Abstract
The aim of the current study was to evaluate the effect of apocynin (APO) on the development of proliferative vitreoretinopathy (PVR). New Zealand-type male rabbits were randomly grouped into three as follows: (1) Sham group rabbits which were applied intraperitoneal (i.p.) vehicle without PVR; (2) PVR group rabbits where PVR was created and an i.p. vehicle was administered for 21 successive days; (3) PVR + APO group rabbits where PVR was created and i.p. APO was administered for 21 successive days. Fundus examination was conducted with an indirect ophthalmoscope before starting the experiments and at each visit afterwards. At the end of the work, the rabbits were sacrificed under high-dose anesthesia and then eye tissues were taken for histopathological analyses. In the PVR + APO group, histopathologic and ophthalmoscopic examination revealed significant decrease in PVR formation. As the result, it has been observed that APO at least partially inhibits PVR formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proliferative vitreoretinopathy (PVR) is an abnormal tissue response that is characterized by the formation of membranes which contract with non-neoplastic cell proliferation on both the inner and outer retinal surface and vitreous [1]. It most often develops after failed retinal detachment surgery and also can occur secondary to proliferative diabetic retinopathy and posttraumatic sequelae. It is one of the main causes of permanent vision loss worldwide [2].
The mainstay of the management of PVR is surgery. Although the surgery resulted in anatomical success, functional results usually do not reach the desired level. The basic event of PVR pathophysiology is retinal detachment and the relevant vitreal changes [3]. However, inflammatory reactions have been shown to predispose to PVR in several studies [4–7]. Preclinical investigators have focused especially on primary treatments or those that are secondary to surgery. However, several pharmacological agents have been investigated that did not enter clinical practice because of ocular and systemic side effects and low therapeutic effect [4–7].
Apocynin (4-hydroxy-3-methoxyacetophenone; APO) is an effective NADPH-oxidase (NOX) inhibitor obtained from the root of the apocynum cannabinum plant [8]. Excessive reactive oxygen species (ROS) formation is known to lead to clinical disorders by increasing oxidative stress and apoptotic cell death. APO decreases ROS and thus oxidative stress through NOX inhibition [9, 10]. Kilic et al. have reported that APO decreases neutrophil oxidative burst and neutrophil chemotaxis, and therefore neutrophil-mediated cell injury. APO was emphasized to have therapeutic and protective effects on pulmonary fibrosis in the same study. Neuroprotective effects of APO have also been demonstrated in the study of Simonyi et al. [8].
The relationship between NOX and vascular endothelial growth factor (VEGF) in ischemic retinopathy was investigated in a study by Al-Shabrawey et al. [11]. In this study, APO was observed to suppress the oxidative stress triggered by ischemia, decrease VEGF to normal levels, and provide protection from retinal neovascularization in rats. Another study found that superoxide radical production was decreased significantly and rod cells were preserved when APO was administered to rats with photoreceptor degeneration [12].
We could not find any study on the effects of APO on PVR in the literature. So, in this study, we aimed to clinically and histopathologically investigate the therapeutic effect of APO administered intraperitoneally against PVR in an experimental PVR model in rabbits.
Materials and methods
New Zealand-type male rabbits aged 1 year with a mean weight of 2.5 kg were kept at 20 ± 2 °C temperature, 55–60 % humidity, and a 12:12 light/dark cycle. All the experiments and animal care procedures were regulated in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Inönü University Local Animal Experimentation Ethics Committee (Ref No: 2015/A-72). The rabbits were randomly divided into three groups: (1) Sham group rabbits that were administered intraperitoneal (i.p.) vehicle without creating PVR; (2) PVR group rabbits where PVR was created and an intraperitoneal (i.p.) vehicle was administered for 21 successive days; (3) PVR + APO group rabbits where PVR was created and i.p. APO 20 mg/kg (Apocynin, Sigma-Aldrich) was administered for 21 successive days. The animals in all groups were then sacrificed with a high anesthetic dose on the 22nd day and the eyes were enucleated.
