Abstract
Neuropathic pain is a common health problem that affects millions of people worldwide. Despite being studied extensively, the cellular and molecular events underlying the central immunomodulation and the pathophysiology of neuropathic pain is still controversial. The idea that ‘glial cells are merely housekeepers’ is incorrect and with respect to initiation and maintenance of neuropathic pain, microglia and astrocytes have important roles to play. Glial cells differentially express opioid receptors and are thought to be functionally modulated by the activation of these receptors. In this review, we discuss evidence for glia-opioid modulation of pain by focusing on the pattern of astrocyte and microglial activation throughout the progress of nerve injury/neuropathic pain. Activation of astrocytes and microglia is a key step in central immunomodulation in terms of releasing pro-inflammatory markers and propagation of a ‘central immune response’. Inhibition of astrocytes before and after induction of neuropathic pain has been found to prevent and reverse neuropathic pain, respectively. Moreover, microglial inhibitors have been found to prevent (but not to reverse) neuropathic pain. As they are expressed by glia, opioid receptors are expected to have a role to play in neuropathic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the central nervous system (CNS) no longer deemed as a passive immune-privileged structure, it is recognised that the CNS can mount innate immune responses, which when chronically activated potentially direct the pathophysiology of a number of neurodegenerative disorders as well as having a central role to play in the development of pathological pain states and opioid drug tolerance. Both neuronal and non-neuronal components of the CNS are recognised as responsible for maintaining the physiologic and pathologic state of the CNS. While the neuronal aspects of pathological pain conditions have historically held the spotlight, attention is now being given to non-neuronal cells, primarily glial cells, and discovering how these cells make fundamental contributions in the development of chronic pain conditions. These same non-neuronal glial cells are also being studied to delineate the mechanism underlying opioid tolerance and opioid withdrawal-induced pain enhancement. New findings show that glial cells are differentially activated to release a variety of signalling molecules, which can have pathological actions, protective actions or both. However, the exact nature of the association between opioids, pain, and alterations in immune functioning remains unclear. In this mini review, we illustrate how glial cells are the focal point in the processes underlying the development and maintenance of chronic pain conditions and the retarded analgesic potency of opioids, and how this point of convergence has important implications for future treatments in pain management.
Peripheral immune function and opioids
Immune regulation encompasses interactions between immune cells and mediators that modulate a variety of stimuli including neuroendocrine modulation of stress (corticosteroids and catecholamines), growth hormones, and opioids. The link between immune function and opioids has been presumed from historical literature, which observed an increase in the incidence and severity of infections in opioid addicts, it has been highlighted that opioids affect the endocrine system. Opioid immune modulatory effects, however, are dependent on a variety of factors including the type of opioid drug, duration of use, as well as patient factors such as genetic background.
The site (s) of action for opioid-mediated immunomodulation is one of current research and debate with potential sites of action including (a) peripheral immunocytes, (b) an effect on the hypothalamic–pituitary–adrenal (HPA) axis and (c) effects on sympathetic tone. A synopsis of the research evidence for these potential sites of immune modulation by opioids is provided in Table 1 and we have reviewed this previously [1]. Our interpretation of these findings leads us to conclude that a direct action on immunocytes is doubtful, that the evidence supporting opioid immunomodulation through the HPA axis is unclear (and species dependent) and it is questionable that the opioid-mediated effect on sympathetic tone would be sufficient to support the immunomodulation described. While the mechanisms for opioid-mediated immunomodulation are not fully elucidated, what can be concluded from the literature is that in MOP (mu :µ) receptor-knockout animals no opioid immune modulation is seen, providing robust evidence that MOP is the biological target. In addition, opioid drugs show variance in immunomodulatory effects and that there are interspecies differences in the immunomodulatory actions of opioids. There is growing evidence that glia are central in pain pathophysiology [53] and emerging evidence that glia are opioid-sensitive targets.
Central immunity
Coupled with the expanding significance of glial cells is a gradual disappearance of the idea that the CNS is an immune-privileged organ. It has been found that an innate immune response can be propagated in the CNS [75] which may indicate that the CNS has the ability to fight and recognise infectious and foreign bodies via pattern recognition receptors. Evolving evidence suggests that the central nervous system is able to process antigens and mount immune responses much like that utilised by peripheral organs and exhibits coordinated innate immune reactions in response to both cerebral injury, and systemic bacterial infection [46, 86]. Such innate immunity is an inflammatory response induced by the detection of immunological proteins, released from microorganisms, and initially this occurs in structures of the brain lacking the normal blood–brain barrier (BBB), such as the circumventricular organs (CVO) of the brain. These structures appear to act as ‘detector’ regions for immunological proteins by a way of constitutive expression of CD14 (Cluster of Differentiation Antigen 14: a pattern recognition receptor) and TLR-4 (Toll-like receptor 4; which recognises pathogen-associated molecular patterns), the activation of which leads to the pro-inflammatory events of innate immunity. Microglial cells are located in these regions reacting to endotoxin in the initial innate responses, along with an advancing effect on microglia across other regions of the brain that may lead to the commencement of adaptive immunity in the CNS.
Central immunity and glia
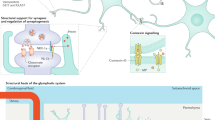
Glial cells were initially thought to be merely supportive elements that surround, protect, and shape the nervous system. In the past decade, glial cells have been recognised to provide neurochemical precursors, supply energy to neurons, regulate the environment of neurons, remove waste products, and control immunity. They are now identified to contribute to the pathophysiology of different disease conditions such as sleep disturbance, fever, disruption of memory and neuroinflammatory/ neurodegenerative diseases. Glial cells are classified into astrocytes, oligodendrocytes, microglia, and ependymocytes, as shown in Fig. 1. Polydendrocytes have also been categorised as glial cells [17, 77].
