Abstract
Arbuscular mycorrhizal (AM) symbiosis plays an important role in ecosystem functioning, particularly in fragile environments. Little is known, however, about how AM fungus community composition responds to slope aspect. Our objective was to compare the AM fungus communities between sunny and shady slopes and to detect factors that influenced the distributions of AM fungi in arid ecosystems of the Daqingshan Mountains, Inner Mongolia, North China. AM fungus communities were evaluated based on small subunit ribosomal RNA genes (SSUs) using Illumina MiSeq sequencing. AM fungus community composition differed significantly between slope aspects, and sunny slopes had significantly higher AM fungus diversity and richness as well as spore density, total root colonization, arbuscule abundance, vesicle abundance, and hyphal colonization than shady slopes. Structural equation modeling (SEM) illustrated that the effects of slope aspect on AM fungus richness likely were mediated by available phosphorus, soil organic carbon, plant cover, and plant diversity. Available phosphorus was the principal factor that influenced AM fungus species richness, and soil organic carbon was the principal factor influencing spore density and total root colonization, suggesting that these factors especially might be responsible for differences between the AM fungus communities of different slope aspects. These findings elucidate the influence of slope aspect on AM fungus communities and may inform use of AM fungi in protection and restoration of vegetation with different slope aspects in arid ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Daqingshan Mountains of Inner Mongolia are an important ecological barrier in northern China, where climate change and human activities cause considerable damage to vegetation resources, and severely soil-eroded and degraded regions are found. Arbuscular mycorrhizal (AM) fungi help plants to survive in the arid conditions that prevail there (Caravaca et al. 2005; Caravaca et al. 2003) by increasing uptake of water and nutrients (Smith and Read 2008) and improving soil structure (Caravaca et al. 2005). In addition, AM fungus diversity may affect the diversity and productivity of plants and the stability and sustainability of the ecosystems (Van Der Heijden et al. 2006; Vogelsang et al. 2006). Recent research has suggested that AM fungus communities together with indigenous plant species are an excellent approach to initiate and promote the re-vegetation of arid ecosystems (Barea et al. 2011; Graf and Frei 2013). Because of the significant roles played by AM fungus communities in plant growth and in the function of ecosystems in arid environments, it is essential to evaluate AM fungus diversity and community composition.
Although a few reports have focused on the ectomycorrhizal fungi associated with several tree species of the Daqingshan Mountains (Bai et al. 2006; Bai et al. 2011), little information is available concerning AM fungi associated with plant communities in this region. In general, however, abiotic and biotic factors affect AM fungus diversity and community composition, such as soil pH (Dumbrell et al. 2010), available phosphate (Xiang et al. 2014), available nitrogen (Camenzind et al. 2014), soil organic carbon (Wu et al. 2012), host plants (Mao et al. 2014; Veresoglou and Rillig 2014), plant diversity (Antoninka et al. 2011), and plant cover (Liu et al. 2011). Apart from these factors, in many mountainous areas, altitude also has been suggested as a primary determinant of AM fungus diversity and community composition (Bonfim et al. 2016; Gai et al. 2012; Senés-Guerrero et al. 2014). Moreover, previous studies have reported that slope aspect can strongly affect soil pH, moisture content, soil organic carbon, total nitrogen, available nitrogen, and available phosphorus (Begum et al. 2013; Kutiel 1992; Sidari et al. 2008) as well as plant diversity and cover in many natural ecosystems (Kutiel 1992; Nadal-Romero et al. 2014). Until now, only one study evaluated the influence of slope aspect on AM fungus communities in forested environments of the Greater Khingan Mountains of China (Chu et al. 2016). We have found no such study of arid ecosystems; while it is well established that AM fungus communities in different habitats can be quite different (Brundrett and Ashwath 2013; Öpik et al. 2013), it is of interest to know the driving factors and potential differences in AM fungus diversity and communities from different slope aspects in an arid environment.
Structural equation modeling (SEM) is a generalization of simultaneous equation procedures originating from path analysis, and SEM is used widely in the general ecological literature to develop causal understanding from observational data (Eisenhauer et al. 2015; Laliberté et al. 2013). Recently, the direct and indirect causal effects of experimental warming, land use, carbon dioxide enrichment, nitrogen fertilization, plant diversity, and host plants on AM fungus communities were evaluated through SEM (Antoninka et al. 2011; Wilson et al. 2016; Xiang et al. 2014; Yang et al. 2012; Zheng et al. 2014). So, SEM will be a useful tool to examine AM fungus communities and their potential mediation by slope aspect, abiotic, and biotic factors in the Daqingshan Mountains.
Additionally, high-throughput sequencing technologies have been recognized as powerful tools for studying environmental microbial diversity. Recently, Illumina MiSeq sequencing has been applied to investigate AM fungus diversity to great success (Cui et al. 2016). Importantly, this method is best with respect to throughput per run and minimizing the number of consensus errors (Jünemann et al. 2013). DNA fragments of 300 bp amplified with the primers AMV4.5NF/AMDGR (Sato et al. 2005) are suitable for the Illumina MiSeq platform (Mahé et al. 2014). These primers produce a higher proportion of AM fungus sequences, a broader spectrum of Glomeromycota, and more high-quality AM fungus sequences than other primer pairs (Lumini et al. 2010; Van Geel et al. 2014). So to study the fungus communities of the Daqingshan Mountains, we will employ Illumina MiSeq sequencing combined with the AMV4.5NF/AMDGR primers.
In this study, we characterize the AM fungus communities of sunny slopes (south facing) and shady slopes (north facing) to investigate two questions: (1) whether slope aspect has an effect on AM fungus diversity and community composition in this region and (2) what soil characteristics and plant community associated with slope aspect are driving factors that shape the AM fungus communities. By addressing these questions, we intend to increase knowledge concerning the effects of slope aspect on the distributions of AM fungus communities in arid environments.