Platelet-rich plasma (PRP) preparation and PVR creation
All rabbits were anesthetized with ketamine hydrochloride at a dose of 50 mg/kg body weight and 2 % xylazine hydrochloride at a dose of 10 mg/kg body weight intramuscularly. A 10-ml blood sample was drawn from the marginal ear vein after anesthesia. This 10-ml blood sample was added to a tube and mixed. The single centrifuge protocol was implemented to obtain PRP [13]. The separation of blood cell elements was realized using a laboratory-type centrifuge at 160 g for 3 min at 3000 rpm and at room temperature, and the blood was separated into three basic components: red blood cells (the bottom layer), PRP (the middle layer of the tube), and platelet-poor plasma (PPP) (the top layer). The PPP layer was removed with a pipette and discarded. We then placed a mark 2 mm below the line separating the bottom layer from the middle layer. All the components above this mark (about 1.2 ml) were removed with a pipette. Tropicamide drops (1 %) (Tropamid forte, Bilim, Turkey) were administered twice to the eyes of the rabbits in group 2 and 3 to provide mydriasis. Then, 0.15 ml (75,000) U PRP was administered intravitreally with the help of an indirect ophthalmoscope using a 30-gauge syringe placed right below the upper temporal limbus. Fundus examination was performed everyday to make sure PVR had developed.
Apocynin treatment
Group 3 was administered i.p. 20 mg/kg APO everyday for 21 days starting with the day after intravitreal PRP administration. The eyes were enucleated and sent to the laboratory for histopathologic analyses following indirect ophthalmoscopic examination on the 22nd day of the study.
Ophthalmoscopic examination
Fundus examination was conducted with a portable indirect ophthalmoscope (Heine Optotechnik, Herrsching, Germany) after the eyes were dilated with tropicamide before starting the experiments and at each visit afterwards. The results were recorded using the PVR classification modified by Machemer et al. [14] in 1991 as grade A: limited to cells in the vitreous and blurring, grade B: tear with rolled or irregular edges or subclinical contractions characterized by wrinkles at the inner retinal surface, or grade C: preretinal or retinal membranes that are anterior (Ca) or posterior to the equator (Cb) [14].
Histological evaluation
Enucleated eyes were fixed in 10 % formalin and embedded in paraffin. Paraffin-embedded specimens were cut into 4-µm-thick sections as horizontal section through the optic disk of the eye and mounted on slides. The sections were stained with hematoxylin and eosin for the evaluation of disruption of inner retina (from the inner plexiform layer to the inner limiting membrane), epiretinal membrane formation, the presence of retinal fold, and abnormal blood vessel growth.
Proliferative changes of PVR were graded according to the classification of Fastenberg except moderate modification [15]. PVR is divided into three progressive grades with grade 1 being the least severe and grade 3 being the severest according to the scoring system (Table 1). Microscopic analysis was carried out at X40 objective with a Leica DFC 280 light microscope (Leica Micros Imaging Solutions Ltd, Cambridge, UK).
Statistical analysis
All data were analyzed with a commercially available statistics software package (SPSS for Windows v. 15.0, Chicago, IL., USA). Distribution of the groups were analyzed with the Kolmogorov–Smirnov test, as with all groups showed a normal distribution. For all parameters, one-way ANOVA was performed with Tukey’s post hoc test. Results were presented as mean ± SD. p < 0.05 were regarded as statistically significant in all data (Table 2).
Results
Histologic results
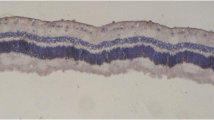
Layers of the retina were observed as intact in sham group using H–E staining methods (Fig. 1). Proliferative changes such as the formation of new blood vessel or preretinal membrane was not observed in this group. However, sections from PVR groups showed markedly edematous inner retina and new blood vessel formation along with retina inner surface. Some of the vessels were filled with blood cells and dilated. Furthermore, epiretinal membrane was also recognized. An epiretinal membrane was a fibrocellular tissue found on the inner surface of the retina (Fig. 2a, b). This membrane tightly adhering to the internal limiting membrane in some areas, in addition to the retina, was convoluted in some places. While the mean PVR stages of the without drug group was found 2.28 ± 0.75, a PVR + drug group was 1.42 ± 0.53. The difference between the PVR group and PVR + drug groups was statistically significant (p = 0.04) (Table 3). On the other hand, although retinal damage was recognized as alleviated in PVR + drug group, the lesions did not completely improve. Degenerative alterations like focal edematous alterations, epiretinal membrane formation, and localized vascular changes were still present in this group (Fig. 3a, b). In addition, a significant difference was observed in both groups (PVR group and PVR + drug) in terms of the score of PVR when compared with the sham group (p = 0.001 and p = 0.04, respectively). Inflammatory cells were not observed in any of PVR groups.
