Abstract
Background
Although serrated polyposis syndrome (SPS) is associated with an increased colorectal cancer (CRC) risk, the carcinogenic mechanisms remain unknown. We investigated clinicopathological characteristics and genetic abnormalities in colorectal polyps and CRC to elucidate carcinogenic mechanisms in SPS.
Methods
We retrospectively analyzed clinicopathological features of colorectal polyps in 44 SPS patients, and examined mutations of genes including APC, RAS, BRAF, and TP53, and microsatellite instability (MSI) in CRC tissues.
Results
Of the 44 patients, 25 (56%) fulfilled WHO criterion 1, 11 (25%) fulfilled criterion 2, and 8 (18%) fulfilled both. A total of 956 polyps were observed; 642 (67%) hyperplastic polyps (HP), 204 (21%) sessile serrated lesions (SSL), 10 (1%) traditional serrated adenoma (TSA), and 100 (11%) adenomas. The median numbers of polyps (/patient) were 10.5 (IQR 2.75–23) HPs, 4.0 (2.0–6.0) SSLs, 0 (0–0) TSA, and 1 (0–3.3) adenoma. SSL and HP located preferentially in the proximal and distal colon, respectively. Twenty-two CRCs were found in 18 patients. Based on the histological coexistence of SSL/TSA, BRAF mutation and MSI, 5 CRCs (26%) were classified as serrated-neoplasia pathway. Conversely, based on the coexistence of adenoma, APC/RAS and TP53 mutations, 11 CRCs (58%) were classified as adenoma–carcinoma pathway. The remaining three were unclassifiable. Most CRCs through adenoma–carcinoma pathway were located in the left-side colorectum and patients bearing those met criterion 2, characterized by many HP and advanced adenomas. Adenoma was a significant risk factor for CRC.
Conclusions
Our results suggest that more than half of the CRCs, particularly those in the left-side colorectum, developed through the adenoma–carcinoma pathway in SPS patients. Adenoma was a risk factor for CRCs, suggesting its importance in colorectal carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serrated polyposis syndrome (SPS), initially termed hyperplastic polyposis syndrome (HPS), is a heterogeneous disease characterized by multiple serrated polyps (SPs), which consist of hyperplastic polyp (HP), sessile serrated lesion (SSL) and traditional serrated adenoma (TSA) [1]. Recently, SPS has received increased attention, because the risk of colorectal cancer (CRC) is reportedly fairly high in SPS patients [2, 3]. Although a single report showed SPS families with a germline RNF43 variant, currently it is recognized that germline RNF43 accounts for only a very small proportion of SPS patients [4, 5]. Therefore, the diagnosis of SPS is currently made in accordance with the World Health Organization (WHO) criteria based on the number, size, and location of the SPs [6].
In recent larger multicenter studies, the prevalence of CRC ranged from 15.8 to 41.9% in SPS patients [2, 3, 7]. The cumulative 5-year incidence of CRC under endoscopic surveillance is reported to range from 1.3 to 7.0% [2, 3, 8, 9]. However, only a few studies have stratified cancer risk in patients with SPS based on clinical risk factors. Ijspeert et al. reported that the risk factors for CRC in SPS patients were fulfillment of both WHO 2010 criteria 1 and 3, at least one SP with dysplasia, or at least one advanced adenoma, while a history of smoking decreased the risk of CRC in these patients [3]. Carballal et al. reported that the presence of more than two sessile serrated polyp/adenomas (SSA/Ps) proximal to the splenic flexure or any proximal SSA/P with dysplasia was a risk factor for CRC [2]. Thus, the risk factors previously reported are inconsistent, and therefore, an effective predictor of CRC in SPS patients has not yet been established.
In general, most CRCs develop from an adenoma through the adenoma–carcinoma pathway, which is associated with APC, KRAS, and TP53 mutations [10], whereas as many as 30% of CRCs reportedly arise from SPs via the serrated-neoplasia pathway [11]. The serrated-neoplasia pathway is associated with BRAF or KRAS mutations and CpG island methylation phenotype (CIMP), as well as MLH1 promoter methylation or TP53 mutation [12]. He et al. reported that 49% (95% CI 33–64%) of CRCs in SPS patients had a BRAF mutation, 3% (95% CI 0–16%) had a KRAS mutation, 40% (95% CI 33–64%) were microsatellite instability-high (MSI-H), and 53% (95% CI 36–71%) were MLH1-deficient [13]. Similarly, Boparai et al. reported that 53% of CRCs had BRAF mutations, 5% had KRAS mutations, 11% had APC mutation, and 32% were MLH1-deficient in CRC with HPS [14]. These results suggest that only half of the CRCs might have developed through the serrated-neoplasia pathway in SPS patients. However, these studies analyzed only a few molecular signatures of serrated-neoplasia related genes in the limited cohort of heterogeneous patients, and the detailed mechanism of carcinogenesis in SPS is unknown [14, 15].
