Interactions between central glial cells and neurons in the pain circuitry are critical contributors to the pathogenesis of chronic pain. In the central nervous system (CNS), two major glial cell types predominate: astrocytes and microglia. Injuries or pathological conditions which evoke pain are concurrently associated with the presence of a reactive microglia or astrocyte state, which is characterized by a variety of changes in the morphological, molecular, and functional properties of these cells. In this review, we highlight the changes that reactive microglia and astrocytes undergo following painful injuries and insults and discuss the critical and interactive role these two cell types play in the initiation and maintenance of chronic pain. Additionally, we focus on several crucial mechanisms by which microglia and astrocytes contribute to chronic pain and provide commentary on the therapeutic promise of targeting these pathways. In particular, we discuss how the inflammasome in activated microglia drives maturation and release of key pro-inflammatory cytokines, which drive pain through neuronal- and glial regulations. Moreover, we highlight several potentially-druggable hemichannels and proteases produced by reactive microglia and astrocytes in pain states and discuss how these pathways regulate distinct phases during pain pathogenesis. We also review two emerging areas in chronic pain research: 1) sexually dimorphic glial cell signaling and 2) the role of oligodendrocytes. Finally, we highlight important considerations for potential pain therapeutics targeting glial cell mediators as well as questions that remain in our conceptual understanding of glial cell activation in pain states.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to detect noxious (painful) stimuli is a highly evolutionarily conserved function of the nervous system designed to alert us to the presence of environmental dangers and potentially harmful stimuli. Peripheral nociceptive sensory neurons are responsible for the initial detection of noxious stimuli [1]. These cells, termed peripheral nociceptors, represent a heterogeneous neuron population that can be further defined according to their somal diameter, degree of myelination, cell surface markers, gene expression, and electrophysiological properties, each of which contributes to the specialized role each nociceptor class plays in detecting a variety of noxious stimuli [2]. The cell bodies of nociceptors are located in the dorsal root ganglia (DRG) and trigeminal ganglia (TG), which project afferent fibers to all the body and the head, respectively, ending in nerve terminals specialized to detect noxious stimuli. These cells also extend centrally-projecting fibers to the dorsal horn of the spinal cord (for DRG neurons) or spinal trigeminal nucleus of the medulla (for TG neurons), where they transmit information to second-order nociceptive neurons through a variety of neurotransmitters and neuropeptides [3].
The normal physiological role of pain during homeostasis can be perturbed following injuries or insults to the sensory nervous system. This can result in the uncoupling of pain from the degree or presence of noxious stimuli, leading to the presence of pain at rest (spontaneous pain), the over-amplification of the response to painful stimuli (hyperalgesia), or pain elicited by normally innocuous stimuli (allodynia). This dysregulation can result in pain that persists well-after the initial causative injury or lesion has healed, producing chronic pain which can last for months or years [4]. While acute pain is an important physiological system serving protective functions, chronic pain is maladaptive, offering no benefits to organismal survival and wellbeing. Chronic pain has become a global health epidemic: it is the leading cause of disability worldwide, and more than 100 million Americans suffer from at least one chronic pain condition annually [5]. Additionally, the emergence of the opioid crisis has necessitated the removal of opioids from the war chest of healthcare providers, restricting these agents to treat acute and chronic pain in only the most severe circumstances, and often at great cost to patients [6, 7]. Thus, patients and healthcare providers have been left with fewer options for pain therapies than existed even a decade ago. For this reason, the need to identify new potential pain targets and to develop novel neurotherapeutics capable of providing safe, efficacious relief from chronic pain is both critical and urgent.
Our understanding of the mechanisms underlying chronic pain has emerged over the last three decades. It is now understood to result from localized neural plasticity in the peripheral nervous system (PNS, peripheral sensitization) or central nervous systems (CNS, central sensitization) [8, 9]. Initially, our knowledge of the synaptic changes resulting in sensitization were thought to involve only the peripheral and central nociceptive neurons themselves. However, a plethora of evidence now exists which demonstrates that painful injuries cause the activation of various non-neuronal cell types along the pain circuitry, including immune cells and glial cells, producing a localized form of inflammation in the PNS and CNS (neuroinflammation). These activated cells, in turn, form bi-directional interactions with nociceptors and play a highly active role in the initiation and maintenance of chronic pain [10]. In this review, we will discuss the non-neuronal cell types that become activated and contribute to chronic pain pathogenesis, with particular focus on central glial cells. Additionally, we will highlight newly emerging targets in the central glial cell which show promise for the development of novel pain neurotherapeutics.
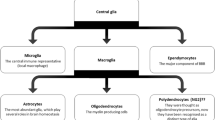
Central Glia in Homeostasis and Disease
Within the central nervous system, three major classes of glial cells are present under normal conditions, with oligodendrocytes being the most abundant, followed by astrocytes, with microglia being the least abundant [11]. While studies have begun to emerge to suggest that oligodendrocytes may contribute to chronic pain pathogenesis [12, 13], our understanding of their role in this process remains in its infancy, which we briefly discuss. Thus, this review will focus primarily on the well-established role of microglia and astrocytes in chronic pain pathogenesis, with a selective emphasis on prospective microglial and astrocytic targets.
Microglia are the archetypical CNS-resident immune cells, emulating peripheral macrophages in their phagocytic capabilities. Like peripheral macrophages, microglia constantly surveil their environment, helping to promote clearance of debris, damaged cells, or infectious agents [14]. However, microglia are also appreciated to possess canonical “glia-like” functions to maintain homeostasis and proper neuronal function in the CNS. To this end, microglia play specialized roles in synaptic pruning during development, neural circuit maintenance and synaptic plasticity in adults, and the regulation of adult neurogenesis under normal physiological functions [15]. Interestingly, Liu et al. recently demonstrated that the morphology and function of microglia changes dynamically in response to neuronal activity. In particular, high noradrenergic tone in awake mice reduced microglial process surveillance, indicating that neuronal function can gate some fundamental activities of microglia [16]. Beyond homeostasis, microglia are associated with disease control and/or pathogenesis in a variety of neurodegenerative (e.g., Alzheimer’s disease, Parkinson’s disease, stroke) and neuropsychiatric (e.g., depression, anxiety) diseases [17, 18]. When microglia become activated in disease states or following injury (e.g., peripheral nerve injury), they undergo “microgliosis,” characterized by profound morphological changes (hypertrophy), proliferation, and functional changes, which correlates with changes in gene expression and function [19, 20].
Compared to microglia, astrocytes are approximately 2–4 times more abundant, accounting for 19–40% of all glial cells in the CNS [11], and are endowed with several unique and non-overlapping homeostatic functions. First, astrocytes are physically coupled to one another through gap junctions, allowing for the permissive exchange of ions and small molecules between adjacent cells [21]. Astrocytes also form unique and extensive contacts with synapses, allowing them to provide structural and metabolic support for neurons to aid in neurotransmission [22]. Under normal physiological conditions, astrocytes play active roles in regulating the extracellular environment, maintaining the proper balance of glutamate, potassium, and water homeostasis [23]. Of particular importance, glutamatergic synaptic transmission is tightly controlled by astrocytic expression of excitatory amino acid transporters EAAT1 and EAAT2 which remove extracellular glutamate, thereby controlling the extent and duration of glutamatergic synaptic transmission [24]. Similar to microglia, astrocytes become activated in a variety of pathological conditions, leading to reactive states (classically termed “astrogliosis”) characterized by morphological changes, profound upregulation of the astrocyte marker glial fibrillary acidic protein (GFAP), and increased proliferation [25, 26]. These changes are also thought to be coupled with a loss of the aforementioned homeostatic functions of astrocytes. However, it is important to note that the activation of astrocytes under pathological conditions is increasingly considered to result in a variety of reactive astrocyte states which vary depending on the initiating disease process [27], although further support for this hypothesis is necessary.
