Abstract
Propranolol is a nonselective, beta-adrenergic receptor blocker with anti-angiogenic and anti-inflammatory effects that could be used in the treatment and control of many vascular disorder-related retinopathies. In this pilot study, the effects and complications of an intra-vitreal injection of propranolol HCl on the rabbit retina were investigated by clinical and histopathological means as the first step to determination the viability of intra-vitreal propranolol administration. Group 1 (n = 8) had 15 μg of propranolol HCL given, via intra-vitreal injection, into the right eye. Group 2 (n = 8) had a matching volume of balanced saline solution also via intra-vitreal injection into the right eye. After 4 weeks, all rabbits were killed humanely and the right eyes from both groups enucleated for histopathological study. Clinical observations and histopathological findings suggest no difference between propranolol and control groups (P ≥ 0.05). As a result of handling, in some cases, the eyes had light inflammation, hyperaemia and oedema with acute degeneration changes. This study shows that 15 μg of propranolol does not cause any significant abnormalities and is not a toxic dose for intra-vitreal injection in rabbits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Propranolol, a nonselective, beta-adrenergic receptor blocking agent, has been used in the treatment of many cardiovascular diseases, such as arterial hypertension, coronary artery disease and heart failure (Degoute 2007; Priviero et al. 2007; Igarashi et al. 2006) and is also used in the treatment of non-cardiovascular disease like severe migraine attacks, some tumours and inflammatory disease (Hoffmann and Reuter 2007; Benish et al. 2008; Kato et al. 2009; Tang et al. 2008). Anti-vascularization and vasoconstriction effects of propranolol were incidentally observed on eyelid haemangioma growth in children treated for heart problems (Léauté-Labrèze et al. 2008). Decreased serum levels of vascular endothelial growth factor (VEGF) following treatment with propranolol in haemangioma have also been observed (Zhao et al. 2011). Beta-adrenergic receptor (AR) blockade with propranolol can lead to downregulation of pro-angiogenic factors like VEGF, reduction of retinal haemorrhage and tufts and interfere with retinal neovascularization in retinopathy of prematurity (ROP) (Filippi et al. 2010). Propranolol also decreased angiogenic factors in leukemic T and monocyte cells on in vitro study (Hajighasemi and Hajighasemi 2009). Due to these properties, propranolol could be an alternative to previous treatment such as cryotherapy, laser therapy and intra-vitreal injections of anti-VEGF drugs (like bevacizumab) to treat neovascularization of retinopathies (Dobson et al. 2006; Kivlin et al. 1996; Early Treatment For Retinopathy Of Prematurity Cooperative Group 2003). Propranolol, compared with previous treatments, is more accessible, cheaper, well tolerated and with fewer side effects (Robinson et al. 2001; Penn et al. 2008).
In this study, we have attempted to investigate the intraocular effects of propranolol following intra-vitreal injection of propranolol HCL; this investigation could be the first step to find the optimum intra-vitreal dosage of propranolol, and we investigated the safety of this route of propranolol administration by ocular examination and histopathology.
Method and materials
Sixteen female New Zealand white rabbits of similar same age and weight were obtained from the Pasteur Institute of Iran. The rabbits were allocated in to two groups and individually housed, with equal feeding and access to water. Group 1 had 15 μg of propranolol HCl administered intra-vitreally into the right eye (propranolol group) (n = 8) and group 2 (control group) (n = 8) received a volume of 15 μl BSS by the same approach. Before intra-vitreal injections, all rabbits were anaesthetized using ketamine hydrochloride (25 mg/kg) and xylazine hydrochloride (2 mg/kg) intramuscularly. All of the intra-vitreal injections were performed in the right eye, using a sterile syringe, with 31-gauge needle (Hamilton syringe), approximately 4 to 5 mm posterior to the limbus in the pars plana area according to the standard model.