Materials and methods
Study area
The study area is located in the Daqingshan Mountains of Inner Mongolia, North China (40° 34′ N–41° 18′ N, 109° 46′ E–113° 04′ E). This region has an altitude range of 1000 to 2000 m and is characterized by a typical temperate continental climate with an average annual temperature range of 3 to 5 °C, average annual rainfall range of 320 to 450 mm, and average annual evaporation range of 1800 to 2300 mm.
Sampling
Sampling was conducted in August 2014. In the Hohhot area of the Daqingshan Mountains, three hills in nearly pristine condition comprising similar altitudes, similar slope angles of the sunny and shady slopes, and similar hill breadths (the straight-line distance from the bottom of the shady slopes to the bottom of the sunny slopes on the opposite side of the hill) were selected (Table 1). The distance between any pair of these hills exceeded 10 km. The shady slopes of the three hills were invariably north facing, and the sunny slopes were south facing. The most common plant species on the sunny slopes were Clematis fruticosa, Prunus pedunculate, Rosa xanthina, Heteropappus altaicus, Lespedeza davurica, Iris dichotoma, Artemisia spp., and Poaceae plants; along the shady slopes, Spiraea pubescens, Ostryopsis davidiana, Quercus mongolica, Rhamnus parvifolia, Prunus sibirica, Pinus tabulaeformis, and Artemisia spp. were common. Among these plants, there were three ectomycorrhizal species, Q. mongolica (Bai et al. 2006), P. tabulaeformis, and O. davidiana (Bai et al. 2009), but the other plants all associated with AM fungi. For each hill, three sampling locations were selected at altitudes of 1200, 1400, and 1600 m on each slope aspect, and at each location, three 5 × 5-m2 quadrats were arranged in a horizontal line. The distance between quadrats within a location was 20 m. Before sampling, the percentage plant cover, plant species identities, and total number of individuals per plant species were assessed in each quadrat. The plant cover was estimated visually for each quadrat, and the Shannon-Wiener index was used to quantify plant diversity. The three ectomycorrhizal species were found only on shady slopes, and we were especially interested in the effects of the AM plants, so we calculated diversity and cover with exclusion of the ectomycorrhizal species. Inclusion of the ectomycorrhizal species (data not shown) made no qualitative difference in our results.
Five soil cores (diameter, 3 cm; depth, 25 cm) were collected from each quadrat based on a quincunx sampling method, and subsequently were mixed well to provide a single representative sample per quadrat, for a total of 54 samples across all three hills. Approximately 20-g soil from each sample was immediately placed in an aluminum container, sealed, and subsequently used to determine the gravimetric soil moisture content. The remaining soil was stored in a sealed bag, transported in an ice box to the laboratory, and divided into two parts. One part was air dried, passed through a 2-mm sieve, and then used to analyze the physicochemical soil properties and spore density of AM fungi, and the other part was stored at −20 °C for using in the molecular analysis. Fine live roots were separated carefully from each soil sample, rinsed with distilled water, and placed in FAA solution (formaldehyde 5 mL, glacial acetic acid 5 mL, and 70 % ethanol 90 mL) before storage at 4 °C and subsequent determination of AM fungus colonization.
Soil physical and chemical analyses
Soil physical and chemical analyses were performed in triplicate for all 54 soil samples. Soil moisture content was measured using the drying method, soil pH was determined with a glass electrode in a ratio of soil to distilled water of 1:2.5, soil organic carbon was evaluated with the potassium dichromate oxidation method (Walkley 1947), total nitrogen content was evaluated using the Kjeldahl procedure (Nelson and Sommers 1982), and total phosphorus was assessed with the sodium hydroxide fusion and Mo-Sb spectrophotometry method (Schmidt et al. 1982). Available phosphorus was extracted with 0.5 mol/L of sodium bicarbonate and determined using the Mo-Sb spectrophotometry method (Olsen 1954), and available nitrogen was determined with the alkali N-proliferation method (Cornfield 1960).
AM fungus spore extraction and root colonization
Approximately 10-g air-dried soil randomly removed from the mixed samples was used to isolate spores by wet sieving, decanting, and centrifugation using sucrose (Li et al. 1991). The number of spores was counted using a stereomicroscope.
The root samples, which represented mixtures of plant species, were washed with distilled water two to three times and then cut into approximately 1-cm pieces, and a random sampling of these roots (>100 pieces) was subjected to staining. The roots were placed into 10 % KOH (w/v) in a 90 °C water bath for approximately 1 h, rinsed with distilled water, and then soaked in a 1 % HCl solution for 3 min, before being stained with 0.05 % trypan-blue in lactoglycerol in a 90 °C water bath for approximately 3 h, and finally destained with a solution of lactic acid and glycerol (1:1). Thirty root segments of each sample were selected randomly and placed onto a slide, and the total root colonization, arbuscule abundance, vesicle abundance, and hyphal colonization were estimated using the magnified intersection method (McGonigle et al. 1990) at ×200 magnification.