Distinct Roles of Microglia and Astrocytes in Chronic Pain
Similar to their involvement in other pathological disease processes, microglia [20, 28] and astrocytes [23, 29, 30] each play an active role in the pathogenesis of chronic pain. Following nerve injury, hallmarks of microgliosis in the ipsilateral dorsal horn of the spinal cord can be observed rapidly [31], although the precise time course may be dependent on the definition of microgliosis (Fig. 1). Interestingly, spinal microglia activation following nerve injury requires the activity of peripheral sensory afferents [32], with unmyelinated C-fibers and large A-fibers playing distinct roles in the initial activation [33] and maintenance [34] of microglia activation, respectively. Mechanistically, the activation of spinal microglia is induced by the release of several sensory neuron-derived pro-inflammatory mediators, including (but not limited to) colony-stimulating factor 1 (CSF1) [35], caspase-6 [36], cytokines such as interleukin-1β [37], and extracellular proteases [20, 38], each of which will be described in depth in subsequent sections. Sterile injury and tissue trauma also lead to the release of a wide variety of damage-associated molecular patterns (DAMPs), endogenous signaling molecules released by damaged or dying cells which bind to pattern-recognition receptors expressed by spinal microglia and astrocytes, contributing to their activation [20, 23, 39]. Critically, inhibiting microglia activation following nerve injury is sufficient to attenuate the development of pain behaviors [40,41,42]. However, nerve injury–induced microgliosis is somewhat transient and self-limiting, diminishing within a few weeks after nerve injury and maintained at reduced levels at later stages [31]. Once microglia activation is reduced at later stages after nerve injury, microglial inhibitors and inhibitors of activated microglia-derived cytokines no longer show efficacy in reducing pain [42]. Importantly, however, despite being reduced from its peak after sciatic nerve injury, ipsilateral microglia activation remains elevated above baseline or contralateral levels in rats, even at late stages (90 days following injury). Interestingly, a recent study employing more aggressive chemo-ablative strategies (rather than inhibition using pharmacologic inhibitors of microglia) found that microglia ablation at 90 days after nerve injury attenuated pain hypersensitivity in male rats despite a substantial reduction in microglia-derived pro-inflammatory cytokines (IL-1β, TNF, IL-6) typically associated with pain hypersensitivity at early stages. Instead, microglia may maintain pain hypersensitivity through the production of brain-derived neurotrophic factor (BDNF), although BDNF expression in late-stage microglia remains to be demonstrated [43]. Thus, the collective weight of evidence suggests that microglia, and by extension, activated microglial-derived cytokine and chemokine pain mediators, are most actively involved in pain initiation, but also play a role in the maintenance of chronic pain resulting from nerve injury through the same and alternative mechanisms.
Microglia activation following peripheral nerve injury. Nerve injury (chronic constriction injury, top; spared nerve injury, bottom) induces microgliosis, characterized by ipsilateral microglia proliferation and morphological changes, as indicated by CX3CR1-GFP mice or CD11b staining (rat tissue). Nerve injury–induced microgliosis is generally self-limiting, returning to basal levels within a few weeks following injury. Scale
In contrast to microglia, astrocytes become activated following nerve injury relatively early (e.g., 1 week), generally following microglial activation, but remain in a reactive state for the duration of the pathological pain condition [30, 44]. Studies have demonstrated signatures of astrogliosis (GFAP upregulation, morphological change; Fig. 2) persist for at least 5 months after nerve injury [44] and 9 months following spinal cord injury [45], each representing the latest timepoint analyzed after injury. Thus, the time course of astrocyte activation appears to coincide with both the acute-to-chronic pain transition and the maintenance phase of chronic pain (Fig. 3). Concordantly, many studies have demonstrated that inhibiting astrocyte activity can attenuate pain behaviors in a variety of pain models [46,47,48,49,50]. Although experimental activation of astrocytes using optogenetic methods is sufficient to induce pain behaviors in mice [51], it is likely that pro-inflammatory cues from both microglia and primary afferents play a role in the initial activation of astrogliosis following injury in vivo [36]. However, the persistence of astrogliosis well beyond the peak of microgliosis [23] as well as the activation of astrogliosis in the absence of microgliosis in some pain conditions [52,53,54] suggests that sensitized primary afferents may act in isolation to initiate and/or sustain the reactive state of astrocytes in some circumstances.
Astrocyte activation following peripheral nerve injury. Chronic constriction injury of the sciatic nerve induces spinal astrocyte activation (astrogliosis) resulting in morphological changes and an upregulation of glial fibrillary acidic protein (GFAP; red) in ipsilateral astrocytes. Interestingly, the robust changes observed by GFAP staining are not observed when assessing astrocyte activation using Aldh1-GFP mice (green), indicating the possibility of different subsets of astrocytes in the spinal cord and heterogeneity in astrocyte reaction following nerve injury
Kinetics of central glia activation following peripheral nerve injury. Following peripheral nerve injury (pink arrow), such as spared nerve injury, it is generally thought that among glia, microglia activation (blue line) precedes astrocyte activation (purple line). Whereas microglia activation is somewhat self-limiting, persisting at reduced levels at later stages, robust astrocyte activation persists throughout the duration of the injury. Mechanistically, nerve injury elicits rapid activation of sensory afferent fibers (C-fibers and large A-fibers), leading to the initial generation of pain (red line). High frequency firing of peripheral fibers causes localized release of pro-inflammatory mediators, activating ipsilateral microglia. Activated microglia, in turn, release pro-inflammatory mediators which sensitize nearby nociceptive neurons and astrocytes, activating astrogliosis. Importantly, activation of sensory afferent fibers themselves are also likely to contribute to the initiation of astrogliosis. Given the kinetics of activation of these cells, it is likely that microglia contribute primarily to the development of pain (acute pain), while astrocytes contribute to the maintenance of pain (chronic pain)
Given the critical physiological roles that microglia and astrocytes play in maintaining CNS homeostasis, it may be unwise to pursue any global therapies which alter the function of these cells under resting conditions. Several glial cell modulators have shown promise in attenuating neuropathic pain in preclinical animal models, including minocycline, propentofylline, and ibudilast [55,56,57]. However, clinical trials employing oral administration of the microglial inhibitor minocycline have shown little or no efficacy in ameliorating pain in humans [58,59,60]. Rather than an indictment of microglia as a poor therapeutic target, this failure may be reflective of the poor pharmacokinetic properties of minocycline, an inability to reach equitable doses compared to those employed in rodent studies, or counter actions on non-microglial targets which antagonize its analgesic effects [61]. In the following sections, we discuss several critical pathways that central glial cells upregulate following injury and directly contribute to the pathogenesis of chronic pain. Further, we discuss the prospect of targeting these pathways with novel pain therapeutics. We also briefly highlight the emerging role oligodendrocytes play in the pathogenesis of chronic pain.
Glial Cell Targets: IL-1β vis-à-vis the Inflammasome
IL-1β is the archetypical pro-inflammatory cytokine and is known to be upregulated in the spinal cord in a variety of acute and chronic pain conditions [62,63,64,65,66]. IL-1β has been shown to be produced by both activated microglia [67, 68] and astrocytes [69, 70] in the dorsal horn in several chronic pain models, including mice with sciatic nerve injury and bone cancer pain. Following peripheral nerve injury, ipsilateral IL-1β upregulation has been detected in the sciatic nerve as early as 1 h after surgery and is maintained for at least 35 days, the latest timepoint analyzed, suggesting IL-1β upregulation is rapid and sustained [71]. Intrathecal administration of IL-1β itself is sufficient to elicit pain hypersensitivity [62, 65], while antagonizing IL-1β signaling via genetic deletion of the IL-1 receptor (IL-1R) or intrathecal injection of IL-1R neutralizing antibodies and antagonists can attenuate pain in a variety of chronic pain models [70, 72,73,74,75,76]. Mechanistically, microglia and astrocyte-derived IL-1β can produce pain by both enhancing excitatory AMPA and NMDA-mediated synaptic transmission and concurrent inhibition of GABAergic and glycinergic neurotransmission in the superficial layers of the dorsal spinal cord [72]. Another inflammation-modulating cytokine and IL-1 family member, interleukin-18 (IL-18), is also produced by activated microglia and astrocytes in the spinal dorsal horn following nerve injury and bone cancer, likely driving central sensitization and pain through similar mechanisms to IL-1β [77,78,79].