Ocular examinations were done before and after injection, and at days 7, 14, 21 and 28. Clinical observation included fundus and slitlamp examinations. After 4 weeks, all animals were anaesthetized and killed humanely by intracardiac injection of 50 % magnesium sulfate. The right eyes of both groups were enucleated and fixed with 10 % neutral buffered formalin solution for shipment to the laboratory of the eye bank of Iran. The eyes were processed for paraffin embedding, sectioned and stained with haematoxylin and eosin (H&E) and glial fibrillary acidic protein (GFAP) separately. All histopathological findings were scored 0 to 5 for statistical evaluation, 0 indicating no change and 5 severe change. Changes within and between two groups were assessed by statistical analysis (SPSS).
Results
In clinical observations, no flare or cells were seen in the anterior chamber or corneal limbus. Mild local changes in the lens (trace cataracts) were seen In three cases from the control group and two cases from the treated group. Fundus examination revealed no detachment or opacity of the retina. Retinal examination for inflammation or any vitreal haemorrhage was negative. Some cases had mild inflammation, hyperaemia and oedema with no severe degeneration changes for 2 to 3 days after intra-vitreal injection.
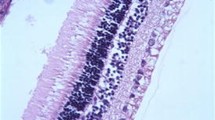
Histological findings on H&E staining revealed no inflammation or necrosis in the retinal layers or choroid layer (Fig. 1). There was no intra-vitreal haemorrhage present in any of the eyes examined, and retinal pigment epithelium (RPE) integrity and sensory retinal integrity were preserved in all eyes.
GFAP staining showed mild reaction in the ganglion cells of the retina of three control eyes (Fig. 1) and one treated eye (Fig. 2). Also, the same reaction was seen in other retinal cells like Müller cells and the photoreceptor layer in some sections of one control eye and one propranolol group eye. All of these histopathological findings are mild, and in both groups considered statistically not significant (P > 0/05) (Figs. 3, 4, 5, 6 and 7).
Discussion
Anti-vascularization and vasoconstriction effects of beta-AR blockers and their effects such as downregulation of pro-angiogenic factors in retinopathies with neovascularization, such as ROP and diabetic retinopathy, inspired us to do this investigation (Hajighasemi and Hajighasemi 2009; Filippi et al. 2010). Although some studies indicated propranolol was not effective against the development of retinopathy in mouse model of oxygen-induced retinopathy (OIR) in three routes of delivery (oral gavage, IP injection and SC injection) (Jing et al. 2012), most investigations showed propranolol was effective in angiogenic abnormalities of the retina such as ROP (Filippi et al. 2010; Early Treatment For Retinopathy Of Prematurity Cooperative Group 2003; Ristori et al. 2011; Martini et al. 2011). The objective of this investigation was to check intra-vitreal injection of propranolol as a new route for propranolol administration that could be more effective on the inner layers of the retina without the systemic side effects of this drug that include bronchospasm, heart failure, bradycardia, hypotension and heart block (Frishman 1988).
These results have shown that propranolol, in this dose, has no complications with respect to the retina and other intraocular tissues. The cataract seen in some eyes of both groups was a side effect of intra-vitreal injection—the large size of lens in a rabbit’s eye can be damaged through intra-vitreal injections.
Toxic complications of propranolol will occur at serum levels above 2000 ng/kg, and we chose 15,000 ng/kg in this investigation for the intra-vitreal injection. According to the results, this dose of propranolol hydrochloride was not toxic for this route of administration, and further study should be done to check higher doses.