Microglia are the mononuclear phagocytes of the CNS that have similar properties and functions to peripheral macrophages [102]. They are of mesodermal origin and derived from myeloid precursor cells [20, 40, 87]. In a healthy mature CNS, microglia in a “resting state” will have a small, ramified morphology with fine cellular processes and perform a surveillance function. Microglial response or “microglial activation” is characterised by rapid and intense changes in cell morphology, function, and gene expression along with several other events. These include (i) migration towards the site of injury mediated by various molecules including CCL2 (chemokine (C-C motif) ligand 2) via CCL2 receptor (CCR2), fractalkine (CX3CL1) via CX3CR1, ATP via P2Y12 receptor, neuregulin1(NRG1) via ErbBR and possibly the complement CC5a (via CC5aR), (ii) proliferation in response to the activation of ErbBR by NRG-1 and possibly macrophage colony stimulating factor (M-CSFR) by M-CSF, (iii) release of pro-inflammatory cytokines (such as Interleukin-1β (IL-1β), brain-derived neurotrophic factor (BDNF), tumour necrosis factor alpha (TNF-α), nitric oxide (NO), cyclooxygenase-2 (COX-2) and interleukin-6 (IL-6)) [11, 27, 28, 42, 45, 103, 110]. Figure 2 summarises the possible events following microglial and astrocyte activation that are expected to develop and/or maintain neuropathic pain.
Proposed roles of astrocytes and microglia in the pathogenesis of neuropathic pain. Astrocytes (adapted from Cancer Research UK/Wikimedia Commons) and microglia (adapted from Blausen.com staff (2014)) are activated in the presence of somatic nerve damage (neuron diagram is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license) and/or infection. Activation of astrocytes and microglia is associated with several events that are mentioned from left to right. Scar formation: although it is well known to be a beneficial event, it can result in neuronal damage and death. Astrocyte and microglial activation releases pro-inflammatory cytokines resulting in an aggravated immune response. In addition, immune disturbance induces microglial proliferation and migration to the site of injury/infection beside antigen presenting and phagocytosis. These events, ultimately result in immunomodulation that may be associated with the development and/or maintenance of neuropathic pain
Local activation of microglia is characterised by the production of signalling molecules including various cytokines, proteases and reactive oxygen species (ROS) [76, 106]. The microenvironments in which microglia are activated determine their phenotype. The classical activation is the early phase that is induced by the presence of lipopolysaccharide and interferon-γ (IFN-γ) [47] and results in M1 phenotype. On the other hand, when microglia are activated (alternative activation) in response to interleukin-4 (IL-4) or interleukin-13 (IL-13), they convert to the M2 phenotype. M1 microglia are characterised by a high level of pro-inflammatory cytokines while the late M2 phenotype is characterised by the production of anti-inflammatory molecules such as interleukin-1 (IL-1) and transforming growth factor 1β (TGFβ) [24, 60].
This activation of central innate immunity does not only occur in response to infection, but also in neuronal damage and ischemia. The reactivity of microglial cells is advantageous permitting neuroprotection, brain homeostasis, and possibly repairs through the release of neurotrophic factors. Despite being a defensive mechanism of immune components, the central immune response is associated with pathological conditions such as meningitis [30, 32], encephalitis [61, 112], multiple sclerosis [13, 81], Alzheimer’s disease [69] and Parkinson’s disease [12]. It is believed that sustained microglial activation leads to such demyelinating and neurodegenerative diseases, most likely through an excessive production of inflammatory mediators modifying the function of structures such as the BBB.
As microglia are the central immune representatives, they were thought to be the only player in the CNS immunity. It is now clear that astrocytes are important regulators of central immune activity. Astroglia have multiple roles in the CNS and are known to regulate local blood flow [7], supply neurons with essential nutrients, and control homeostasis [6, 65, 72]. They control the endothelial elements of BBB, and have important implications on brain pathology [15]. Astrocytes have been found to be involved in a variety of neurological disorders through a process known as reactive astrogliosis. This can have both beneficial and detrimental effects, and is characterised by the upregulation of glial fibrillary acidic protein (GFAP) which is seen in a range of neuropathologies including brain ischemia, brain haemorrhage, chronic CNS infections, epilepsy, diabetic retinopathy, Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. It is understood from rodent models that activation of astrocytes is mediated by a variety of cytokines including transforming TGF-α, ciliary neurotrophic factor (CNTF), and IL-6 [71, 96]. Overall, it is believed that the extent of reactive astrogliosis, and subsequent changes in the networks of astrocytes, is disease specific with different neuropathologies having distinct molecular and cellular features. In addition, astrocytes are responsible for scar formation, an event that protects fragile neurons [35], improves axon regeneration [5], and maintains BBB integrity [16]. However, this might result in neuronal damage or death as Tysseling-Mattiace et al. found that the inhibition of scar formation promotes axon elongation after spinal cord injury [109], Fig. 2.
Glial modifiers
Glial cells have been found to respond to several molecules. These compounds have been used in the study of glial cell behaviour in a range of disease conditions experimentally and clinically. Minocycline is a member of tetracyclines, a group of antibacterial agents. Minocycline has been found to act as a microglial inhibitor, immunomodulator with anti-inflammatory, and neuroprotective actions. The proposed mechanism(s) are multifactorial and include the inhibition of key enzymes such as inducible nitric oxide synthase (iNOS) [4] and Phospholipase A2 (PLA2) [82], ability to inhibit caspase-1 and caspase-3 [23], peroxynitrite-scavenging activity (so it reduces protein tyrosine nitration) [114], and inhibition of p38 MAPK activity.