Soil DNA extraction, PCR amplification, and sequencing
Approximately a 0.5-g soil sample was used to extract DNA using the FastDNA® SPIN Kit (MP Biomedicals, Santa Ana, CA) according to the manufacturer’s instructions. The extracted soil DNA was first detected by agarose gel electrophoresis (1.0 % agarose in 0.5× TAE) to examine its integrity and approximate concentration, and then, it was quantified using a Qubit2.0® Fluorometer (Life Technologies, New York, USA) to determine the quantity of DNA to be amplified. In the current study, the primers MV4.5NF/AMDG (Sato et al. 2005) were used to amplify the most variable part of the small subunit (SSU) rRNA gene region. The mixed primers contained Illumina MiSeq sequencing adapters that were designed for use with the Illumina MiSeq platform and a 7-bp sample-specific molecular identifier barcode capable of identifying reads originating from different samples between the adapters and the forward primer. The PCR was conducted with a mixture containing 5 μL PCR buffer, 0.1 μL dNTP (10 mM), 0.5 μL primers (50 μM of each), 0.5 μL Taq DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA), 50 ng genomic DNA, and double-distilled H2O to a total volume of 50 μL. The PCR program was as follows: initial denaturing at 94 °C for 2 min; 35 cycles of 45 s at 94 °C, 45 s at 65 °C, and 45 s at 72 °C; and a final elongation of 10 min at 72 °C. The PCR products were separated and purified by gel electrophoresis (1.5 % agarose in 0.5× TAE) using the SanPrep Column DNA Gel Extraction Kit (Sangon Biotech, Shanghai, China). The amount of DNA in the purified PCR products was measured using the Qubit2.0® DNA Detection Kit. The PCR products from all of the samples were mixed at equimolar concentrations and then subjected to sequencing on an Illumina MiSeq™ System platform.
According to the relationships between the paired-end reads and overlapping region, pairs of reads from the original DNA fragments were merged using FLASH 1.2.3 (Magoč and Salzberg 2011), and the sequences were subjected to quality control using Prinseq 0.20.4 (Schmieder and Edwards 2011). Sequences with a quality score < 20 and short reads were removed and excluded from further analysis. Chimeras were detected using the “chimeras.seqs” commanded in “Mothur” (Schloss et al. 2009), and then, the sequences were binned into operational taxonomic units (OTUs) with 97 % similarity using the unsupervised Bayesian clustering algorithm CROP (Hao et al. 2011). The most abundant sequence from each OTU was selected as the representative sequence, and a Basic Local Alignment Search Tool (BLAST) search against the GenBank database at the National Center for Biotechnology Information (NCBI) was used to detect non-Glomeromycota sequences. To accurately identify the obtained AM fungus OTUs, representative sequences of each OTU were further queried against the MaarjAM database (Öpik et al. 2010) by BLAST. Representative sequences of each OTU in this study were deposited in GenBank and are accessible using the following accession numbers: KX058691–KX058876.
Data analysis
To determine whether the numbers of samples were adequate, species accumulation curves were generated using the vegan package (Oksanen et al. 2007) in R 3.2. To compare the diversity (Shannon-Wiener index) and richness (Chao 1 index and observed species) between different slope aspects, rarefaction curves of these indicators were generated in Mothur 1.30.1. Percentage data (including the total root colonization, arbuscule abundance, vesicle abundance, hyphal colonization, moisture content, and plant cover) were arcsine-square root transformed for normalization, while the others (including richness and diversity indices of AM fungi, soil pH, soil organic carbon, total nitrogen, total phosphorus, available phosphorus, available nitrogen, and the Shannon-Wiener index of plants) were log-transformed. Comparisons between sunny slopes and shady slopes regarding spore density, mycorrhizal colonization rate, richness and diversity indices of AM fungi, and soil and plant factors were conducted using paired t tests with SPSS 20.0 software.
Based on the relative abundances of the OTUs per sample and Bray-Curtis distances (unless otherwise stated), a series of AM fungus community-related analyses were performed. To test whether the AM fungus community composition between sunny and shady slopes differed significantly, a principal coordinate analysis (PCoA) was conducted using CANOCO 5.0, and a PERMANOVA was conducted using the vegan package in R 3.2. An indicator species analysis was conducted to examine whether particular OTUs were more abundant along the sunny slopes or shady slopes, and the significance of the values was determined using Monte Carlo tests in PCORD 5.0 (Dufrêne and Legendre 1997). If an indicator value was ≥ 40 and P < 0.05 (da Silva et al. 2014), the OTUs were considered to be good indicators for a particular slope aspect.
All of the environmental factors were standardized (range 0–1), and Mantel tests based on Euclidean distances were performed to evaluate the relationships between the AM fungus communities and the environmental factors using the ecodist package (Goslee and Urban 2007) of R 3.2. Because a total of 10 environmental factors were tested, a Bonferroni correction was implemented to test for significance that effects were considered not significant if P values were higher than 0.005 (0.05/10).
To determine the direct and indirect effects of the slope aspect on the AM fungi, we used LISERL 8.72 (Du Toit et al. 2001) to construct a structural equation model. Those environmental variables (among soil pH, soil organic carbon, available phosphorus, available nitrogen, moisture content, plant cover, and the Shannon-Wiener index of plants) that were significantly associated with slope aspect by a correlation analysis were considered for inclusion in the model. A principle component analysis (PCA) among these factors was conducted, and the explanatory variables were assessed according to their scores on the first component of the PCA, which explained 83.9 % of the total variance. On those bases, for inclusion in the SEM, we selected the available phosphorus, soil organic carbon, plant cover, and the Shannon-Wiener index of plants. We tested how well the SEM model fit the data using the following criteria: χ 2/df < 2, P > 0.05, root-mean-square error of approximation (RMSEA) < 0.05, and goodness of fit index (GFI) > 0.9 (Hooper et al. 2008).
Results
Overall sequencing information
A total of 478,439 sequences (ranging from 2637 to 20,214 reads per sample) were obtained from all 54 soil samples with Illumina MiSeq sequencing combined with the AMV4.5NF/AMDGR primers after quality control. Based on a 97 % similarity level, a total of 260 OTUs were detected. Most of the sequences and OTUs belonged to Glomeromycota (364,927 sequences, 76.27 %; 186 OTUs, 71.54 %); the second group was Chytridiomycota (91,498 sequences, 19.12 %; 53 OTUs, 20.38 %); and the remaining groups were Basidiomycota (15,380 sequences, 3.21 %; 9 OTUs, 3.46 %), Entomophthoromycota (3392 sequences, 0.71 %; 7 OTUs, 2.69 %), and Chlorophyta (3242 sequences, 0.68 %; 5 OTUs, 1.92 %), except for unidentified microorganisms.