Both IL-1β and IL-18 are initially produced as cytosolic pro-protein forms which require proteolytic cleavage at specific sites to activate their biological function and enable their extracellular release. The caspase family of cysteine proteases governs the cleavage and subsequent secretion of these factors, which function within a specialized inflammation-driving multimeric protein complex called the inflammasome [80]. Pro-caspase-1 is a component of the canonical inflammasome, linked to one of many specialized inflammasome sensor proteins through an adapter protein, ASC. The inflammasome sensor proteins are variable, conveying functional specificity leading to unique inflammasome subtypes [81]. These inflammasome sensor proteins recognize signals associated with infection, toxic chemicals, cell damage, and stress, driving sensor protein oligomerization, ASC recruitment, and polymerization. This process subsequently enables docking of pro-caspase-1 at the inflammasome, enabling dimerization and subsequent autocleavage to generate the fully active cleaved form, caspase-1. Mature caspase-1 is subsequently active and present, enabling cleavage of pro-IL-1β and pro-IL-18 to their mature forms, which are rapidly released into the extracellular environment to drive inflammation and pain [80, 81].
Several core inflammasome components have been demonstrated to play a role in inflammatory and neuropathic pain. Mice lacking caspase-1 demonstrated attenuated mechanical allodynia in an acute inflammatory pain model induced by intraplantar carrageenin, with a corresponding reduction in hindpaw IL-1β [82], supporting a role of peripheral inflammasome-derived IL-1β the induction of acute pain. In a nerve injury model of neuropathic pain, Li et al. demonstrated the activation of a specialized NALP1+ inflammasomes in spinal astrocytes and neurons in the superficial dorsal horn, which corresponded with heightened IL-1β production. Additionally, administration of a caspase-1 inhibitor could attenuate nerve injury–induced IL-1β production and chronic pain [83]. NALP1+ inflammasomes have also been observed in activated microglia and astrocytes following spinal cord injury (SCI) and traumatic brain injury [84, 85], but their consequence on chronic pain in these models has not been determined. In addition, the NOD-like receptor protein 3 (NLRP3)-containing inflammasome, the prototypical inflammasome driving IL-1β-mediated inflammation in response to sterile injury, has emerged as a novel contributor to pain pathogenesis [86]. Formation of the NLRP3 inflammasome in peripheral sensory neurons has been demonstrated to contribute to acute inflammatory pain, postoperative pain, and neuropathic pain in the chemotherapy-induced peripheral neuropathy (CIPN) model [87,88,89,90]. However, further studies are warranted to investigate whether the NLRP3 inflammasome is induced in central glia during some neuropathic pain states to initiate and sustain central neuroinflammation.
Therapeutics targeting IL-1β or the IL-1Ra have been proposed for a variety of pathologies, including autoinflammatory conditions, rheumatoid arthritis, and atherosclerosis, among others [91]. The FDA approved an anti-IL-1β monoclonal antibody (Canakinumab) to treat a spectrum of autoinflammatory syndromes known as cryopyrin-associated periodic syndromes (CAPS). More recently, clinical trials explored the ability of Canakinumab to protect against atherosclerotic disease (the CANTOS study). Interestingly, the results demonstrated that anti-IL-1β therapy via subcutaneous administration could prevent recurrence of cardiovascular disease while also protecting against arthritis, gout, and cancer [92]. Additionally, subcutaneous administration of an IL-1Ra (Anakinra) neutralizing antibody has shown promise for treating patients with rheumatoid arthritis, acute stroke, and autoinflammation-associated epilepsy syndrome [91, 93, 94]. Of note, both Canakinumab and Anakinra appear to be relatively well tolerated [91], and both achieve a reasonably high degree of bioavailability (75–92%) when administered subcutaneously [95, 96]. The mean terminal half-life of the two agents appears to differ considerably, with Canakinumab achieving approximately 26 days [95], compared to 4–6 h for Anakinra [96], although this may be quite different if administered directly into the CNS environment (e.g., intrathecal administration) for direct targeting of CNS nociceptors or glia. Given the promise of IL-1R/IL-1β antagonists/inhibitors in preclinical models of acute and chronic pain, testing whether Canakinumab or Anakinra can provide relief to patients suffering from severe chronic pain warrants investigation.
Glial Cell Targets: Gap Junction Hemichannels
A unique characteristic of astrocytes is their intrinsic ability to form intercellular networks using gap junction proteins [21]. The gap junction proteins connexin-43 (Cx43) is selectively expressed by astrocytes, allowing exchange of ions, water, and small molecules between adjacent cells during homeostasis. Interestingly, Cx43 expression is dramatically upregulated by spinal astrocytes following nerve injury or SCI [97, 98], and inhibition of gap junction function with intrathecal carbenoxolone, a nonselective gap junction inhibitor, can reverse mechanical allodynia in various rodent models of neuropathic pain [99]. Similarly, mice lacking Cx43 exhibit reduced ATP release and astrocyte activation following spinal cord injury [100], suggesting Cx43 plays an active role in this model. These findings were also confirmed in studies in which intrathecal injection of Cx43 biomimetic peptides achieved selective Cx43 inhibition, which attenuated nerve injury–induced neuropathic pain [97].
It is noteworthy that in addition to changes in expression level, Cx43 functionality is also altered in neuropathic pain states. Whereas Cx43 gap junction channels directly oppose one another to allow cell-to-cell communication under homeostatic conditions, following nerve injury Cx43 exists as unopposed hemichannels, allowing the release of small nociceptive mediators such as ATP, glutamate, and chemokines (CCL2, CXCL1) into the extracellular space, where they can directly activate nociceptive neurons [97, 98, 101, 102]. In addition, Cx43-induced ATP release can also serve as a means of crosstalk between astrocytes and microglia, binding to purinergic receptors present on microglia [103]. ATP activation of microglial purinergic receptors can lead to the release of BDNF, which drives neuropathic pain through disinhibition [104, 105].
Beyond connexins, microglia and astrocytes also express a unique gap junction-like protein that exists as a transmembrane hemichannel, pannexin 1 (Panx1), which allows passage of ions and small molecules between the intracellular and extracellular space when open [106]. Interestingly, there is substantial convergence of these pathways. Activation of the purinergic receptors by ATP has been demonstrated to activate the inflammasome in some immune cell types, leading to subsequent IL-1β production and release through Panx1 [107]. Indeed, Panx1-mediated IL-1β contributes to the development of mechanical allodynia in an osteoarthritis model of chronic joint pain in mice [68], suggesting that purinergic signaling can drive Panx1-mediated IL-1β to contribute to the pathogenesis of chronic pain. In addition, Burma et al. recently demonstrated that Panx1-mediated ATP release from microglia is required for morphine withdrawal in rodents, and blocking Panx1 reduced the severity of withdrawal without affecting opioid analgesia [108]. A second study confirmed that Panx1 contributes to opioid withdrawal, but demonstrated it does not contribute to the development of opioid-induced hyperalgesia or opioid tolerance [109], suggesting that Panx1 only regulates some aspects of the negative sequelae associated with sustained opioid treatment.
Although many reagents exist that inhibit Cx43 and Panx1 in vitro or in preclinical animal models, there has only been one Cx43 targeting therapeutic that has completed clinical trials in humans. An antisense oligodeoxynucleotide blocking translation of Cx43 was tested in a small cohort of treatment-refractory patients suffering from persistent ocular epithelial defect wounds secondary to ocular surface burns. Notably, inhibition of Cx43 via intraocular application reduced inflammation within 2 days and promoted complete vascularization and re-epithelialization of the cornea in all 5 patients [110]. Unfortunately, this study lacked placebo controls, but provides encouraging data to suggest that antisense oligodeoxynucleotides may be a safe and efficacious Cx43-targeting therapy in human patients. Future studies evaluating the safety and efficacy of Cx43 antisense therapeutics or the plethora of Cx43 mimetic peptide inhibitors [111] are warranted, but could hold promise in treating late-stage chronic pain in human patients.