Ophthalmoscopy results
Data recorded according to the clinical PVR grading system are summarized in (Table 4). Briefly, PVR + APO group showed improvement in a statistically significant level according to the grading system of Machemer et al.[14], and there was no grade C-specific PVR finding (p = 0.001).
Discussion
The most common reason for PVR is unsuccessful or inadequate retinal detachment surgery. PVR is usually related to giant retinal tears, posterior segment trauma, vascular proliferative retinopathy, and excessive cryotherapy (Table 5). It is believed to be the result of a recovery from biological injury and is characterized by the proliferation of retinal pigment epithelium cells, glial cells, and fibroblasts [16].
A more detailed explanation of PVR pathogenesis is as follows: a cascade occurs during the recovery from a tissue injury. This recovery from the injury process can be divided into three basic phases: inflammation, proliferation, and scar formation. The inflammation phase in the vitreoretinal region develops because the blood–retina barrier is damaged. The platelets active in this region secrete chemotactic factors such as platelet-derived growth factor, transforming growth factor β (TGF-β), and epidermal growth factor leading to monocyte and macrophage influx to the region. Monocytes and macrophages also produce proliferative factors such as VEGF, fibroblast growth factor, insulin-like growth factor, and hepatocyte growth factor [17]. In conclusion, a proliferation phase that results in connective tissue cells and granulation tissue formation in the area is realized [18]. In the following scar phase, there is a reorganization of extracellular matrix formation, fibroblast contraction, and development of intensive granulation tissue [19]. This is clinically observed with cellular membranes on both retinal surfaces and hyaloid. These membranes apply traction to the retina and macula that can lead to new tears, persistently detached areas, granulation tissue formation in all retinal layers, and a distorted macula [16].
Surgery has been the standard treatment for PVR. Anatomic success is achieved with successful surgery. However, the functional results are not at the desired level3. Investigators have tried various medical treatments for this purpose, but only some of them could be used in clinical studies due to the low degree of effect and/or high incidence of side effects [20, 21].
Corticosteroids and antimetabolites such as 5-fluorouracil and mytomycin-c, which are thought to be able to prevent PVR pathogenesis, have been used in animal and human studies [7, 22]. The effects of corticosteroids have been shown to be weak outside the early period or preoperative use [23]. Antimetabolite use has not become popular due to the serious side effects [7]. Drugs and molecules such as retinoic acid, retinol, carmustine, silicone oil tamponade, interleukin-4, nilvadipine, and taxol have also been tried for treatment in various experimental models [24–27].
In this study, we created a successful PVR model with intravitreal PRP administration and started intraperitoneal treatment with APO to investigate its therapeutic effects in rabbits. We thought that APO could be used as an alternative or additional treatment for surgical procedures when planning our study as it suppresses VEGF production via NOX inhibition, protects structures such as photoreceptor cells directly relevant to vision physiology, and it has been proven antioxidant and neuroprotective effects [12, 28, 29]. APO is known as a strong NOX inhibitor and it has been shown to have a positive therapeutic effect on photoreceptors in a study conducted on mice with photoreceptor degeneration [12]. The authors of that study stated that neuronal cells and those related to vision physiology were especially negatively affected by the NOX activation in retinal dystrophies and that NOX inhibitors could give positive results [12]. NOX activation is thought to trigger the choroidal endothelial cell migration that has critical importance in age-related macular degeneration and it was suppressed with APO in an in vitro model with ultimately a significant reduction in choroidal neovascularization due to the powerful antioxidant effects [28]. APO showed strong neuroprotective effects in monkeys with experimental Parkinson’s disease and decreased Parkinson’s symptoms in a study evaluating the neuroprotective effects [29].
Most of the previous studies which were evaluated the APO on the eye are realized its effects via NOX inhibition. Positive effects of APO and similar NOX inhibitors have been shown in conditions such as choroidal neovascularization and diabetic retinopathy where the intraocular placental growth factor (PGF), tumor necrosis factor (TNF), VEGF, and similar enzymes have increased [30, 31]. These effects were also proven histopathologically and biochemically. It is well established that intraocular levels of PGF, TNF, VEGF, and similar enzymes increase in PVR developing secondarily to vitreoretinal surgery or advanced proliferative diabetic retinopathy [32, 33]. We believe that APO could at least partially decrease unwanted inflammation, granulation, and scar formation through NOX inhibition.