AM fungus community composition
Among the 364,927 sequences and 186 OTUs of Glomeromycota, the majority of sequences belonged to Glomus at 187,794 sequences (51.46 %); followed by Diversispora at 72,544 sequences (19.88 %), Rhizophagus at 39,078 sequences (10.71 %), uncultured Glomeromycota at 31,916 sequences (8.75 %), and Scutellospora at 24,114 sequences (6.61 %); and the remaining sequences presented low values (Fig. 1). For 186 of the Glomeromycota OTUs, Glomus was the dominant genus (105 OTUs, 56.45 %), uncultured Glomeromycota was the second largest (31 OTUs, 16.67 %), and Diversispora was the third largest (20 OTUs, 10.75 %; Fig. 1).
Species accumulation curve and rarefaction curve analyses of the AM fungi on different slope aspects
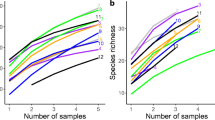
The species accumulation curves (Fig. 2a) tended to approach the saturation plateau with increasing sample numbers, which indicated that the sampling intensity was sufficient. The rarefaction curves of the Shannon-Wiener index (Fig. 2b), the Chao 1 index (Fig. 2c), and observed species (Fig. 2d) were used to estimate and compare the AM fungus diversity and richness between different slope aspects. All of the rarefaction curves reached a plateau when the sequences were over 60,000, which indicated that the sequence-derived diversity and richness in this study were sufficient to characterize the AM fungus species in each sample. In addition, the Shannon-Wiener index, the Chao 1 index, and observed species were significantly higher on the sunny slopes than on the shady slopes (paired t tests, P < 0.05). Specifically, values of the Shannon-Wiener index, Chao 1 index, and observed species significantly decreased from 3.76, 175.28, and 168 for the sunny slopes to 3.06, 151.39, and 145 for the shady slopes, respectively (Fig. 2b–d).
Species accumulation curves (a) and rarefaction curves for the Shannon-Wiener index (b), Chao 1 index (c), and observed species (d) of arbuscular mycorrhizal (AM) fungi colonizing sunny slopes and shady slopes of the Daqingshan Mountains, Inner Mongolia, North China. The number of detected operational taxonomic units (OTUs) is presented by observed species; the estimated asymptotic AM fungus taxon richness is presented by the Chao 1 index
AM fungus colonization and community composition from different slope aspects
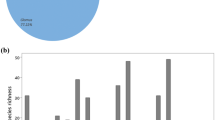
Spore density, total root colonization, arbuscule abundance, vesicle abundance, and hyphal colonization were significantly higher on the sunny slopes than on the shady slopes (Fig. 3).
Arbuscular mycorrhizal (AM) fungus root colonization and spore density from sunny slopes and shady slopes of the Daqingshan Mountains, Inner Mongolia, North China. Comparisons between the two slope aspects were performed with paired t tests. The double asterisks indicate that the differences are significant at P < 0.01; the error bars indicate SE. SD spore density, TRC total root colonization, A arbuscule abundance, V vesicle abundance, HC hyphal colonization
A total of 207,708 AM fungus sequences were obtained from the sunny slopes and were assigned to 168 OTUs, whereas a total of 168,987 sequences were obtained from the shady slopes and were assigned to 145 OTUs. PCoA resulted in a clear separation of the AM fungus community composition of the sunny slopes and shady slopes (Fig. 4). This observation was further confirmed by the PERMANOVA (F = 2.069, P = 0.007). In total, the following 10 taxa of AM fungi were shared between the two slope aspects: Glomus, uncultured Glomeromycota, Diversispora, Scutellospora, Claroideoglomus, Rhizophagus, Funneliformis, Archaeospora, Paraglomus, and Ambispora (Fig. 1). Glomus was the most dominant genus on both sunny and shady slopes, while Septoglomus was detected only on the sunny slopes. In addition, a distinct AM fungus assembly was observed for the different slope aspects, with a significantly elevated proportion of Glomus and Diversispora observed on the sunny slopes and a significantly elevated proportion of Archaeospora observed on the shady slopes (paired t tests, P < 0.05). Of the total 186 OTUs from both slope aspects, 13 were significant indicator species of the sunny slopes or shady slopes according to the indicator species analysis (Table 2). The sunny slopes contained 10 indicator species that belonged to Glomus, uncultured Glomeromycota, and Diversispora, whereas the shady slopes contained 3 indicator species that belonged to Scutellospora and Rhizophagus.
Relationships among AM fungus parameters and soil and plant factors
The PERMANOVA indicated that the soil characteristics (F = 12.88, P = 0.0001) and plant parameters (F = 14.99, P = 0.0001) were significantly different between sunny and shady slopes. The paired t tests further showed that soil organic carbon, available phosphorus, available nitrogen, moisture content, plant cover, and the Shannon-Wiener index of plants were significantly higher on the shady slopes than on the sunny slopes. The soil on the sunny slopes, however, had a significantly higher pH than that of the shady slopes. There were no significant differences in total nitrogen and total phosphorus between the two slope aspects (Table 3).
We used SEM to quantify the relative effects of the slope aspect, available phosphorus, soil organic carbon, plant cover, and the Shannon-Wiener index of plants on AM fungus richness, spore density, and total root colonization (Fig. 5). The model fitted the data well (χ 2 = 9.92, df = 6, P = 0.128, RMSEA = 0.012, GFI = 0.94). Our SEM results revealed that the slope aspect had significant direct effects on soil available phosphorus, soil organic carbon, plant cover, and the Shannon-Wiener index of plants but showed no significant direct effects on AM fungus richness, spore density, and total root colonization. AM fungus richness was significantly affected by available phosphorus, soil organic carbon, plant cover, and the Shannon-Wiener index of plants. Moreover, available phosphorus, soil organic carbon, and plant cover significantly influenced spore density and total root colonization. Available phosphorus was the primary factor that influenced AM fungus richness, presenting a path coefficient of −0.69. Soil organic carbon was the driving factor on spore density and total root colonization, with path coefficients of −0.57 and −0.61, respectively. In addition, the effects of plant cover on AM fungus richness, spore density, and total root colonization were always stronger than effects of the Shannon-Wiener index of plants.