References
Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, Ben-Eliyahu S (2008) Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol 15(7):2042–2052
Degoute CS (2007) Controlled hypotension: a guide to drug choice. Drugs 67(7):1053–1076
Dobson V, Quinn GE, Summers CG, Hardy RJ, Tung B (2006) Cryotherapy for Retinopathy of Prematurity Cooperative Group: Visual acuity at 10 years in Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) study eyes: effect of retinal residua of retinopathy of prematurity. Arch Ophthalmol 124:199–202
Early Treatment For Retinopathy Of Prematurity Cooperative Group (2003) Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 121:1684–1694
Filippi L, Cavallaro G, Fiorini P et al (2010) Study protocol: safety and efficacy of propranolol in newborns with Retinopathy of Prematurity (PROP-ROP): ISRCTN18523491 (Abstract). BMC Pediatr 10:83
Frishman WH (1988) Beta-adrenergic receptor blockers. Adverse effects and drug interactions. Hypertension 11:II21–II29
Hajighasemi F, Hajighasemi S (2009) Effect of propranolol on angiogenic factors in human hematopoietic cell lines in vitro. Iran Biomed J 13(4):223–228
Hoffmann J, Reuter U (2007) Treatment of migraine. Dtsch Med Wochenschr 132(41):2153–2158
Igarashi N, Nozawa T, Fujii N, Suzuki T, Matsuki A, Nakadate T, Igawa A, Inoue H (2006) Influence of beta-adrenoceptor blockade on the myocardial accumulation of fatty acid tracer and its intracellular metabolism in the heart after ischemia-reperfusion injury. Circ J 70(11):1509–1514
Jing Chen, Jean-Sebastian Joyal, Colman J. Hatton, Aimee M. Juan, Dorothy T. Pei, Christian G. Hurst, Dan Xu, Andreas Stahl, Ann Hellstrom, Lois E. H. Smith (2012) Propranolol Inhibition of b-Adrenergic Receptor Does Not Suppress Pathologic Neovascularization in Oxygen-Induced Retinopathy. IOVS Vol. 53, No. 6
Kato H, Kawaguchi M, Inoue S, Hirai K, Furuya H (2009) the effects of beta-adrenoceptor antagonists on proinflammatory cytokine concentrations after subarachnoid hemorrhage in rats. Anesth Analg 108(1):288–295
Kivlin JD, Biglan AW, Gordon RA, Dobson V, Hardy RA, Palmer EA, Tung B, Gilbert W, Spencer R, Cheng KP, Buckley E (1996) Early retinal vessel development and iris vessel dilatation as factors in retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) Cooperative Group. Arch Ophthalmol 114:150–154
Léauté-Labrèze C, DumasdelaRoque E, Hubiche T et al (2008) Propranolol for severe hemangiomas of infancy. N Engl J Med 358:2649–2651
Martini D, Dal Monte M, Ristori C et al (2011) Antiangiogenic effects of β2-adrenergic receptor blockade in a mouse model of oxygen induced retinopathy. J Neurochem 119:1317–1329
Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME (2008) Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 27:331–371
Priviero FB, Teixeira CE, Claudino MA, De Nucci G, Zanesco A, Antunes E (2007) Vascular effects of long-term propranolol administration after chronic nitric oxide blockade. Eur J Pharmacol 571(2–3):189–196
Ristori C, Filippi L, Dal Monte M et al (2011) Role of the adrenergic system in a mouse model of oxygen-induced retinopathy: antiangiogenic effects of beta-adrenoreceptor blockade. Invest Ophthalmol Vis Sci 52:155–170
Robinson GS, Ju M, Shih SC, Xu X, McMahon G, Caldwell RB, Smith LE (2001) Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J 15:1215–1217
Tang FK, Hua N, Lu H, Xiao J, Tang XZ, Qi Z (2008) Effects of bisoprolol on serum interleukin-6 and tumor necrosis factor-alpha level in patients with congestive heart failure. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 24(12):1177–1179
Zhao ZF, Lü RR, Huo R et al (2011) The change of serum vascular endothelial growth factor and matrix metalloproteinases-9 in proliferative hemangioma treated with propranolol. Zh Zheng Xing Wai Ke Za Zhi 27(5):359–361
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taghavidinani, S.I., Aldavood, S.J., Mashhadirafii, S. et al. Histopathologic examination of the retina following intra-vitreal propranolol administration in rabbits. Comp Clin Pathol 24, 981–984 (2015). https://doi.org/10.1007/s00580-014-2015-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-014-2015-6