 expression. BMC Complementary and Alternative Medicine 16: 1.
Sayes, C.M., A.M. Gobin, K.D. Ausman, J. Mendez, J.L. West, and V.L. Colvin. 2005. Nano-C 60 cytotoxicity is due to lipid peroxidation. Biomaterials 26: 7587–7595.
Dou, T., M. Yan, X. Wang, W. Lu, L. Zhao, D. Lou, C. Wu, X. Chang, and Z. Zhou. 2016. Nrf2/ARE pathway involved in oxidative stress induced by paraquat in human neural progenitor cells. Oxidative Medicine and Cellular Longevity 2015.
Salazar-Montes, A., L. Ruiz-Corro, A. López-Reyes, E. Castrejón-Gómez, and J. Armendáriz-Borunda. 2008. Potent antioxidant role of pirfenidone in experimental cirrhosis. European Journal of Pharmacology 595: 69–77.
Toygar, M., I. Aydin, M. Agilli, F. Aydin, M. Oztosun, H. Gul, E. Macit, Y. Karslioglu, T. Topal, and B. Uysal. 2014. The relation between oxidative stress, inflammation, and neopterin in the paraquat-induced lung toxicity. Human & Experimental Toxicology. https://doi.org/10.1177/0960327114533808.
Lee, I.T., C.C. Lin, Y.C. Wu, and C.M. Yang. 2010. TNF-α induces matrix metalloproteinase-9 expression in A549 cells: Role of TNFR1/TRAF2/PKCα-dependent signaling pathways. Journal of Cellular Physiology 224: 454–464.
Leask, A., and D.J. Abraham. 2004. TGF-β signaling and the fibrotic response. The FASEB Journal 18: 816–827.
Mukherjee, S., M.R. Kolb, F. Duan, and L.J. Janssen. 2012. Transforming growth factor–β evokes Ca2+ waves and enhances gene expression in human pulmonary fibroblasts. American Journal of Respiratory Cell and Molecular Biology 46: 757–764.
Patel, A.S., L. Lin, A. Geyer, J.A. Haspel, C.H. An, J. Cao, I.O. Rosas, and D. Morse. 2012. Autophagy in idiopathic pulmonary fibrosis. PloS One 7: e41394.
Takeda, Y., K. Tsujino, T. Kijima, and A. Kumanogoh. 2014. Efficacy and safety of pirfenidone for idiopathic pulmonary fibrosis. Patient Preference and Adherence 8: 361–370.
Wynn, T.A., and T.R. Ramalingam. 2012. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature Medicine 18: 1028–1040.
Dancer, R., A. Wood, and D. Thickett. 2011. Metalloproteinases in idiopathic pulmonary fibrosis. European Respiratory Journal 38: 1461–1467.
Visse, R., and H. Nagase. 2003. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circulation Research 92: 827–839.
Woessner, J.F. 1991. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. The FASEB Journal 5: 2145–2154.
Nagase, H., R. Visse, and G. Murphy. 2006. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular Research 69: 562–573.
Ruiz, V., R.M. Ordóñez, J. Berumen, R. Ramirez, B. Uhal, C. Becerril, A. Pardo, and M. Selman. 2003. Unbalanced collagenases/TIMP-1 expression and epithelial apoptosis in experimental lung fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology 285: L1026–L1036.
Acknowledgements
We wish to thank Prof. Hamid Rajaian for helpful discussion. The production of this manuscript has been funded by the Shiraz University and Iran National Science Foundation INSF (grant number 93021576).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s10753-017-0695-1.
Rights and permissions
About this article
Cite this article
Rasooli, R., Pourgholamhosein, F., Kamali, Y. et al. Combination Therapy with Pirfenidone plus Prednisolone Ameliorates Paraquat-Induced Pulmonary Fibrosis. Inflammation 41, 134–142 (2018). https://doi.org/10.1007/s10753-017-0671-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0671-9