A structural equation model (SEM) showing causal relationships among the slope aspect, available phosphorus, soil organic carbon, plant cover, the Shannon-Wiener index of plants, AM fungus richness, spore density, and total root colonization. The width of the arrows indicates the strength of the causal effects, and the numbers near the arrows indicate the standardized path coefficients (*correlation is significant at P < 0.05, **correlation is significant at P < 0.01, ***correlation is significant at P < 0.001). χ 2 chi-squared test, df degrees of freedom, RMSEA root-mean-square error of approximation, e the values of residuals
Mantel tests were used to compare the effects of variables on the AM fungus community composition (Table 4). The results showed that available phosphorus had the strongest effect on AM fungus community composition, and plant cover, soil organic carbon, and the Shannon-Wiener index of plants also significantly influenced AM fungus community composition. The other factors, especially slope aspect, had no significant influence on AM fungus community composition (Table 4).
Discussion
The present study was the first to compare AM fungus communities between different slope aspects in arid ecosystems of the Daqingshan Mountains, Inner Mongolia, North China. The results indicated that the AM fungus community composition clearly differed between the two slope aspects, and AM fungus diversity and richness were significantly higher on the sunny slopes than on the shady slopes, which might be explained in several ways. First, AM fungi may be drought tolerant and increase the drought tolerance of their host plants (Caravaca et al. 2005; Caravaca et al. 2003) by increasing the supply of mineral nutrients and water (Smith and Read 2008) and improving soil structure (Caravaca et al. 2002). Therefore, in poor water and nutrient conditions, such as sunny slopes, plants may be most dependent on AM fungi to meet their demands for growth, leading to a stronger association between AM fungi and plants to overcome the harsh conditions. In the relatively rich water and nutrient conditions of shady slopes, however, plants may take up sufficient mineral nutrients from the soil without the help of AM fungi, leading to a gradual reduction of the dependency of plants on AM fungi. Second, there are some ectomycorrhizal plants on the shady slopes potentially suppressing AM fungus diversity (Tyndall 2005) and AM fungus root colonization (Becklin et al. 2011). Third, the shady slopes have reduced light levels and understory plants have less opportunity to obtain light because of the presence of some ectomycorrhizal trees. Therefore, low light availability may decrease photosynthesis and carbon translocation to roots, thereby limiting carbon availability to AM fungi (Bennett and Bever 2009). In contrast, on the sunny slopes, there are higher light levels and longer light duration than on the shady slopes and no overstory trees are present. Although the diversity and richness of plants on the sunny slopes are lower than on the shady slopes, because of high light levels, the plants on sunny slopes might produce abundant carbohydrates to support a diverse AM fungus community. Nevertheless, further studies are needed to confirm our explanations by simulating natural environments in the laboratory to investigate the effects of the ectomycorrhizal plants and different availabilities of light, soil water, and mineral nutrient contents on AM fungus communities.
This study was inconsistent with the only research concerning the influence of slope aspect on AM fungus communities that we found in the literature (Chu et al. 2016), which reported that AM fungus diversity and community composition did not differ significantly between north facing (shady) slopes and south facing (sunny) slopes in the forest ecosystems of North China. Previous studies have demonstrated that slope aspect leads to the differences in biotic and abiotic factors, but those differences usually differ among ecosystems (Begum et al. 2013; Nadal-Romero et al. 2014; Sidari et al. 2008), including forest versus arid ecosystems, which may partly explain the different results. In addition, methodological differences are likely another reason contributing to the disparate findings. Recent studies have indicated that Illumina MiSeq sequencing has higher sequencing depth and throughput, higher-quality read cover, and lower rate of erroneous sequences compared with 454 pyrosequencing. Therefore, the Illumina MiSeq sequencing that we used can collect a tremendous amount of amplicon data and obtain an accurate depiction of AM fungus diversity within samples.
At the genus level, Glomus is dominant genus, in agreement with the results of other studies in arid areas (Alguacil et al. 2011; da Silva et al. 2014; Torrecillas et al. 2012). Glomus includes a wide range of genetic diversity and shows a broad ecological amplitude (Öpik et al. 2013), and it has the ability to colonize via pieces of mycelium or mycorrhizal root fragments so that it survives and propagates easily (Brenda and Linderman 1983). These factors partially explain the dominance of Glomus in arid ecosystems. In addition, Septoglomus was detected only from sunny slopes. It is tempting to speculate that adaptations to aridity might exist within this genus, while further studies are needed to explore this issue.