Fluorocitrate is an astrocyte inhibitor that is considered as a fluoroacetate precursor. It has the ability to inhibit aconitase, an enzyme that is responsible for isomerization of citrate to isocitrate in the tricarboxylic acid cycle (TCA). Astrocytes have been described as an acetate/glutamate-specific compartment and have been found to be inhibited by this agent [for more details about mechanism, see ref: [36]].
Pain and glial cells
Pain has been defined by the International Association for the Study of Pain (IASP) as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” [64]. It involves both sensory and affective components. Pain is propagated by a process of nociception that includes the transmission of harmful stimuli by nociceptors (afferent neurons) from the periphery to the CNS via the spinal cord. The transmitted stimulus is processed centrally and results in pain sensation and reflex. In general, pain is classified as acute and chronic; chronic pain is different from acute pain in terms of duration and the presence/absence of tissue damage. In general, when it is maintained for more than 3 months after the disappearance of the causative tissue damage, pain is chronic. Neuropathic pain is a chronic pain that is caused by somatosensory system damage [64]. It is spontaneous pain with a low pain threshold (hypersensitivity and allodynia) and a poor response to traditional analgesics.
The neuronal processes and plasticity underlying different chronic pain states are historically known to involve both peripheral sensitisation—the hyperexcitability of primary sensory neurons and central sensitisation, the increased excitatory transmission at the level of spinal cord, brainstem, and cortex. However, there is growing understanding that non-neural mechanisms are important in the commencement and maintenance of chronic pain states with glial cells being recognised as central to these non-neuronal processes. Pain researchers have been interested in the role of glia in the regulation of pain since 1991, when Garrison et al. [38] noticed an elevated GFAP staining density in chronic pain state, an event that was correlated with hyperalgesia and indicated astrocyte participation in peripheral nerve injury-induced neuropathy. Serious injuries and not minor acute pains are able to stimulate dynamic alterations in glial cell functioning [91].
Glia show a clear heterogeneity not only in receptor expression but also in their regional response profiles. For example, cortical and cerebellar astrocytes are activated by a profile of peptides which differ to those peptides stimulating spinal cord astrocytes [78]. Similarly, there are regional differences in microglial responses between spinal and supraspinal sites following injury [117]. Such regional differences in glial cell responses make interpreting findings from region to region and from brain to spinal cord challenging.
Supraspinally, in the rostral ventromedial medulla (RVM), hyperactivation of microglia is seen following chronic constriction injury of the infraorbital nerve, this occurs 1–3 days after injury, and is followed by a prolonged hyperactivation of astrocytes lasting for 28 days [113]. The hyperactivation of glial cells in the rostral ventromedial medulla (RVM) following nerve injury is known to release cytokines (IL-1β and TNF-α) leading to subsequent glutamate receptor phosphorylation in descending pain-modulating pathways leading to an overall facilitation in neuropathic pain. Cytokines released from hyperactivated RVM glial cells act as mediators leading to neuronal hyperexcitability and the development of neuropathic pain [113]. The sequence of glial activation seen supraspinally in the RVM sees microglia cells as important in the initiation phase and astrocytes in maintaining hyperalgesia following nerve injury, similar to the activation chain seen spinally. However, Zhang et al. [117] found that microglial contribution in chronic pain conditions is limited to the spinal cord but not all supraspinal regions.
Even in the spinal cord, microglial activation is variable. For example, following peripheral nerve injury, the activation and proliferation of microglia is seen on the ipsilateral dorsal horn with the contralateral dorsal horn having weak activation [108]. Glial cells rarely divide under resting conditions and their proliferation in the spinal cord is a crucial aspect of glial cell activation. Using a spared nerve injury model, cell proliferation determined using bromodeoxyuridine (5-bromo-2′-deoxyuridine, BrdU), was seen in the dorsal and ventral horn of the spinal cord on the ipsilateral side, those cells positive for BrdU were also labelled for IBA1 (microglial marker), demonstrating predominately microglial propagation [31].
Surface marker expression following microglial and astroglial activation, at the transcriptional level has been studied. Four hours following L5 nerve transection, microglial activation was determined by an upregulation of microglial surface markers, integrin alpha M (ITGAM), TLR4 and CD14; this was followed later (4 days) by increased and sustained upregulation of GFAP mRNA, indicative of astrocyte activation [29]. In the same laboratory, the pre-emptive use of minocycline decreased the increased expression of ITGAM and TLR4 and reduced nerve injury induced mechanical allodynia. When minocycline was administered 5 days post injury, its effect on behavioural hypersensitivity and mRNA levels of ITGAM and TLR4 was limited [85], therefore both spinal microglia and astrocytes are progressively involved in the spinal sensitisation following nerve injury.
Following painful injury, glia go through a variety of activation states which include (i) up-regulation of glial markers associated with glial cell activation CCR3 and CD11b, IBA1, GFAP and related morphological changes such as hypertrophy, (ii) increased expression and activation of TLRs involved in innate immunity and chemokine receptors on glial cells [19, 70], (iii) stimulation of intracellular MAPK cascades and (iv) subsequent increase in growth factors, cytokines, and chemokines that mediate the glial function.