Glial Cell Targets: Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) have emerged as powerful regulators of tissue remodeling due to their ability to enzymatically degrade a variety of extracellular matrix proteins. In addition, MMPs can catalyze proteolytic cleavage of cell surface receptors and other bioactive molecules, including cytokines and chemokines, thereby serving as positive or negative regulators of inflammation depending on the substrate and the cellular context [112]. MMPs have been demonstrated to play a role in the pathogenesis of many disease processes, including periodontitis, arthritis, cancer metastasis, and recently, neuropathic pain [113, 114]. MMP-9 was reported to be upregulated by injured DRG neurons rapidly after nerve injury, and intrathecal administration of MMP-9 was sufficient to induce neuropathic pain and microgliosis through IL-1β cleavage. Conversely, antagonizing MMP-9 function using small molecule inhibitors or siRNA knockdown, or TIMP-1 (tissue inhibitor of MMP-1), can attenuate the onset of microgliosis and pain behaviors after nerve injury [114, 115]. In a recent study, IgG L13, a monoclonal antibody selectively targeting MMP-9 was also shown to attenuate the development of pain behaviors when administered intrathecally in the CIPN mouse model of neuropathic pain [116]. MMP-2, on the other hand, is induced in spinal astrocytes following nerve injury, and siRNA-mediated knockdown of MMP-2 can attenuate spinal IL-1β cleavage and persistent mechanical allodynia in the maintenance stage of neuropathic pain. Thus, neuron-derived MMP-9 appears to contribute selectively to the initiation of neuropathic pain and the early onset of microgliosis, while astrocyte-derived MMP-2 contributes to the late phase. However, both MMP-9 and MMP-2 contribute to pain pathogenesis through cleavage and subsequent activation of IL-1β [115].
Drugs targeting MMPs, originating in the 1990s and early 2000s for the treatment of cancer, have historically produced disappointing results. This is thought to be largely due to the relatively non-specific affinity of early drugs for individual MMP targets [113, 117]. As our understanding of MMP biology has improved and we have come to appreciate that distinct MMPs have unique and often antagonistic functions [112], highly specific drugs selectively targeting specific MMPs have been developed. To date, these are limited to inhibitors of MMP-1 (Rebimastat) and MMP-9 (Andecaliximab, AB0041, DX-2400) [117]. Andecaliximab, administered intravenously, recently completed phase I–III clinical trials, where it achieved target engagement and showed no dose-limiting toxicities but failed to improve survival in patients with untreated gastric gastroesophageal junction cancer [117]. Thus, despite the failures in cancer therapy, the developmental pipeline to create MMP inhibitors has resulted in several drugs which appear to be relatively well tolerated and could represent a therapeutic strategy to treat neuropathic pain in a stage-specific manner.
In addition to MMP-9, C-fiber primary afferents also upregulate caspase-6 in response to acute inflammation, and acute inflammatory pain behaviors were attenuated in mice lacking caspase-6 or following administration of caspase-6 inhibitors. Intrathecal injection of caspase-6 led to a dramatic increase in TNF-α in spinal microglia, which was found to potentiate excitatory synaptic transmission in the spinal cord, producing pain behaviors [36]. Additionally, TNF-α can also act on nearby astrocytes, leading to their subsequent activation and release of pro-inflammatory mediators such as CCL2 [118]. In addition, spinal microglia also dramatically upregulate the lysosomal cysteine protease cathepsin S (CatS) following peripheral nerve injury. Administration of an intrathecal CatS inhibitor attenuated microglia activation and neuropathic pain behaviors, while exogenous CatS could produce pain behaviors in naïve rats in a mechanism dependent on cleavage and subsequent liberation of CX3CL1 (fractalkine), further activating microglia [119, 120]. Thus, nociceptor- and microglia-derived proteases can each contribute to pain through cytokine/chemokine signaling.
Glial Cell Targets: β-Adrenergic Signaling
Catecholamines such as epinephrine and norepinephrine are the primary mediators of adrenergic signaling and a principal component of the stress response [121]. Notably, the association between stress and a diverse array of chronic pain conditions has long been appreciated, and elevated catecholamine levels have been demonstrated in patients with functional pain syndromes such as fibromyalgia (FM) and temporomandibular disorder (TMD) [122, 123]. Human genetic variants of COMT, an enzyme that metabolizes catecholamines, have been extensively explored as moderators of pain perception [124], and preclinical studies utilizing COMT inhibitors, which elevate systemic catecholamines, have contributed to our understanding of the role of β-adrenergic receptor (β-AR) signaling in pain. Converging lines of evidence suggest that β-adrenergic pathways can promote nociception through distinct actions on peripheral nociceptors and central glia, including microglia and astrocytes. In rats, intraplantar application of epinephrine or the β-AR-selective agonist isoproterenol induces rapid and dose-dependent mechanical allodynia, which is attenuated by treatment with propranolol, a non-selective beta-adrenergic receptor antagonist. Furthermore, epinephrine treatment increases action potential firing in cultured DRG nociceptors within 3 min of perfusion [125], suggesting activation of β-adrenergic signaling can directly sensitize peripheral nociceptors. In addition, sustained increases in β2 and β3-adrenergic signaling using an implanted osmotic mini-pump to deliver the COMT inhibitor OR486 leads to persistent mechanical hypersensitivity and elevated levels of several pro-nociceptive cytokines, including IL-1β, TNF-α, and CCL2, in the systemic circulation and spinal cord of rats [126]. Notably, this also leads to an increase in the proportion of reactive microglia and astrocytes and a corresponding increase in p-p38 in spinal nociceptive neurons, and these effects were abolished by systemic administration of β2AR and β3AR antagonists [127]. The finding that enhanced catecholaminergic tone contributes to glia activation and spinal central sensitization is complemented by a recent study demonstrating β-AR signaling promotes microglia activation [128]. These data, taken together with the well-established association between chronic pain and elevated stress, suggest that prolonged elevated stress can potentiate or sustain neuroinflammatory changes in central glia, thereby driving pain pathogenesis. Further studies are needed to define the relative contributions of glial β-AR signaling in this context.
Given the multifaceted pro-nociceptive actions of β-AR signaling, acting through both peripheral and central mechanisms in neurons and glia, β-AR antagonists have been tested as another potential avenue to treat acute and chronic pain conditions. The use of β-blockers is bolstered by the availability of cost-effective options and an abundance of data which support their safety. In a large cohort of 873 patients with osteoarthritis, β-blockers showed efficacy in attenuating chronic joint pain and lowered opioid use in these patients [129]. Additional studies have observed that β-blockers do not themselves significantly reduce postoperative pain after surgery but do reduce opioid requirements, thereby reducing negative opioid-related treatment outcomes [130]. At present, there is a paucity of evidence as to whether β-blockers are effective in treating neuropathic pain. Interestingly, the S-enantiomer of the nonselective β-blocker bupranolol (S-bupranolol) was reported to attenuate mechanical allodynia in mice in both acute inflammatory pain models and in neuropathic pain following nerve injury [131]. Thus, given the important role of β-AR signaling in pain processing coupled with the safety and cost-effective nature of β-AR antagonists, future clinical studies exploring the efficacy of β-blockers in neuropathic pain conditions and functional pain syndromes are warranted and may offer a simple but powerful treatment approach.