Slope aspect had no significant direct effects on AM fungus diversity and communities, but instead, effects on the latter were mediated by aspect-induced changes in soil characteristics and plant communities, in accordance with the study by Chu et al. (2016). Soil pH, soil organic carbon, available phosphorus, available nitrogen, moisture content, plant cover, and the Shannon-Wiener index of plants differed significantly between sunny and shady slopes in the Daqingshan Mountains, and these factors similarly have been shown to be vital in shaping AM fungus communities in different natural ecosystems (Antoninka et al. 2011; Dumbrell et al. 2010; Lin et al. 2012; Liu et al. 2011; Xiang et al. 2014). An important question is which factors principally contribute to the differences in AM fungus communities on different slope aspects. We revealed that available phosphorus was the major driving factor of the AM fungus communities between different slope aspects, having found a significant negative correlation between AM fungus richness and available phosphorus. Phosphorus uptake is considered the main role of AM fungal symbiosis, but high phosphorus availability can reduce the dependency of plants on AM fungi (Lin et al. 2012), which may decrease carbohydrate supply from plants for AM fungi in roots and lead to a decline of the fungus community. In addition, soil organic carbon had a significant negative relationship with AM fungus richness. In previous studies, Oehl et al. (2010) found no relationship between soil organic carbon in soil and richness of AM fungus genera or spore populations, Xiang et al. (2014) detected a significant positive correlation between soil organic carbon and AM fungus richness, while Lin et al. (2012) reported that organic matter addition significantly decreased AM fungus diversity in arable soil in North China, indicating that the responses of AM fungi to soil organic carbon can be highly variable. We did not detect significant relationships between AM fungus communities and soil pH, total nitrogen, total phosphorus, available nitrogen, and moisture content, suggesting that they were not important factors in the assembly of the AM fungus communities in our study.
We detected a negative relationship between plant diversity and AM fungus richness in the present study. It is interesting that data about the relationships between plant communities and AM fungus communities in natural ecosystems still is scarce. Studies from natural ecosystems revealed positive relationships between plant diversity and AM fungus richness (Hiiesalu et al. 2014; Liu et al. 2011; Vogelsang et al. 2006), in agreement with the “plant diversity hypothesis”, which suggests that the higher the plant diversity, the greater of the range of niches or habitats available for AM fungi, thereby enhancing their opportunities to find a suitable host. The plant diversity hypothesis, however, is not generally supported. Some studies have indicated that plant and AM fungus richness were not interrelated (Lekberg et al. 2013; Öpik et al. 2010; Xiang et al. 2014), but similar to our study, Antoninka et al. (2011) also detected a negative relationship between AM fungus richness and plant diversity. Therefore, the relationships between the richness of AM fungi and their plant hosts lacked consistency, and the inconsistency might be related to soil mineral nutrient content (Yang et al. 2014; Zangaro et al. 2012), host plant species (Martínez-García et al. 2011), and the functional traits of AM fungi and plants (Chagnon et al. 2013) in different ecosystems.
Conclusions
This paper is the first report to reveal differences in AM fungus communities between sunny and shady slopes in the Daqingshan Mountains, Inner Mongolia, North China. Slope aspects have no significant direct effects on AM fungus richness and communities, but the latter are mediated by aspect-induced changes in soil characteristics and plant communities, such as soil available phosphorus, soil organic carbon, plant cover, and the Shannon-Wiener index of plants. Our results deepen insight into the impacts of slope aspect on AM fungus communities in arid ecosystems, but further investigation should be performed to explore the influences of AM fungi on the survival of plants on different slope aspects. By building upon the present work, we hope to find especially appropriate AM fungi for protecting and restoring vegetation on different slope aspects in this arid region in the future.
References
Alguacil M, Torres M, Torrecillas E, Díaz G, Roldán A (2011) Plant type differently promote the arbuscular mycorrhizal fungi biodiversity in the rhizosphere after revegetation of a degraded, semiarid land. Soil Biol Biochem 43:167–173
Antoninka A, Reich PB, Johnson NC (2011) Seven years of carbon dioxide enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem. New Phytol 192:200–214
Bai SL, Li GL, Liu Y, Kasten Dumroese R, Lv RH (2009) Ostryopsis davidiana seedlings inoculated with ectomycorrhizal fungi facilitate formation of mycorrhizae on Pinus tabulaeformis seedlings. Mycorrhiza 19:425–434
Bai SL, Liu Y, Zhou J (2006) Resources investigation and ecological study on ectomycorrhizal fungi in Daqingshan Mountains, Inner Mongolia. Acta Ecol Sin 26:837–841
Bai SL, Yan W, Hu YJ (2011) Mycorrhizal studies and resources investigation on ectomycorrhizal fungi in Daqingshan Mountains, Inner Mongolia. Inner Mongolian People’s Publishing House, Huhhot
Barea JM, Palenzuela J, Cornejo P, Sánchez-Castro I, Navarro-Fernández C, Lopéz-García A, Estrada B, Azcón R, Ferrol N, Azcón-Aguilar C (2011) Ecological and functional roles of mycorrhizas in semi-arid ecosystems of Southeast Spain. J Arid Environ 75:1292–1301
Becklin KM, Pallo ML, Galen C (2011) Willows indirectly reduce arbuscular mycorrhizal fungal colonization in understorey communities. J Ecol 100:343–351