Glia are non-axonal and cannot directly relay nociceptive signals to the brain from the spinal cord, instead glial activation states are believed to shape pathological pain conditions mediated through the release of glial pro-inflammatory products which have a direct effect on nociceptive neurons to increase their excitability and hence firing. Importantly, it has been shown that the inhibition of activated astrocytes and microglia results in attenuated experimental neuropathic pain. Interestingly, minocycline has been shown to prevent this process although it is unable to reverse neuropathic pain once established, while the inhibition of astrocytes using fluorocitrate (an astrocyte inhibitor) has been shown to reverse neuropathic pain. Together these findings suggest that the tempo of microglia and astrocyte activation differs and is important in the establishment and maintenance of neuropathic pain states.
Activation of microglia seems to occur during the early phase of pain [44] which may further contribute to the activation of astrocytes [41, 88], (Fig. 3). However, astrocytes release microglial activation factors such as TNF-α, lymphotoxin, IL-6 [3, 62, 63], alpha- and beta-interferons, monocyte chemoattractant protein-1 (MCP-1), CC5, and RANTES (regulated on activation, normal T cell expressed and secreted) [55]. Therefore, it seems that astrocytes initially enhance the activation of the already activated microglia during the early phase of neuropathic pain. Neurons are not only influenced by these events but also they are able to exert their own modulation on the orchestration of central immunity. Several actions are attributed to the activation of neurons including the inhibition of microglia and induction of microglial apoptosis along with other cellular components [25]. Unlike microglia, astrocytes are resistant to apoptosis [98], which might explain the effectiveness of astroglial but not microglial inhibitors in the reversal of neuropathic pain (Fig. 3).
Microglial, astroglial and neuronal responses during the progression of neuropathic pain and/or opioid use. A Microglial activation is the early phase following nerve injury, characterised by classical M1 phenotype features including the release of astrocyte activating cytokines. B Activation of astrocytes in response to microglial markers released. C Further (peak) activation of microglia produced in response to activated astrocytes. D Microglial activation includes the targeting of affected neurons (engulfment of injured neurons). E The neurons themselves try to counteract the phagocytic and damaging activity of microglia by releasing microglial inhibitors and apoptotic factors. F A possible event is that microglia switch to the anti-inflammatory M2 phenotype. Alternatively, the microglial activity declines in response to the neuron-induced apoptosis (G) and/or prolonged use of opioids (H)
Glia and opioids
Opioid receptors are GPCR members that are classified into classical (MOP, DOP, and KOP) and are antagonised by naloxone, and non-classical (NOP) which has no affinity to naloxone. They are used extensively in the management of pain. However, their use is associated with unwanted effects including tolerance, dependence, respiratory depression, and immunomodulation. Morphine is the prototypical opioid. Beitner-Johnson et al. [9] determined that the beneficial and unwanted effects of opioids are not limited to neurons, and extend to non-neuronal glial cells based on findings that showed an increased expression of GFAP in the ventral tegmental area following prolonged systemic administration of morphine. Subsequent studies have now shown that opioids are able to activate glial cells leading to up-regulation and release of pro-inflammatory cytokines/chemokines and that repeated dosing of opioid drugs strengthens this activation. In addition, opioid-mediated activation of glia results in an enhancement in nociceptive transmission that subsequently surpasses its analgesic actions [29, 85]. Therefore, long-term exposure to opioids results in processes that enable nociceptive transmission and it is believed that this action contributes towards opioid drug tolerance.
Glial cell activation would appear to be a central mechanistic mediator in chronic pain states and opioid drug tolerance, indeed there is strong evidence highlighting the mechanistic parallels between morphine tolerance and neuropathic pain; both conditions see glia increase extracellular concentrations of neuroexcitiatory substances such as nitric oxide and prostaglandins. Both conditions result in the facilitation of pain and reduction in morphine analgesia and see a reduction in glial glutamate transporters in the dorsal horn leading to increased neuronal excitability. Furthermore, it has been found that long-term use of opioids is associated with increased microglial apoptosis and reduced microglial activity [49]. As mentioned before, microglial activation throughout the progress of neuropathic pain involves an early “classical” pro-inflammatory M1 phenotype and late (alternative) anti-inflammatory M2 phenotype. Minocycline has been found to inhibit M1 but not M2 phenotype [59], findings that support the proposed explanation of the effectiveness of microglial inhibitors in prevention but not reversion of neuropathic pain. Figure 3 shows the progress of neuropathic pain and/or opioid use from the early phase until the development of neuropathic pain and/or opioid tolerance along with the profile of microglial, astroglial, and neuronal responses.
The findings presented here suggest that glial cell activation, whether induced by opioids, inflammation or tissue injury leads to similar consequences including the release of pro-inflammatory cytokines such as TNF-α, IL-1 and IL-6, which subsequently lead to the release of neuroexcitiatory mediators including nitric oxide, prostaglandins, and excitatory amino acids enhancing pain transmission. The recognition that opioid tolerance and neuropathic pain could be attributed to similar mechanisms is strengthened by the longstanding clinical awareness that opioid drugs poorly treat neuropathic pain conditions [66, 67].
Glial cells and opioid receptor expression
Given that opioid activation of glia leads to enhancement of tolerance diminishing opioid analgesia and augmentation of dependence and reward. It is important to understand whether glial sensitivity to opioids is through a direct or indirect effect. Some studies have indicated that MOP opioid receptor agonists may bind to MOP receptors on spinal glial cells. Indeed numerous studies have shown that opioid receptor expression on glia is variable from one cell line to another, from established cell lines to primary cells, from in vitro to in vivo and from region to region in the CNS. For example, MOP receptor mRNA detected in cultured cortical astrocytes was higher than in cultured cerebellar and striatal astrocytes and was absent in hippocampal astrocytes [90]. Low expression of MOP receptor was detected in vivo in a limited area of the CNS under normal conditions [101]. The absence of MOP receptor from astrocytes in rats was reported by Kao et al. [56]. In regards to the forskolin-stimulated cAMP accumulation, Eriksson et al. [33, 34] found an antagonist-reversed effect of DOP and KOP, but not MOP agonists in rat cerebral cortex astrocytes. However, they found MOP-induced inhibitions of cAMP in other regions of the brain [34].