Kilic et al. have shown that APO decreases granulation and fibrosis in bleomycin-induced pulmonary fibrosis [34]. The fibroblastic activity increase and granulation tissue formation provide anatomic healing during there recovery process after injury but have negative effects on functional recovery. This granulation tissue that develops in disorders like PVR that involve all layers of the retina replaces structures such as photoreceptors and ganglion cells following recovery and it has a negative effect on vision [16]. The effects of APO on the hepatotoxicity induced by cisplatin were investigated in a recent study and it showed positive effects on inflammation, granulation tissue formation, and vascular structures [35]. The positive effects of APO on apoptosis and histopathological changes were also demonstrated in a study which evaluated the effects of APO in radiotherapy-induced intestinal damage where a decrease in granulation tissue and scar formation [36]. In our study, we found a significant improvement in histopathological parameters such as retinal folds, epiretinal membrane formation, and neovascularization. There was almost no clinically significant pathology on ophthalmoscopic examination in the group that received treatment other than one rabbit with membranes at the posterior equator and four rabbits with vitreous haze. In addition, retinal tears were not observed in any of treatment group eyes. At least one retinal tears were observed in all of non-treatment group eyes.
One of the limitations of our study is that the current study could not include oxidative stress parameters biologically as APO is known to be a strong antioxidant, and not photographing the fundi due to our limited equipment.
In conclusion, we believe that APO can be used to treat PVR both histopathologically and clinically, at least to some extent. Considering all the described features of APO, it could be used primarily or in addition to the primary treatment in eye disorders without a definite treatment, such as PVR. However, further experimental and clinical studies are needed to verify these results before its use in clinical practice.
References
The Retina Society Terminology Committee (1983) The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 90:121–125
Weller M, Wiedemann P, Heimann K (1990) Proliferative vitreoretinopathy—is it anything more than wound healing at the wrong place? (review). Int Ophthalmol 14(2):105–117
Charteris DG (1995) Proliferative vitreoretinopathy: pathobiology, surgical management and adjunctive treatment. Br J Ophthalmol 79:953–960
Araiz JJ, Refojo MF, Arroyo MH, Leong FL, Albert DM, Tolentino FI (1993) Antiproliferative effect of retinoic acid in intravitreous silicone oil in an animal model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 34:522–530
Blumenkranz MS, Hernandez E, Ophir A, Norton EWD (1984) 5-Fluorouracil: new applications in complicated retinal detachment for an established antimetabolite. Ophthalmology 91:122–130
Berman DH, Gombos GM (1989) Proliferative vitreoretinopathy: does low-dose colchicine have an inhibitory effect? A controlled study in humans. Ophthalmic Surg 20:268–272
Berger AS, Cheng CK, Pearson PA, Ashton P, Crooks PA, Cynkowski T et al (1996) Intravitreal sustained release corticosteroid-5-fluorouracil conjugate in the treatment of experimental proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 37:2318–2325
Simonyi A, Serfozo P, Lehmidi TM, Cui J, Gu Z, Lubahn DB et al (2011) The neuroprotective effects of apocynin. Front Biosci (Elite Edition) 4(1):2183–2193
El-Sawalhi MM, Ahmed LA (2014) Exploring the protective role of apocynin, a specific NADPH oxidase inhibitor, in cisplatin-induced cardiotoxicity in rats. Chem Biol Interact 207:58–66
Ozbek O, Altintas R, Polat A, Vardi N, Parlakpinar H, Sagir M et al (2015) The protective effect of apocynin on testicular ischemia-reperfusion injury. J Urol 193:1417–1422
Al-Shabrawey M, Bartoli M, El-Remessy AB, Platt DH, Matragoon S, Behzadian MA et al (2005) Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol 167(2):599–607
ZengH DingM, ChenXX LuQ (2014) Microglial NADPH oxidase activation mediates rod cell death in the retinal degeneration in rd mice. Neuroscience 275(5):54–61