Begum F, Bajracharya RM, Sitaula BK, Sharma S (2013) Seasonal dynamics, slope aspect and land use effects on soil mesofauna density in the mid-hills of Nepal. Int J Biodivers Sci Ecosyst Serv Manag 9:290–297
Bennett AE, Bever JD (2009) Trade-offs between arbuscular mycorrhizal fungal competitive ability and host growth promotion in Plantago lanceolata. Oecologia 160:807–816
Bonfim JA, Vasconcellos RLF, Gumiere T, Mescolotti DDLC, Oehl F, Cardoso EJBN (2016) Diversity of arbuscular mycorrhizal fungi in a Brazilian Atlantic forest toposequence. Microb Ecol 71:164–177
Brenda B, Linderman RG (1983) Use of vesicular arbuscular mycorrhizal roots, intraradical vesicles and extraradical vesicles as inoculum. New Phytol 95:97–105
Brundrett MC, Ashwath N (2013) Glomeromycotan mycorrhizal fungi from tropical Australia III. Measuring diversity in natural and disturbed habitats. Plant Soil 370:419–433
Camenzind T, Hempel S, Homeier J, Horn S, Velescu A, Wilcke W, Rillig MC (2014) Nitrogen and phosphorus additions impact arbuscular mycorrhizal abundance and molecular diversity in a tropical montane forest. Glob Change Biol 20:3646–3659
Caravaca F, Alguacil M, Barea J, Roldán A (2005) Survival of inocula and native AM fungi species associated with shrubs in a degraded Mediterranean ecosystem. Soil Biol Biochem 37:227–233
Caravaca F, Alguacil M, Figueroa D, Barea J, Roldán A (2003) Re-establishment of Retama sphaerocarpa as a target species for reclamation of soil physical and biological properties in a semi-arid Mediterranean area. For Ecol Manag 182:49–58
Caravaca F, Barea J, Figueroa D, Roldan A (2002) Assessing the effectiveness of mycorrhizal inoculation and soil compost addition for enhancing reafforestation with Olea europaea subsp. sylvestris through changes in soil biological and physical parameters. Appl Soil Ecol 20:107–118
Chagnon PL, Bradley RL, Maherali H, Klironomos JN (2013) A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci 18:484–491
Chu HY, Xiang XJ, Yang J, Adams JM, Zhang KP, Li YT, Shi Y (2016) Effects of slope aspects on soil bacterial and arbuscular fungal communities in a boreal forest in China. Pedosphere 26:226–234
Cornfield A (1960) Ammonia released on treating soils with N sodium hydroxide as a possible means of predicting the nitrogen-supplying power of soils. Nature 187:260–261
Cui XC, Hu JL, Wang JH, Yang JS, Lin XG (2016) Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in eastern China as revealed by Illumina sequencing. Appl Soil Ecol 98:140–149
da Silva IR, de Mello CMA, Neto RAF, da Silva DKA, de Melo AL, Oehl F, Maia LC (2014) Diversity of arbuscular mycorrhizal fungi along an environmental gradient in the Brazilian semiarid. Appl Soil Ecol 84:166–175
Du Toit M, Du Toit SHC, Hawkins DM (2001) Interactive LISREL: user’s guide. Scientific Software International
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Dumbrell AJ, Nelson M, Helgason T, Dytham C, Fitter AH (2010) Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J 4:337–345
Eisenhauer N, Bowker MA, Grace JB, Powell JR (2015) From patterns to causal understanding: structural equation modeling (SEM) in soil ecology. Pedobiologia 58:65–72
Gai JP, Tian H, Yang FY, Christie P, Li XL, Klironomos JN (2012) Arbuscular mycorrhizal fungal diversity along a Tibetan elevation gradient. Pedobiologia 55:145–151
Goslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19
Graf F, Frei M (2013) Soil aggregate stability related to soil density, root length, and mycorrhiza using site-specific Alnus incana and Melanogaster variegatus s.L. Ecol Eng 57:314–323
Hao XL, Jiang R, Chen T (2011) Clustering 16S rRNA for OTU prediction: a method of unsupervised Bayesian clustering. Bioinformatics 27:611–618
Hiiesalu I, Pärtel M, Davison J, Gerhold P, Metsis M, Moora M, Öpik M, Vasar M, Zobel M, Wilson SD (2014) Species richness of arbuscular mycorrhizal fungi: associations with grassland plant richness and biomass. New Phytol 203:233–244
Hooper D, Coughlan J, Mullen M (2008) Structural equation modelling: guidelines for determining model fit. Electron J Bus Res Methods 6:53–60
Jünemann S, Sedlazeck FJ, Prior K, Albersmeier A, John U, Kalinowski J, Mellmann A, Goesmann A, von Haeseler A, Stoye J (2013) Updating benchtop sequencing performance comparison. Nat Biotechnol 31:294–296
Kutiel P (1992) Slope aspect effect on soil and vegetation in a Mediterranean ecosystem. Isr J Bot 41:243–250
Laliberté E, Grace JB, Huston MA, Lambers H, Teste FP, Turner BL, Wardle DA (2013) How does pedogenesis drive plant diversity? Trends Ecol Evol 28:331–340
Lekberg Y, Gibbons SM, Rosendahl S, Ramsey PW (2013) Severe plant invasions can increase mycorrhizal fungal abundance and diversity. ISME J 7:1424–1433
Li XL, George E, Marschner H (1991) Phosphorus depletion and pH decrease at the root-soil and hyphae-soil interfaces of VA mycorrhizal white clover fertilized with ammonium. New Phytol 119:397–404
Lin XG, Feng YZ, Zhang HY, Chen RR, Wang JH, Zhang JB, Chu HY (2012) Long-term balanced fertilization decreases arbuscular mycorrhizal fungal diversity in an arable soil in North China revealed by 454 pyrosequencing. Environ Sci Technol 46:5764–5771
Liu YJ, He JX, Shi GX, An LZ, Öpik M, Feng HY (2011) Diverse communities of arbuscular mycorrhizal fungi inhabit sites with very high altitude in Tibet Plateau. FEMS Microbiol Ecol 78:355–365
Lumini E, Orgiazzi A, Borriello R, Bonfante P, Bianciotto V (2010) Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ Microbiol 12:2165–2179
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963
Mahé F, Mayor J, Bunge J, Chi J, Siemensmeyer T, Stoeck T, Wahl B, Paprotka T, Filker S, Dunthorn M (2014) Comparing high-throughput platforms for sequencing the V4 region of SSU-rDNA in environmental microbial eukaryotic diversity surveys. J Eukaryot Microbiol 62:338–345
Mao L, Liu YJ, Shi GX, Jiang SJ, Cheng G, Li XM, An LZ, Feng HY (2014) Wheat cultivars form distinctive communities of root-associated arbuscular mycorrhiza in a conventional agroecosystem. Plant Soil 374:949–961