Other studies have shown that MOP, DOP and KOP mRNA are differentially expressed in rat primary astroglial cultures from different areas of the brain including cortical, striatal, cerebellar, hippocampal and hypothalamic regions [90]. In five glial cultures, Ruzicka et al. found that KOP and DOP expression is higher than MOP. Although these receptors were expressed on astrocytes from all of these sites, it was found that the abundance of opioid receptors was as follows: MOP in cortical, DOP in cortical/hypothalamic, KOP in cortical/hypothalamic/ cerebellar astrocytes.
Other factors may affect cell behaviour and the expression of opioid receptors. Culture confluence might influence the pattern of opioid receptor expression. Stiene-Martin et al. [100] found that cell confluence could change the expression of opioid receptors on astroglia taken from the cerebral cortex, hippocampus, cerebellum, and striatum of 1-day-old mice. Using flow cytometry and agonist-induced changes in intracellular calcium, they found that low-density cultures resulted in greater expression of MOP in the cerebral cortex and hippocampus and low expression of DOP. At confluence, MOP expression was still the greatest while DOP expression declined in the cerebellum but increased in the hippocampus. It was found that confluence did not affect the expression of KOP and no difference was found between low-density and confluent cultures. However, in confluent cortical cultures, the proportion of KOP expressing cells is less than at low-density.
The expression of opioid receptors on oligodendrocytes is also differential. According to Knapp and colleges, primary mouse oligodendrocytes express both MOP and KOP but not DOP receptors. However, the expression of MOP and KOP was found to be influenced by stage of development and level of stimulation [58]. Higher expression was seen in the early stage of development; this decreased in mature cells. Furthermore, they found a differential response of opioid receptor activation namely a MOP-induced proliferation and KOP-induced growth and differentiation [57]. Progressive down-regulation of MOP receptor on primary mouse oligodendrocytes was reported in relation to developmental stages [107] that might indicate a direct effect of maturation on the expression patterns of opioid receptors on oligodendrocytes. However, in rat primary oligodendrocytes, it was found that MOP and DOP antagonists inhibit oligodendrocyte proliferation [79].
Some uncertainty surrounds opioid expression on the central immune representative cells, microglia. For example, in terms of RNA, radioligand binding assays and immunofluorescence assays, it was found that primary neonatal human microglia constitutively express KOP receptor [21]. Inhibitory effect of MOP receptor on microglial cell chemotaxis has been reported, suggesting MOP expression [22]. However, the absence of classical opioid receptors on microglia and/or opioid receptor-independent actions has been reported by other studies [56, 84]. A possible interpretation of morphine-induced microglial response is the cross talk between opioid receptors and toll-like receptors (TLRs; well known to be expressed by microglia and other immune cells). Nevertheless, most studies are reporting that morphine (as a prototypical opioid) has a naloxone reversible effect on microglia and the question to be raised here is whether naloxone can reverse the effect of morphine on TLR-4 receptors. Interestingly, it has been reported that naloxone does reverse the effect of morphine on TLR-4 [50].
Given how the activation of glia by opioids essentially instigates a limiting factor in their analgesic efficacy, and that chronic pain states and inflammation share a common activation of glia, what are the possible clinical implications for the use of opioids in the treatment of different conditions? In animal models, intraperitoneal injection of the bacterial endotoxin LPS can activate spinal glia and that a loss of analgesic efficacy for morphine is seen [54]. Further studies have revealed how neuropathic pain decreases morphine efficacy [66], a finding linked to the increased production pro-inflammatory cytokines, by glia, when opioids are administered to an established neuropathic environment.
Inhibitors of glial cell activity could therefore represent an approach for reducing opioid tolerance, and it has been suggested that suppression of activated microglia attenuates neuropathic pain. Indeed, both fluorocitrate and minocycline, which inhibit the actions of pro-inflammatory cytokines, have been demonstrated to enhance morphine analgesia and reduce morphine tolerance.
In conclusion, glia cells play central (and interacting) roles in several immune-related actions including inflammation and neuropathic pain. Moreover, the use of opioids is associated with addiction, tolerance, immunosuppression, astrogliosis, and microglial apoptosis. Glial cells represent targets for use in the pain clinic and development of novel selective drugs for both opioid receptors and glia is an exciting challenge for the future.