Sex Dimorphism in Glial Signaling and Pain
Sex dimorphism is an emerging and somewhat controversial area of pain research. Mounting evidence suggests different immune cell types as well as different signaling pathways within a given cell type each contribute to pain pathogenesis in males and females. In preclinical models, intrathecal administration of the microglial inhibitors minocycline and propentofylline and microglial ablation using MAC-1 conjugated to saporin toxin is sufficient to attenuate nerve injury–induced pain in male but not female mice [132]. However, in the absence of microglia, female mice may utilize a mechanism driven by adaptive immune cells [132]. Subsequently, nerve injury–induced phosphorylation of p38 MAPK, a critical microglial mechanism involved in the generation of neuropathic and inflammatory pain, was observed predominantly in male microglia. Critically, intrathecal administration of skepinone, a p38 inhibitor, was found to reduce pain behaviors in male, but not female mice [133]. Thus, despite the observation by both studies that comparable levels of spinal dorsal horn microgliosis and neuropathic pain exist in males and females, only male microglia rely on the p38 MAPK pathway for pain generation, and p38 activation was predominantly observed in male microglia. Interestingly, male microglia that are primed with ATP and injected intrathecally induce pain behaviors in rats, but this is not true of female microglia primed with ATP [134]. This has been attributed to male-dominant expression of P2X4Rs, a well-characterized contributor to chronic pain in males (recently reviewed in depth by Halievski et al. [135]. P2X4R activation in spinal microglia leads to release of BDNF via p38 activation, and male mice lacking BDNF selectively in CX3CR1+ spinal microglia exhibit attenuated nerve injury–induced pain hypersensitivity, an effect not observed in females, and intrathecal inhibition of BDNF attenuates pain selectively in males, indicating microglial BDNF may be yet another sexually dimorphic pain regulator [134, 136]. However, there is some controversy surrounding the localization and function of BDNF in pain regulation, as BDNF has been challenging to detect in spinal microglia but can readily be detected in DRG neurons and primary afferents in the spinal dorsal horn [137]. Moreover, Sikander et al. demonstrated that sensory neuron-derived BDNF contributes to the acute-to-chronic pain transition in both male and female mice [138], raising the possibility that the sexual dimorphic contributions of BDNF may also be dependent on the pain model analyzed.
Interestingly, while sex appears to dictate the contribution of microglia to pain pathogenesis, inhibition of astrocyte activation attenuates pain in preclinical models equally in males and females, indicating astrocytes may contribute equally [20, 23, 139]. These studies demonstrate that in addition to neuropathic pain model, stage, and genetic background, our understanding of the glial/immune cell contribution to pain pathogenesis is also influenced by sex, underscoring the importance of sex as a biological variable in pain research. It is important to note, however, that clinical studies in humans have yet to conclude that microglia contribute to pain in a sex-specific manner, and both microglia and astrocytes have been found to be activated in both males and females [136]. In future studies, clarification of whether microglia or other immune cells contribute to pain in a sexually dimorphic manner in humans is an important question. Moreover, it will be interesting to identify additional cell types and mechanisms that differentially contribute to pain in males and females through future preclinical and clinical studies.
Emerging Role of Oligodendrocytes in Pain
Oligodendrocytes are the most abundant glial cell type in the CNS, accounting for 45–75% of all glia. However, relatively few studies have focused on the role of oligodendrocytes in the pathogenesis of chronic pain. In patients with HIV-associated peripheral neuropathy, a condition characterized by chronic pain, markers of oligodendrocyte precursor cells (NG2 and Olig2) and mature oligodendrocytes (PDGFRa and MBP) were upregulated in the spinal dorsal horn of human postmortem tissues [13]. Additionally, a subset of patients suffering from neuromyelitis optica, a painful demyelinating disorder affecting the optic nerve and spinal cord, have autoantibodies targeting myelin oligodendrocyte glycoprotein (MOG) [140]. Notably, chronic pain occurs in many patients with multiple sclerosis [141], another demyelinating disorder characterized by autoimmune-mediated loss of oligodendrocytes, suggesting a possible interaction between oligodendrocyte destruction and pain in humans. Experimental ablation of oligodendrocytes in adult mice using diphtheria toxin was found to trigger neuropathic pain for several weeks, which was found to be independent of adaptive immune cells or reactive microglia and astrocytes [142], indicating oligodendrocytes may contribute to the maintenance of pain during homeostasis. Additionally, Zarpelon et al. found that sciatic nerve injury in mice resulted in the upregulation of IL-33 primarily by oligodendrocytes in the dorsal spinal cord, and mice lacking the IL-33 receptor ST2 exhibit reduced pain. Additionally, intrathecal administration of IL-33 evoked hypersensitivity in naïve mice and potentiated mechanical allodynia following nerve injury, which was dependent on TNF-α and IL-1β [12]. Thus, the role of oligodendrocyte in pain modulation is likely interwoven with that of primary afferents, microglia, and astrocytes.
Conclusions and Future Directions
In summary, we have reviewed several targets which have dual functions in the pathogenesis of chronic pain: 1) a direct role in activating sensory neurons in the nociceptive circuitry, thereby acutely promoting pain; and 2) in activating or sustaining the activation of central glia, such as microglia and astrocytes, which themselves produce inflammatory mediators that sensitize nociceptive sensory neurons, leading to pain chronification. Thus, a therapeutic strategy aimed at the pro-inflammatory mediators contributing to both acute and chronic aspects of persistent pain is, in theory, a tenable approach to achieving both acute pain relief and promoting pain resolution. To this end, this review focused on a small cohort of particularly promising, and potentially “druggable,” targets to treat chronic pain. IL-1β is a critical regulator of sensitization and neuroinflammation in the PNS and CNS and thus may be one promising target, especially given the relatively recent emergence of IL-1β-targeting therapies. However, given the protective role of IL-1β in host immunity, targeting IL-1β may yield undesirable consequences such as increased infection or immunosuppression [80, 86, 91]. To avoid such effects, central blockade (e.g., intrathecal administration) of spinal cord microglia and astrocyte-derived IL-1β could be one strategy, also enabling the administration of lower doses of drugs. In addition, targeting other central glia pathways involved in IL-1β synthesis, maturation, or release, such as MMP-2 or MMP-9, Cx43, Panx1, or selective components of the inflammasome machinery may also be potential strategies (summarized in Fig. 4). Several of these potential targets have already been the target of considerable research and development efforts, resulting in inhibitors and antagonists in various stages of clinical trials for human disease, which may facilitate their application to testing their efficacy in chronic pain conditions in humans. The list of targets covered in this review is in no way all-inclusive, and many other therapeutic strategies have been discussed elsewhere, including neuromodulation [143], intrathecal cell therapy [144], glial modulators (e.g., ibudilast) [145], TLR4 antagonists, IL-10 gene therapy [146, 147], and β-blockers, each of which represents an exciting prospective strategy to treat pain through modulation of central glia and neuronal function.
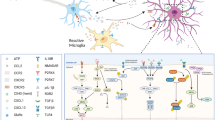
Sensory neuron interactions with central glial cells in neuropathic pain. (a) Following nerve injury, primary afferent nerve fibers release pro-inflammatory mediators such as Csf1, caspase-6 and MMP-9, activating microglia (microgliosis). In turn, activated microglia produce and release mature IL-1β and IL-18 and TNF-α, which act on primary afferent fibers and spinal dorsal horn nociceptive neurons and contribute to sensitization. Microglial-derived TNF-α also acts on nearby astrocytes, contributing to their activation. Activation of astrocytes causes release of mature IL-1β and IL-18, as well as Cx43-mediated release of ATP, glutamate, and chemokines such as CXCL1 and CCL2, all of which induce sensitization of primary afferents and excitatory spinal dorsal horn nociceptors. IL-1β can also suppress GABAergic and glycinergic synaptic transmission in inhibitor spinal dorsal horn neurons (not shown), thus producing central sensitization through both enhanced excitation and central disinhibition. Activation of microglial purinergic receptors also leads to their production and release of BDNF, which drives neuropathic pain via central disinhibition. (b) Astrocyte-derived ATP can also amplify microglial activation by binding to microglial purinergic receptors, contributing to inflammasome activation and subsequent IL-1β and IL-18 maturation (cleavage) and release by the pore-forming inflammasome as well as by microglial Panx1 channels. In addition to caspase-1/inflammasome-mediated activation of IL-1β and IL-18, activated microglia and astrocytes can activate these cytokines through alternative mechanisms involving MMP-9 (microglia) or MMP-2 (astrocytes)
For each therapeutic agent, a consideration must be made on where the therapeutic effects are most advantageous (e.g., peripheral or central route of administration), where likely side effects would be least desirable, and the pharmacokinetic properties of each agent, including the CNS penetrance. Small nonpolar molecules, for example, are typically much more adept at gaining CNS entry than large biologics such as neutralizing antibodies. It is important to note, however, that blood–brain barrier (BBB) permeability can be altered in many disease states, including chronic pain states [148], and penetrance of large biologics into the CNS can be increased [149]. However, even if BBB permeability is increased, whether systemic administration of large biologics could reach sufficient concentrations to achieve their therapeutic effects is questionable, emphasizing the need to develop new small molecule antagonists which can penetrate the BBB and/or consider alternative routes of administration (e.g., intrathecal).