Anitua E (1999) Plasma rich in growth factors: preliminary results of use in the preparation of sites for implants. Int J Oral Maxillofac Implants 14:529–535
Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM (1991) An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol 112(2):159–165
Fastenberg DM, Diddie KR, Sorgente N, Ryan SJ (1982) A comparison of different cellular inocula in an experimental model of massive periretinal proliferation. Am J Ophthalmol 93:559–564
Pastor JC (1998) Proliferative vitreoretinopathy: an overview. Surv Ophthalmol 43(1):3–18
Wiedemann P (1992) Growth factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy and retinal degeneration. Surv Ophthalmol 36:373–384
Johnston R (1988) Immunology: monocytes and macrophages. N Engl J Med 318:747–752
Wilkins RB, Kulwin DR (1979) Wound healing. Ophthalmology 86:507–510
Ryan SJ (1993) Traction retinal detachment. XLIX Edward Jackson Memorial Lecture. Am J Ophthalmol 115:1–20
Weller M, Wiedemann P, Heimann K (1990) Proliferative vitreoretinopathy. Is it anything more than wound healing at the wrong place? Int Ophthalmol 14:105–117
Yang CS, Khawly JA, Hainsworth DP, Chen SN, Ashton P, Guo H et al (1998) An intravitreal sustained-release triamcinolone and 5-fluorouracil codrug in the treatment of experimental proliferative vitreoretinopathy. Arch Ophthalmol 116(1):69–77
Chandler DB, Hida T, Sheta S, Proia AD, Machemer R (1987) Improvement in efficacy of corticosteroid therapy in an animal model of proliferative vitreoretinopathy by pretreatment. Graefes Arch Clin Exp Ophthalmol 225:259–265
Arroyo MH, Refojo MF, Araiz JJ, Tolentino FI, Cajita VN, Elner VM (1993) Silicone oil as a delivery vehicle for BCNU in rabbit proliferative vitreoretinopathy. Retina 13(3):245–250
Daniels S, Coonley K, Yoshizumi M (1990) Taxol treatment of experimental proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol 228:513–516
Hart PH, Vitti GF, Burgess DR, Whitty GA, Piccoli DS, Hamilton JA (1989) Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor-b, interleukin-1, and prostaglandin-E2. Proc Natl Acad Sci USA 86:3803–3807
Nomoto A, Mutoh S, Hagihara H, Yamaguchi I (1988) Smooth muscle cell migration induced by inflammatory cell products and its inhibition by a potent calcium antagonist, nilvadipine. Atherosclerosis 72:213–219
Monaghan-Benson E, Hartmann J, Vendrov AE, Budd S, Byfield G, Parker A et al (2010) The role of vascular endothelial growth factor-induced activation of NADPH oxidase in choroidal endothelial cells and choroidal neovascularization. Am J Pathol 177(4):2091–2102
Philippens IH, Wubben JA, Finsen B, ‘t Hart BA (2013) Oral treatment with the NADPH oxidase antagonist apocynin mitigates clinical and pathological features of parkinsonism in the MPTP marmoset model. J Neuroimmune Pharmacol 8(3):715–726
Wang H, Han X, Wittchen ES, Hartnett ME (2016) TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol Vis 22(3):116–128
Wang H, Fotheringham L, Wittchen ES, Hartnett ME (2015) Rap1 GTPase inhibits tumor necrosis factor-α-induced choroidal endothelial migration via NADPH oxidase- and NF-κB-dependent activation of Rac1. Am J Pathol 185(12):3316–3325
Vaziri K, Schwartz SG, Relhan N, Kishor KS, Flynn HW Jr (2015) New therapeutic approaches in diabetic retinopathy. Rev Diabet Stud 12(1–2):196–210
Wang H, Hartnett ME (2016) Regulation of signaling events involved in the pathophysiology of neovascular AMD. Mol Vis 22(27):189–202
Kilic T, Parlakpinar H, Taslidere E, Yildiz S, Polat A, Vardi N et al (2015) Protective and therapeutic effect of apocynin on bleomycin-induced lung fibrosis in rats. Inflammation 38(3):1166–1180
Cagin YF, Erdogan MA, Sahin N, Parlakpinar H, Atayan Y, Polat A et al (2015) Protective effects of apocynin on cisplatin-induced hepatotoxicity in rats. Arch Med Res 46(7):517–526
Cagin YF, Parlakpinar H, Polat A, Vardi N, Atayan Y, Erdogan MA et al (2016) The protective effects of apocynin on ionizing radiation-induced intestinal damage in rats. Drug Dev Ind Pharm 42(2):317–324
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozer, M.A., Polat, N., Ozen, S. et al. Histopathological and ophthalmoscopic evaluation of apocynin on experimental proliferative vitreoretinopathy in rabbit eyes. Int Ophthalmol 37, 599–605 (2017). https://doi.org/10.1007/s10792-016-0318-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-016-0318-0