Abbreviations
- ATP:
-
Adenosine triphosphate
- BBB:
-
Blood–brain barrier
- BDNF:
-
Brain-derived neurotrophic factor
- BrdU:
-
Bromodeoxyuridine (5-bromo-2′-deoxyuridine)
- CC5a:
-
Complement component 5a
- CC5aR:
-
Complement component 5a receptor
- CCL2:
-
Chemokine (C-C motif) ligand 2
- CCR2:
-
Chemokine (C-C motif) Receptor 2
- CCR3:
-
C-C chemokine receptor type 3
- CD11B:
-
Cluster of differentiation molecule 11B
- CD14:
-
Cluster of differentiation antigen 14
- CNS:
-
Central nervous system
- CNTF:
-
Ciliary neurotrophic factor
- COX-2:
-
Cyclooxygenase-2
- CVO:
-
Circumventricular organs
- CX3CL1:
-
CX3C chemokine
- CX3CR1:
-
CX3C chemokine receptor
- DOP:
-
Delta (\(\delta\)) opioid receptor
- ErbB2:
-
Similar to ErbB (avian erythroblastosis oncogene B)
- GFAP:
-
Glial fibrillary acidic protein
- HIV:
-
Human immunodeficiency virus
- HPA:
-
Hypothalamic–pituitary–adrenal
- IASP:
-
International Association for the Study of Pain
- IBA1:
-
Ionized calcium-binding adapter molecule 1
- IFN-γ:
-
Interferon-γ
- IL-1:
-
Interleukin-1
- IL-13:
-
Interleukin-13
- IL-1β:
-
Interleukin-1β
- IL-4:
-
Interleukin-4
- IL-6:
-
Interleukin-6
- iNOS:
-
Inducible nitric oxide synthase
- ITGAM:
-
Integrin alpha M
- KOP:
-
Kappa (k) opioid receptor
- L5:
-
Fifth Lumbar vertebra
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MCP-1:
-
Monocyte chemoattractant protein-1
- M-CSF:
-
Macrophage-colony stimulating factor
- M-CSFR:
-
Macrophage-colony stimulating factor receptor
- MOP:
-
Mu (µ) opioid receptor
- NO:
-
Nitric oxide
- NOP:
-
Nociceptin/orphanin FQ (N/OFQ) opioid receptor
- NRG-1:
-
Neuregulin1
- P2Y12:
-
Platelet P2Y12 receptor
- PLA2:
-
Phospholipase A2
- RANTES:
-
Regulated on activation, normal T cell expressed and secreted
- ROS:
-
Reactive oxygen species
- RVM:
-
Rostral ventromedial medulla
- TGFβ:
-
Transforming growth factor 1β
- TLR-4:
-
Toll-like receptor 4
- TNF-α:
-
Tumour necrosis factor alpha
References
Al-Hashimi M, Scott S, Thompson JP, Lambert D. Opioids and immune modulation: more questions than answers. Br J Anaesth. 2013;111:80–88.
Al-Hashimi M, Mcdonald J, Thompson JP, Lambert DG. Evidence for nociceptin/orphanin FQ (NOP) but not µ (MOP), δ (DOP) or κ (KOP) opioid receptor mRNA in whole human blood. Br J Anaesth. 2016;116:423–9.
Aloisi F, Carè A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–66.
Amin AR, Patel RN, Thakker GD, Lowenstein CJ, Attur MG, Abramson SB. Post-transcriptional regulation of inducible nitric oxide synthase mRNA in murine macrophages by doxycycline and chemically modified tetracyclines. FEBS Lett. 1997;410:259–64.
Anderson MA, Burda JE, Ren Y, Ao Y, O’shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195.
Araque A, Navarrete M. Glial cells in neuronal network function. Philos Trans R Soc Lond B Biol Sci. 2010;365:2375–81.
Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43.
Beck M, Mirmohammadsadegh A, Franz B, Blanke J, Hengge UR. Opioid receptors on white blood cells: effect of HIV infection and methadone treatment. Pain. 2002;98:187–94.
Beitner-Johnson D, Guitart X, Nestler EJ. Glial Fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and lewis-fischer strain differences in the rat ventral tegmental area. J Neurochem. 1993;61:1766–73.
Bell SP, Sack MN, Patel A, Opie LH, Yellon DM. Delta opioid receptor stimulation mimics ischemic preconditioning in human heart muscle. J Am Coll Cardiol. 2000;36:2296–302.
Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69.
Bonifati DM, Kishore U. Role of complement in neurodegeneration and neuroinflammation. Mol Immunol. 2007;44:999–1010.
Bsibsi M, Ravid R, Gveric D, Van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–21.
Buckingham JC, Cooper TA. Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinol. 1984;38:411–7.
Bundgaard M, Abbott NJ. All vertebrates started out with a glial blood-brain barrier 4–500 million years ago. Glia. 2008;56:699–708.
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308.
Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207:695–706.
Caldiroli E, Leoni O, Cattaneo S, Rasini E, Marino V, Tosetto C, Mazzone A, Fietta AM, Lecchini S, Frigo GM. Neutrophil function and opioid receptor expression on leucocytes during chronic naltrexone treatment in humans. Pharmacol Res. 1999;40:153–8.
Carpentier PA, D’anne SD, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun. 2008;22:140–7.
Chan W, Kohsaka S, Rezaie P. The origin and cell lineage of microglia—new concepts. Brain Res Rev. 2007;53:344–54.
Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu D-F, Archer S, Bidlack JM, Peterson PK. 1996. Kappa opioid receptors in human microglia downregulate human immunodeficiency virus 1 expression. Proc Natl Acad Sci, 93, 8051–8056.
Chao CC, Hu S, Shark KB, Sheng WS, Gekker G, Peterson PK. Activation of mu opioid receptors inhibits microglial cell chemotaxis. J Pharmacol Exp Ther. 1997;281:998–1004.
Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801.
Chhor V, Le Charpentier T, Lebon S, Oré M-V, Celador IL, Josserand J, Degos V, Jacotot E, Hagberg H, Sävman K. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85.
Choi C, Benveniste EN. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Rev. 2004;44:65–81.
Chuang TK, Killam KF Jr, Chuang LF, Kung HF, Sheng WS, Chao CC, Yu L, Chuang RY. Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun. 1995;216:922–30.
Colton CA, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets (Formerly Current Drug Targets-CNS Neurological Disorders). 2010;9:174–91.
Davoust N, Vuaillat C, Androdias G, Nataf S. From bone marrow to microglia: barriers and avenues. Trends Immunol. 2008;29:227–34.