This review also highlights the crucial role that central glial cells play in the pathogenesis of chronic pain. While the role of oligodendrocytes is still emerging, the contribution of microglia and astrocytes to pain pathogenesis is substantial and irrefutable. Importantly, neither microgliosis nor astrogliosis exists as an “all-or-none” phenomenon. Similarly, the absence of immunohistochemical signatures of microgliosis or astrogliosis does not necessarily imply the absence of a functional change in microglia and astrocytes. In addition, neither reactive microglia nor reactive astrocytes are likely to exist as a homogeneous cell state, but rather, as a dynamic and heterogeneous functional state that is dependent on the evoking stimulus and the local cellular environment [20, 23]. Indeed, even under resting conditions, profound region-specific heterogeneity has been observed when analyzing microglia [150] and astrocytes [151] using single-cell sequencing techniques. Thus, future studies aimed at understanding the molecular and phenotypic changes in microglia and astrocytes at baseline and in various chronic pain states—beyond simple parameters of glial cell activation—will greatly enhance our understanding of the pathophysiological mechanisms involved. Towards this goal, Renthal et al. recently conducted a comprehensive analysis of the transcriptional changes occurring within DRGs by performing single nucleus RNA sequencing on over 100,000 single cells across several different injury models [152]. Similar studies analyzing transcriptomic changes in the central nervous system are needed to facilitate the identification of new glial cell targets for pain treatment.
Abbreviations
- β-AR:
-
Beta-adrenergic receptor
- ASC:
-
Apoptosis-associated speck-like protein containing CARD
- ATP:
-
Adenosine triphosphate
- CCI:
-
Chronic constriction injury
- CIPN:
-
Chemotherapy-induced peripheral neuropathy
- CNS:
-
Central nervous system
- COMT:
-
Catechol-O-methyltransferase
- Csf1:
-
Colony-stimulating factor-1
- Cx43:
-
Connexin 43
- DRG:
-
Dorsal root ganglia
- GFAP:
-
Glial fibrillary acidic protein
- IL-1β:
-
Interleukin 1β
- IL-18:
-
Interleukin 18
- MBP:
-
Myelin basic protein
- MMP:
-
Matrix metalloproteinase
- NLRP3:
-
NOD-like receptor protein 3
- Panx1:
-
Pannexin 1
- PNS:
-
Peripheral nervous system
- SCI:
-
Spinal cord injury
- SNI:
-
Spared nerve injury
- TNF:
-
Tumor necrosis factor
References
Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–10.
Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends in neurosciences. 2012;35(6):373–81.
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–84.
Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. AnnuRevNeurosci. 2009;32:1–32.
Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology. 2018.
Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med. 2017;377(4):391–4.
Price TJ, Basbaum AI, Bresnahan J, Chambers JF, De Koninck Y, Edwards RR, et al. Transition to chronic pain: opportunities for novel therapeutics. Nat Rev Neurosci. 2018.
Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–8.
Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. JPain. 2009;10(9):895–926.
Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572–7.
von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. The Journal of comparative neurology. 2016;524(18):3865–95.
Zarpelon AC, Rodrigues FC, Lopes AH, Souza GR, Carvalho TT, Pinto LG, et al. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J. 2016;30(1):54–65.
Shi Y, Shu J, Liang Z, Yuan S, Tang SJ. EXPRESS: Oligodendrocytes in HIV-associated pain pathogenesis. Molecular pain. 2016;12.
Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. NatNeurosci. 2007;10(11):1387–94.
Kierdorf K, Prinz M. Microglia in steady state. The Journal of clinical investigation. 2017;127(9):3201–9.
Liu YU, Ying Y, Li Y, Eyo UB, Chen T, Zheng J, et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nature neuroscience. 2019;22(11):1771–81.
Crotti A, Ransohoff RM. Microglial Physiology and Pathophysiology: Insights from Genome-wide Transcriptional Profiling. Immunity. 2016;44(3):505–15.
Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nature medicine. 2017;23(9):1018–27.
Gilmore SA. Proliferation of non-neuronal cells in spinal cords of irradiated, immature rats following transection of the sciatic nerve. Anat Rec. 1975;181(4):799–811.
Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron. 2018;100(6):1292–311.
Giaume C, McCarthy KD. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19(8):319–25.
Haydon PG. GLIA: listening and talking to the synapse. NatRevNeurosci. 2001;2(3):185–93.
Ji RR, Donnelly CR, Nedergaard M. Astrocytes in chronic pain and itch. Nat Rev Neurosci. 2019.
Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Physiol Rev. 2013;93(4):1621–57.
Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565(1):1–7.
Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). NeurochemRes. 2000;25(9-10):1439–51.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7.
Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. MolPain. 2007;3:33.
Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24(8):450–5.
Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013.
Kohno K, Kitano J, Kohro Y, Tozaki-Saitoh H, Inoue K, Tsuda M. Temporal Kinetics of Microgliosis in the Spinal Dorsal Horn after Peripheral Nerve Injury in Rodents. Biological & pharmaceutical bulletin. 2018;41(7):1096–102.
Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience. 2009;160(4):847–57.
Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain. 2009;144(1-2):110–8.
Suter MR, Berta T, Gao YJ, Decosterd I, Ji RR. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. MolPain. 2009;5:53.
Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nature neuroscience. 2016;19(1):94–101.
Berta T, Park CK, Xu ZZ, Xie RG, Liu T, Lu N, et al. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J ClinInvest. 2014;124(3):1173–86.
Abbadie C, Bhangoo S, De KY, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain ResRev. 2009;60(1):125–34.
Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation–driven chronic pain. NatRevDrug Discov. 2014;13(7):533–48.
Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112.
Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America. 2016.
Grace PM, Wang X, Strand KA, Baratta MV, Zhang Y, Galer EL, et al. DREADDed microglia in pain: Implications for spinal inflammatory signaling in male rats. Exp Neurol. 2018.
Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. JPharmacolExpTher. 2003;306(2):624–30.
Echeverry S, Shi XQ, Yang M, Huang H, Wu Y, Lorenzo LE, et al. Spinal microglia are required for long-term maintenance of neuropathic pain. Pain. 2017;158(9):1792–801.
Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. JNeurochem. 2006;97(3):772–83.
Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. ExpNeurol. 2012;234(2):362–72.
Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33(11):1471–8.
Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71(3):225–35.
Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. JNeurosci. 2006;26(13):3551–60.
Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, et al. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. JNeurosci. 2007;27(34):9068–76.
Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, et al. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. JNeurosci. 2009;29(36):11161–71.
Nam Y, Kim JH, Kim JH, Jha MK, Jung JY, Lee MG, et al. Reversible Induction of Pain Hypersensitivity following Optogenetic Stimulation of Spinal Astrocytes. Cell Rep. 2016;17(11):3049–61.
Shi Y, Gelman BB, Lisinicchia JG, Tang SJ. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci. 2012;32(32):10833–40.
Robinson CR, Zhang H, Dougherty PM. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience. 2014;274:308–17.
Hald A, Nedergaard S, Hansen RR, Ding M, Heegaard AM. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur J Pain. 2009;13(2):138–45.
Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. JPharmacolExpTher. 2001;297(3):1210–7.
Walters ET. Neuroinflammatory contributions to pain after SCI: roles for central glial mechanisms and nociceptor-mediated host defense. Exp Neurol. 2014;258:48–61.