Martínez-García LB, Armas C, Miranda JDD, Padilla FM, Pugnaire FI (2011) Shrubs influence arbuscular mycorrhizal fungi communities in a semi-arid environment. Soil Biol Biochem 43:682–689
McGonigle T, Miller M, Evans D, Fairchild G, Swan J (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Nadal-Romero E, Petrlic K, Verachtert E, Bochet E, Poesen J (2014) Effects of slope angle and aspect on plant cover and species richness in a humid Mediterranean badland. Earth Surf Process Landf 39:1705–1716
Nelson D, Sommers L (1982) Total carbon, organic carbon, and organic matter. In: Page A (ed) Methods of soil analysis. Part 2. Chemical and microbiological properties. pp 539–579
Oehl F, Laczko E, Bogenrieder A, Stahr K, Bösch R, van der Heijden M, Sieverding E (2010) Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem 42:724–738
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests M (2007) The vegan package. Community Ecology Package 10
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. United States Department of Agriculture, Washington
Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij J, Reier Ü, Zobel M (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol 188:223–241
Öpik M, Zobel M, Cantero JJ, Davison J, Facelli JM, Hiiesalu I, Jairus T, Kalwij JM, Koorem K, Leal ME (2013) Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi. Mycorrhiza 23:411–430
Sato K, Suyama Y, Saito M, Sugawara K (2005) A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl Sci 51:179–181
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Schmidt EL, Belser L, Page A, Miller R, Keeney D (1982) Methods of soil analysis, Part 2, chemical and microbiological properties. In: Methods of soil analysis: Part 2: Chemical and microbiological properties
Schmieder R, Edwards R (2011) Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864
Senés-Guerrero C, Torres-Cortés G, Pfeiffer S, Rojas M, Schüßler A (2014) Potato-associated arbuscular mycorrhizal fungal communities in the Peruvian Andes. Mycorrhiza 24:405–417
Sidari M, Ronzello G, Vecchio G, Muscolo A (2008) Influence of slope aspects on soil chemical and biochemical properties in a Pinus laricio forest ecosystem of Aspromonte (southern Italy). Eur J Soil Biol 44:364–372
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic press, New York
Torrecillas E, del Mar AM, Roldán A (2012) Differences in the AMF diversity in soil and roots between two annual and perennial gramineous plants co-occurring in a Mediterranean, semiarid degraded area. Plant Soil 354:97–106
Tyndall RW (2005) Twelve years of herbaceous vegetation change in oak savanna habitat on a Maryland serpentine barren after Virginia Pine removal. Castanea 70:287–297
Van Der Heijden MG, Streitwolf-Engel R, Riedl R, Siegrist S, Neudecker A, Ineichen K, Boller T, Wiemken A, Sanders IR (2006) The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol 172:739–752
Van Geel M, Busschaert P, Honnay O, Lievens B (2014) Evaluation of six primer pairs targeting the nuclear rRNA operon for characterization of arbuscular mycorrhizal fungal (AMF) communities using 454 pyrosequencing. J Microbiol Methods 106:93–100
Veresoglou SD, Rillig MC (2014) Do closely related plants host similar arbuscular mycorrhizal fungal communities? A meta-analysis. Plant Soil 377:395–406
Vogelsang KM, Reynolds HL, Bever JD (2006) Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytol 172:554–562
Walkley A (1947) A critical examination of a rapid method for determining organic carbon in soils-effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–264
Wilson H, Johnson BR, Bohannan B, Pfeifer-Meister L, Mueller R, Bridgham SD (2016) Experimental warming decreases arbuscular mycorrhizal fungal colonization in prairie plants along a Mediterranean climate gradient. Peer J 4:e2083
Wu QS, He XH, Zou YN, He KP, Sun YH, Cao MQ (2012) Spatial distribution of glomalin-related soil protein and its relationships with root mycorrhization, soil aggregates, carbohydrates, activity of protease and β-glucosidase in the rhizosphere of Citrus unshiu. Soil Biol Biochem 45:181–183
Xiang D, Verbruggen E, Hu YJ, Veresoglou SD, Rillig MC, Zhou WP, Xu TL, Li H, Hao ZP, Chen YL (2014) Land use influences arbuscular mycorrhizal fungal communities in the farming-pastoral ecotone of northern China. New Phytol 204:968–978
Yang GW, Liu N, Lu WJ, Wang S, Kan HM, Zhang YJ, Xu L, Chen YL (2014) The interaction between arbuscular mycorrhizal fungi and soil phosphorus availability influences plant community productivity and ecosystem stability. J Ecol 102:1072–1082
Yang HS, Zang YY, Yuan YG, Tang JJ, Chen X (2012) Selectivity by host plants affects the distribution of arbuscular mycorrhizal fungi: evidence from ITS rDNA sequence metadata. BMC Evol Biol 12:1–13
Zangaro W, Rostirola LV, Souza PBD, Alves RDA, Rondina ABL, Nogueira MA, Carrenho R (2012) Root colonization and spore abundance of arbuscular mycorrhizal fungi in distinct successional stages from an Atlantic rainforest biome in southern Brazil. Mycorrhiza 23:221–233
Zheng Y, Kim YC, Tian XF, Chen L, Yang W, Gao C, Song MH, Xu XL, Guo LD (2014) Differential responses of arbuscular mycorrhizal fungi to nitrogen addition in a near pristine Tibetan alpine meadow. FEMS Microbiol Ecol 89:594–605
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31360125), the Scientific Innovation Team of Inner Mongolia Agricultural University (No. NDDYTD 2013-7), and the Natural Science Foundation of Inner Mongolia (No. 2016MS0343).
Author information
Authors and Affiliations
Corresponding author
Additional information
Min Liu and Rong Zheng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, M., Zheng, R., Bai, S. et al. Slope aspect influences arbuscular mycorrhizal fungus communities in arid ecosystems of the Daqingshan Mountains, Inner Mongolia, North China. Mycorrhiza 27, 189–200 (2017). https://doi.org/10.1007/s00572-016-0739-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-016-0739-7