Deleo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. The Neuroscientist. 2004;10:40–52.
Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2–deficient mice are highly susceptible to streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806.
Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. PAIN®. 2008;135:37–47.
Emonts M, Hazelzet J, De Groot R, Hermans P. Host genetic determinants of Neisseria meningitidis infections. The Lancet Infect Dis. 2003;3:565–77.
Eriksson PS, Hansson E, Ronnback L. Delta and kappa opiate receptors in primary astroglial cultures from rat cerebral cortex. Neurochem Res. 1990;15:1123–6.
Eriksson PS, Hansson E, Ronnback L. Mu and delta opiate receptors in neuronal and astroglial primary cultures from various regions of the brain–coupling with adenylate cyclase, localisation on the same neurones and association with dopamine (D1) receptor adenylate cyclase. Neuropharmacology. 1991;30:1233–9.
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–55.
Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia. 1997;21:106–13.
Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124–37.
Garrison C, Dougherty P, Kajander K, Carlton S. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565:1–7.
Gavériaux-Ruff C, Matthes HW, Peluso J, Kieffer BL. 1998. Abolition of morphine-immunosuppression in mice lacking the µ-opioid receptor gene. Proc Natl Acad Sci, 95, 6326–6330.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5.
Giulian D, Li J, Leara B, Keenen C. Phagocytic microglia release cytokines and cytotoxins that regulate the survival of astrocytes and neurons in culture. Neurochem Int. 1994;25:227–33.
Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105.
Guo C-J, Li Y, Tian S, Wang X, Douglas SD, Ho W-Z. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of β-chemokines and CCR5 receptor. J Investig Med. 2002;50:435–42.
Hald A, Nedergaard S, Hansen RR, Ding M, Heegaard AM. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur J Pain. 2009;13:138–45.
Hanisch U-K, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94.
Hendriks JJ, Teunissen CE, De Vries HE, Dijkstra CD. Macrophages and neurodegeneration. Brain Res Rev. 2005;48:185–95.
Hernandez-Ontiveros DG, Tajiri N, Acosta S, Giunta B, Tan J, Borlongan CV. Microglia activation as a biomarker for traumatic brain injury. Front Neurol. 2013;4:30.
Hernandez MC, Flores LR, Bayer BM. Immunosuppression by morphine is mediated by central pathways. J Pharmacol Exp Ther. 1993;267:1336–41.
Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–36.
Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci. 2008;28:20–9.
Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95.
Jessop DS, Fassold A, Wolff C, Hofbauer R, Chover-Gonzalez A, Richards LJ, Straub RH. Endomorphins in rheumatoid arthritis, osteoarthritis, and experimental arthritis. Ann N Y Acad Sci. 2010;1193:117–22.
Ji R-R, Berta T, Nedergaard M. 2013. Glia and pain: is chronic pain a gliopathy? PAIN®, 154, S10-S28.
Johnston IN, Westbrook RF. Inhibition of morphine analgesia by LPS: role of opioid and NMDA receptors and spinal glia. Behav Brain Res. 2005;156:75–83.
Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93:182–93.
Kao S-C, Zhao X, Lee C-Y, Atianjoh FE, Gauda EB, Yaster M, Tao Y-X. Absence of mu opioid receptor mRNA expression in astrocytes and microglia of rat spinal cord. Neuroreport. 2012;23:378.
Knapp PE, Hauser KF. µ-Opioid receptor activation enhances DNA synthesis in immature oligodendrocytes. Brain Res. 1996;743:341–5.
Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: µ and κ opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22:189–201.
Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4:e525.
Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26:1191–201.
Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA. 2004;101:1315–20.
Lau LT, Yu AC-H. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18:351–9.
Lieberman AP, Pitha PM, Shin HS, Shin ML. 1989. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci, 86, 6348–6352.
Loeser JD, Treede R-D. The kyoto protocol of IASP basic pain terminology? Pain. 2008;137:473–77.
Magistretti PJ. Neuron–glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–11.
Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353–64.
Mayer DJ, Mao J, Holt J, Price DD. 1999. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci, 96, 7731–7736.
McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Dependnce. 2001;62:111–23.
Mcgeer PL, Mcgeer EG. The possible role of complement activation in Alzheimer disease. Trends Mol Med. 2002;8:519–23.
Mckimmie CS, Fazakerley JK. In response to pathogens, glial cells dynamically and differentially regulate Toll-like receptor gene expression. J Neuroimmunol. 2005;169:116–25.
Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–8.
Mulligan SJ, Macvicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–9.
Nair M, Schwartz S, Polasani R, Hou J, Sweet A, Chadha K. Immunoregulatory effects of morphine on human lymphocytes. Clin Diagn Lab Immunol. 1997;4:127–32.
Nelson CJ, Schneider GM, Lysle DT. Involvement of central µ-but Not δ-or κ-opioid receptors in immunomodulation. Brain Behav Immun. 2000;14:170–84.
Nguyen MD, Julien J-P, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216–27.
Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8.
Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13:62–76.
Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. In: Milner R (eds) Astrocytes, vol 814. Humana Press, New York, pp 23–45 2012.
Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS. Mu-and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003;17:1159–72.
Peterson PK, Molitor TW, Chao CC. The opioid–cytokine connection. J Neuroimmunol. 1998;83:63–9.
Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, Piesche M, Schroers R, Weiss E, Kirschning CJ. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Investig. 2006;116:456–64.
Pruzanski W, Greenwald RA, Street IP, Laliberte F, Stefanski E, Vadas P. Inhibition of enzymatic activity of phospholipases A2 by minocycline and doxycycline. Biochem Pharmacol. 1992;44:1165–70.