Ellis A, Wieseler J, Favret J, Johnson KW, Rice KC, Maier SF, et al. Systemic administration of propentofylline, ibudilast, and (+)-naltrexone each reverses mechanical allodynia in a novel rat model of central neuropathic pain. J Pain. 2014;15(4):407–21.
Sumitani M, Ueda H, Hozumi J, Inoue R, Kogure T, Yamada Y, et al. Minocycline Does Not Decrease Intensity of Neuropathic Pain Intensity, But Does Improve Its Affective Dimension. J Pain Palliat Care Pharmacother. 2016;30(1):31–5.
Curtin CM, Kenney D, Suarez P, Hentz VR, Hernandez-Boussard T, Mackey S, et al. A Double-Blind Placebo Randomized Controlled Trial of Minocycline to Reduce Pain After Carpal Tunnel and Trigger Finger Release. J Hand Surg Am. 2017;42(3):166–74.
Vanelderen P, Van Zundert J, Kozicz T, Puylaert M, De Vooght P, Mestrum R, et al. Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology. 2015;122(2):399–406.
Haight ES, Forman TE, Cordonnier SA, James ML, Tawfik VL. Microglial Modulation as a Target for Chronic Pain: From the Bench to the Bedside and Back. Anesth Analg. 2019;128(4):737–46.
Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. JNeurosci. 2008;28(52):14062–73.
Takeda M, Kitagawa J, Takahashi M, Matsumoto S. Activation of interleukin-1beta receptor suppresses the voltage-gated potassium currents in the small-diameter trigeminal ganglion neurons following peripheral inflammation. Pain. 2008;139(3):594–602.
Gustafson-Vickers SL, Lu VB, Lai AY, Todd KG, Ballanyi K, Smith PA. Long-term actions of interleukin-1beta on delay and tonic firing neurons in rat superficial dorsal horn and their relevance to central sensitization. MolPain. 2008;4:63.
Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain ResRev. 2009;60(1):57–64.
Yan X, Weng HR. Endogenous interleukin-1beta in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic NMDA receptors. J BiolChem. 2013.
Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–55.
Mousseau M, Burma NE, Lee KY, Leduc-Pessah H, Kwok CHT, Reid AR, et al. Microglial pannexin-1 channel activation is a spinal determinant of joint pain. Sci Adv. 2018;4(8):eaas9846.
Lu Y, Jiang BC, Cao DL, Zhang ZJ, Zhang X, Ji RR, et al. TRAF6 upregulation in spinal astrocytes maintains neuropathic pain by integrating TNF-alpha and IL-1beta signaling. Pain. 2014;155(12):2618–29.
Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, et al. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135(3):232–9.
Ruohonen S, Khademi M, Jagodic M, Taskinen HS, Olsson T, Röyttä M. Cytokine responses during chronic denervation. J Neuroinflammation. 2005;2:26.
Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. JNeurosci. 2008;28(20):5189–94.
Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, et al. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. JNeurosci. 2001;21(8):2808–19.
Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103(2):529–39.
Gabay E, Wolf G, Shavit Y, Yirmiya R, Tal M. Chronic blockade of interleukin-1 (IL-1) prevents and attenuates neuropathic pain behavior and spontaneous ectopic neuronal activity following nerve injury. European journal of pain (London, England). 2011;15(3):242–8.
Wolf G, Gabay E, Tal M, Yirmiya R, Shavit Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain. 2006;120(3):315–24.
Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. JNeurosci. 2008;28(48):12775–87.
Chu YX, Zhang YQ, Zhao ZQ. Involvement of microglia and interleukin-18 in the induction of long-term potentiation of spinal nociceptive responses induced by tetanic sciatic stimulation. Neurosci Bull. 2012;28(1):49–60.
Yang Y, Li H, Li TT, Luo H, Gu XY, Lu N, et al. Delayed Activation of Spinal Microglia Contributes to the Maintenance of Bone Cancer Pain in Female Wistar Rats via P2X7 Receptor and IL-18. JNeurosci. 2015;35(20):7950–63.
Chan AH, Schroder K. Inflammasome signaling and regulation of interleukin-1 family cytokines. The Journal of experimental medicine. 2020;217(1).
Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annual review of immunology. 2011;29:707–35.
Cunha TM, Talbot J, Pinto LG, Vieira SM, Souza GR, Guerrero AT, et al. Caspase-1 is involved in the genesis of inflammatory hypernociception by contributing to peripheral IL-1beta maturation. Molecular pain. 2010;6:63.
Li Q, Tian Y, Wang ZF, Liu SB, Mi WL, Ma HJ, et al. Involvement of the spinal NALP1 inflammasome in neuropathic pain and aspirin-triggered-15-epi-lipoxin A4 induced analgesia. Neuroscience. 2013;254:230–40.
de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, et al. P2X4 receptors influence inflammasome activation after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(9):3058–66.
de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29(7):1251–61.
Cowie AM, Dittel BN, Stucky CL. A Novel Sex-Dependent Target for the Treatment of Postoperative Pain: The NLRP3 Inflammasome. Frontiers in neurology. 2019;10:622.
Chen L, Li X, Huang L, Wu Q, Chen L, Wan Q. Chemical stimulation of the intracranial dura activates NALP3 inflammasome in trigeminal ganglia neurons. Brain research. 2014;1566:1–11.
Wahlman C, Doyle TM, Little JW, Luongo L, Janes K, Chen Z, et al. Chemotherapy-induced pain is promoted by enhanced spinal adenosine kinase levels through astrocyte-dependent mechanisms. Pain. 2018;159(6):1025–34.
Jia M, Wu C, Gao F, Xiang H, Sun N, Peng P, et al. Activation of NLRP3 inflammasome in peripheral nerve contributes to paclitaxel-induced neuropathic pain. Molecular pain. 2017;13:1744806917719804.
Cowie AM, Menzel AD, O'Hara C, Lawlor MW, Stucky CL. NOD-like receptor protein 3 inflammasome drives postoperative mechanical pain in a sex-dependent manner. Pain. 2019;160(8):1794–816.
Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity. 2019;50(4):778–95.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England journal of medicine. 2017;377(12):1119–31.
Wong R, Lenart N, Hill L, Toms L, Coutts G, Martinecz B, et al. Interleukin-1 mediates ischaemic brain injury via distinct actions on endothelial cells and cholinergic neurons. Brain, behavior, and immunity. 2019;76:126–38.
DeSena AD, Do T, Schulert GS. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. Journal of neuroinflammation. 2018;15(1):38.
Chakraborty A, Tannenbaum S, Rordorf C, Lowe PJ, Floch D, Gram H, et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1β monoclonal antibody. Clin Pharmacokinet. 2012;51(6):e1–18.
Yang BB, Gozzi P, Sullivan JT. Pharmacokinetics of Anakinra in Subjects of Heavier vs. Lighter Body Weights. Clin Transl Sci. 2019;12(4):371–8.
Chen G, Park CK, Xie RG, Berta T, Nedergaard M, Ji RR. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain. 2014;137(Pt 8):2193–209.
Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, et al. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012;60(11):1660–70.
Wang H, Cao Y, Chiang CY, Dostrovsky JO, Sessle BJ. The gap junction blocker carbenoxolone attenuates nociceptive behavior and medullary dorsal horn central sensitization induced by partial infraorbital nerve transection in rats. Pain. 2013.
Cronin M, Anderson PN, Cook JE, Green CR, Becker DL. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. MolCell Neurosci. 2008;39(2):152–60.
Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, et al. Connexin 43 hemichannels are permeable to ATP. JNeurosci. 2008;28(18):4702–11.
Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. JNeurosci. 2009;29(13):4096–108.
Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain ResRev. 2010;63(1-2):222–32.
Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–21.
Hildebrand ME, Xu J, Dedek A, Li Y, Sengar AS, Beggs S, et al. Potentiation of Synaptic GluN2B NMDAR Currents by Fyn Kinase Is Gated through BDNF-Mediated Disinhibition in Spinal Pain Processing. Cell reports. 2016;17(10):2753–65.
Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte "hemichannels". JNeurosci. 2009;29(21):7092–7.
Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. The Journal of biological chemistry. 2009;284(27):18143–51.
Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M, et al. Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nature medicine. 2017;23(3):355–60.
Burma NE, Leduc-Pessah H, Trang T. Genetic deletion of microglial Panx1 attenuates morphine withdrawal, but not analgesic tolerance or hyperalgesia in mice. Channels (Austin). 2017;11(5):487–94.
Ormonde S, Chou CY, Goold L, Petsoglou C, Al-Taie R, Sherwin T, et al. Regulation of connexin43 gap junction protein triggers vascular recovery and healing in human ocular persistent epithelial defect wounds. The Journal of membrane biology. 2012;245(7):381–8.
Willebrords J, Maes M, Crespo Yanguas S, Vinken M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacology & therapeutics. 2017;180:144–60.
Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nature reviews Molecular cell biology. 2007;8(3):221–33.
Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. NatRevDrug Discov. 2007;6(6):480–98.
Ji RR, Xu ZZ, Wang X, Lo EH. Matrix metalloprotease regulation of neuropathic pain. Trends in pharmacological sciences. 2009;30(7):336–40.
Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. NatMed. 2008;14(3):331–6.
Lopez T, Mustafa Z, Chen C, Lee KB, Ramirez A, Benitez C, et al. Functional selection of protease inhibitory antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(33):16314–9.
Winer A, Adams S, Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Molecular cancer therapeutics. 2018;17(6):1147–55.
DeLeo JA, Rutkowski MD, Stalder AK, Campbell IL. Transgenic expression of TNF by astrocytes increases mechanical allodynia in a mouse neuropathy model. Neuroreport. 2000;11(3):599–602.
Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. ProcNatlAcadSciUSA. 2007;104(25):10655–60.
Clark AK, Malcangio M. Microglial signalling mechanisms: Cathepsin S and Fractalkine. ExpNeurol. 2012;234(2):283–92.
Kambur O, Männistö PT. Catechol-O-Methyltransferase and Pain. In: Nissinen E, editor. International Review of Neurobiology. 95: Academic Press; 2010. p. 227–79.
Evaskus DS, Laskin DM. A Biochemical Measure of Stress in Patients with Myofascial Pain-Dysfunction Syndrome. Journal of dental research. 1972;51(5):1464–6.
Torpy DJ, Papanicolaou DA, Lotsikas AJ, Wilder RL, Chrousos GP, Pillemer SR. Responses of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis to interleukin-6: A pilot study in fibromyalgia. Arthritis and Rheumatism. 2000;43(4):872–80.
Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic architecture of human pain perception. Trends in genetics : TIG. 2007;23(12):605–13.
Khasar SG, McCarter G, Levine JD. Epinephrine Produces a β-Adrenergic Receptor-Mediated Mechanical Hyperalgesia and In Vitro Sensitization of Rat Nociceptors. Journal of neurophysiology. 1999;81(3):1104–12.
Hartung JE, Ciszek BP, Nackley AG. β2- and β3-adrenergic receptors drive COMT-dependent pain by increasing production of nitric oxide and cytokines. Pain. 2014;155(7):1346–55.
Zhang X, Hartung JE, Bortsov AV, Kim S, O'Buckley SC, Kozlowski J, et al. Sustained stimulation of β2- and β3-adrenergic receptors leads to persistent functional pain and neuroinflammation. Brain, behavior, and immunity. 2018;73:520–32.
Sugama S, Takenouchi T, Hashimoto M, Ohata H, Takenaka Y, Kakinuma Y. Stress-induced microglial activation occurs through β-adrenergic receptor: noradrenaline as a key neurotransmitter in microglial activation. Journal of neuroinflammation. 2019;16(1):266.
Valdes AM, Abhishek A, Muir K, Zhang W, Maciewicz RA, Doherty M. Association of Beta-Blocker Use With Less Prevalent Joint Pain and Lower Opioid Requirement in People With Osteoarthritis. Arthritis Care & Research. 2017;69(7):1076–81.
Bahr MP, Williams BA. Esmolol, Antinociception, and Its Potential Opioid-Sparing Role in Routine Anesthesia Care. Regional Anesthesia and Pain Medicine. 2018;43(8).
Martin LJ, Piltonen MH, Gauthier J, Convertino M, Acland EL, Dokholyan NV, et al. Differences in the Antinociceptive Effects and Binding Properties of Propranolol and Bupranolol Enantiomers. J Pain. 2015;16(12):1321–33.
Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. NatNeurosci. 2015;18(8):1081–3.
Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, et al. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain, behavior, and immunity. 2016;55:70–81.
Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, et al. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain. 2018;159(9):1752–63.
Halievski K, Ghazisaeidi S, Salter MW. Sex-dependent mechanisms of chronic pain: A focus on microglia and P2X4R. J Pharmacol Exp Ther. 2020.
Mapplebeck JC, Beggs S, Salter MW. Molecules in pain and sex: a developing story. Mol Brain. 2017;10(1):9.
Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, et al. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. MolCell Neurosci. 2006;31(3):539–48.
Sikandar S, Minett MS, Millet Q, Santana-Varela S, Lau J, Wood JN, et al. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain. 2018.
Chen G, Luo X, Qadri MY, Berta T, Ji RR. Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neuroscience bulletin. 2018;34(1):98–108.
Asseyer S, Schmidt F, Chien C, Scheel M, Ruprecht K, Bellmann-Strobl J, et al. Pain in AQP4-IgG-positive and MOG-IgG-positive neuromyelitis optica spectrum disorders. Multiple sclerosis journal - experimental, translational and clinical. 2018;4(3):2055217318796684.
Urits I, Adamian L, Fiocchi J, Hoyt D, Ernst C, Kaye AD, et al. Advances in the Understanding and Management of Chronic Pain in Multiple Sclerosis: a Comprehensive Review. Current pain and headache reports. 2019;23(8):59.
Gritsch S, Lu J, Thilemann S, Wortge S, Mobius W, Bruttger J, et al. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nature communications. 2014;5:5472.
Tao X, Lee, M.S., Donnelly, C.R., Ji, R.R. Neuromodulation, Specialized Pro-resolving Mediators, and Resolution of Pain Neurotherapeutics. 2020. https://doi.org/10.1007/s13311-020-00892-9.
Buchheit T, Huh Y, Maixner W, Cheng J, Ji RR. Neuroimmune modulation of pain and regenerative pain medicine. J Clin Invest. 2020;130(5):2164–76.
Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16(7):935–50.
Vanderwall AG, Milligan ED. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front Immunol. 2019;10:3009.
Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. 2015;96(Pt A):55–69.
Wolka AM, Huber JD, Davis TP. Pain and the blood-brain barrier: obstacles to drug delivery. Adv Drug Deliv Rev. 2003;55(8):987–1006.
Ronaldson PT, Davis TP. Targeting blood-brain barrier changes during inflammatory pain: an opportunity for optimizing CNS drug delivery. Ther Deliv. 2011;2(8):1015–41.
Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity. 2019;50(1):253–71 e6.
Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, et al. Molecular Architecture of the Mouse Nervous System. Cell. 2018;174(4):999–1014 e22.
Renthal W, Tochitsky I, Yang L, Cheng Y-C, Li E, Kawaguchi R, et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. bioRxiv. 2019.
Acknowledgments
This work was supported by Duke University funds. C.R.D. was supported by the John J. Bonica Trainee Fellowship from the International Association for the Study of Pain and NIH T32 GM08600. Schematic figures were created by A.S.A. and C.R.D. using BioRender.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guest Editors: Christine Sang and William Schmidt
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Neurotherapeutics Special Issue: Aligning New Approaches to Accelerate the Development of Analgesic Therapies
Rights and permissions
About this article
Cite this article
Donnelly, C.R., Andriessen, A.S., Chen, G. et al. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 17, 846–860 (2020). https://doi.org/10.1007/s13311-020-00905-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-020-00905-7