Przewlocki R, Hassan A, Lason W, Epplen C, Herz A, Stein C. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociception. Neuroscience. 1992;48:491–500.
Qian L, Tan KS, Wei S-J, Wu H-M, Xu Z, Wilson B, Lu R-B, Hong J-S, Flood PM. Microglia-mediated neurotoxicity is inhibited by morphine through an opioid receptor-independent reduction of NADPH oxidase activity. J Immunol. 2007;179:1198–209.
Raghavendra V, Tanga F, Deleo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–30.
Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Investig. 2012;122:1164.
Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Ann Rev Immunol. 2009;27:119–45.
Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–92.
Roy S, Barke RA, Loh HH. Mu-opioid receptor-knockout mice: role of µ-opioid receptor in morphine mediated immune functions. Mol Brain Res. 1998;61:190–4.
Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially express mu, delta and kappa opioid receptor mRNA. Brain Res Mol Brain Res. 1995;34:209–20.
Samikkannu T, Rao KV, Salam AaA, Atluri VS, Kaftanovskaya EM, Agudelo M, Perez S, Yoo C, Raymond AD, Ding H. HIV subtypes B and C gp120 and methamphetamine interaction: dopaminergic system implicates differential neuronal toxicity. Sci Rep. 2015;5:11130.
Schultz JEJ, Hsu AK, Gross GJ. Morphine mimics the cardioprotective effect of ischemic preconditioning via a glibenclamide-sensitive mechanism in the rat heart. Circ Res. 1996;78:1100–4.
Schultz JJ, Hsu AK, Gross GJ. Ischemic Preconditioning and morphine-induced cardioprotection Involve the delta (δ)-opioid receptor in the intact rat heart. J Mol Cell Cardiol. 1997;29:2187–95.
Sharp BM. Opioid receptor expression and function. J Neuroimmunol. 2004;147:3–5.
Shavit Y, Depaulis A, Martin FC, Terman GW, Pechnick RN, Zane CJ, Gale RP, Liebeskind JC. 1986. Involvement of brain opiate receptors in the immune-suppressive effect of morphine. Proc Natl Acad Sci, 83, 7114–7117.
Shrikant P, Weber E, Jilling T, Benveniste EN. Intercellular adhesion molecule-1 gene expression by glial cells. Differential mechanisms of inhibition by IL-10 and IL-6. J Immunol. 1995;155:1489–501.
Singhal PC, Sharma P, Kapasi AA, Reddy K, Franki N, Gibbons N. Morphine enhances macrophage apoptosis. J Immunol. 1998;160:1886–93.
Song JH, Bellail A, Margaret C, Yong VW, Hao C. Human astrocytes are resistant to Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. J Neurosci. 2006;26:3299–308.
Steele AD, Henderson EE, Rogers TJ. µ-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107.
Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in mu, delta, and kappa-opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–59.
Stiene-Martin A, Knapp PE, Martin K, Gurwell JA, Ryan S, Thornton SR, Smith FL, Hauser KF. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia. 2001;36:78–88.
Streit WJ. Microglia and the Response to Brain Injury. In: Kettenmann H, Burton GA, Moenning UJ, Neuroinflammation—from bench to bedside. Berlin: Springer 2002.
Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system’s immune response. Neurol Res. 2005;27:685–91.
Suzuki S, Chuang TK, Chuang LF, Doi RH, Chuang RY. Morphine upregulates kappa-opioid receptors of human lymphocytes. Adv Exp Med Biol. 2001;493:81–7.
Tasiemski A, Verger-Bocquet M, Cadet P, Stefano G, Salzet M. Proenkephalin and innate immunity in invertebrates: the antibacterial peptide, peptide B. Mol Brain Res. 2000;76:237–52.
Toklu HZ, Tümer N. Oxidative Stress, brain edema, blood–brain barrier permeability, and autonomic dysfunction from traumatic brain injury. In: Kobeissy FH (eds) Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects, vol 5. Boca Raton: CRC Press/Taylor & Francis 2015.
Tryoen-Toth P, Gavériaux-Ruff C, Labourdette G. Down-regulation of mu-opioid receptor expression in rat oligodendrocytes during their development in vitro. J Neurosci Res. 2000;60:10–20.
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A. P2 × 4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778.
Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–23.
Van Rossum D, Hanisch U-K. Microglia Met Brain Dis. 2004;19:393–411.
Vuong C, Van Uum SHM, O’dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132.
Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–73.
Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial–neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–95.
Whiteman M, Halliwell B. Prevention of peroxynitrite-dependent tyrosine nitration and inactivation of alpha1-antiproteinase by antibiotics. Free Radic Res. 1997;26:49–56.
Williams JP. 2008. Opioids and a neuro-vascular-immune axis. University of Leicester, Leicester.
Williams JP, Thompson JP, Mcdonald J, Barnes TA, Cote T, Rowbotham DJ, Lambert DG. Human peripheral blood mononuclear cells express nociceptin/orphanin FQ, but not µ, δ, or κ opioid receptors. Anesthes Analg. 2007;105:998–1005.
Zhang F, Vadakkan KI, Kim SS, Wu L-J, Shang Y, Zhuo M. Selective activation of microglia in spinal cord but not higher cortical regions following nerve injury in adult mouse. Mol Pain. 2008;4:15.
Acknowledgements
SK is funded by a scholarship from Higher Committee for Education Development in Iraq.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kadhim, S., McDonald, J. & Lambert, D.G. Opioids, gliosis and central immunomodulation. J Anesth 32, 756–767 (2018). https://doi.org/10.1007/s00540-018-2534-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-018-2534-4