Therefore, in this study, we first analyzed clinicopathological features of each type of colorectal polyp in SPS patients. We then performed molecular analyses of CRC lesions in SPS to clarify the detailed mechanism of carcinogenesis in patients with SPS. Moreover, we analyzed risk factors for CRC in SPS patients.
Methods
Patients
We retrospectively screened and enrolled SPS patients from the endoscopy filing system and medical records who met the 2019 WHO diagnostic criteria for SPS [6] in Tokushima University Hospital (Tokushima, Japan) between January 2009 and November 2021. Patients with hereditary CRC syndrome, including familial adenomatous polyposis (FAP), Lynch syndrome, gastric adenocarcinoma and proximal polyposis syndrome (GAPPS), and patients with inflammatory bowel disease were excluded. General information on the patients including age, gender, smoking status, body mass index (BMI), personal history, and family history of malignancies at the time of diagnosis of SPS were collected from medical records. This study was approved by the ethics committee of Tokushima University Hospital and registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR; study number UMIN000030189). Written informed consent was obtained from all the patients.
Polyp review
Colonoscopy reports, surgery records, and the corresponding pathology reports were used to obtain information regarding the number, distribution, size, and type of colorectal polyps. All resected polyps and cancers were formalin-fixed, paraffin-embedded (FFPE), and used for HE staining, immunohistochemical staining, and gene analysis. The histological diagnosis of colorectal lesions was made independently by two pathologists (Y.B. and K.T.) according to WHO Classification 2019 [15]. All polyps were classified as SPs and conventional adenomas. SPs were further classified as HP, SSL, and TSA. The mixed polyp was excluded from the analysis. We classified intramucosal carcinoma (Tis) as cancer according to Japanese diagnostic guideline [16]. Advanced adenoma was defined as an adenoma > 10 mm or with tubulovillous architecture.
Somatic gene mutation analysis
Genomic DNA was extracted from FFPE tissues using a QIAamp DNA FFPE Tissue Kit (Qiagen, Venlo, Netherlands) and analyzed for each gene. Mutational analysis of codons 12, 13, 59, 61, 117, and 146 in KRAS, and NRAS and codon 600 in BRAF, was performed using the PCR-reverse sequence specific oligonucleotide (PCR-rSSO) method, as previously described [17]. Subsequently, the mutation was confirmed by direct sequencing of the PCR product, as described previously [18]. Mutational analysis of APC (mutation cluster region: MCR), β-catenin (exon 3), RNF43 (exons 2–5, 7–9), and TP53 (exons 4–9) was performed by direct sequencing of the PCR products, as previously described [19,20,21].
MSI analysis
Microsatellite instability (MSI) analysis was performed as previously described [22]. Alternatively, in cases without normal tissue, MSI analysis was performed using the quasi-monomorphic variation range (QMVR) with a new MSI kit (Falco Biosystems Ltd., Kyoto, Japan). Tumors with instability at ≥ 2 markers were classified as high-degree MSI (MSI-H); at 1 marker as low-degree MSI (MSI-L); and at no markers as microsatellite stable (MSS).
Immunohistochemistry
Immunohistochemistry (IHC) for MLH1 and p53 in CRC tissue was performed using EnVision method (Envision-PO, Agilent Technologies, Inc., Santa Clara, CA) as previously described [23]. Antigen retrieval was performed using an electric pressure cooker in 0.05% citraconic anhydride solution, pH7.4 at 98ºC for 45 min. Rabbit anti-human MLH1 monoclonal antibody (diluted 1:100, Abcam Co. Ltd., Tokyo, Japan) and rabbit anti-human p53 polyclonal antibody (diluted 1:100, Novocastra Laboratories Ltd., Newcastle upon Tyne, UK) were used as primary antibodies.
Definition of CRCs via adenoma–carcinoma pathway and serrated-neoplasia pathway
Carcinogenesis via the adenoma–carcinoma pathway has been well documented, since it was first reported by Vogelstein et al. [10, 24,25,26]. Based on the histological findings and gene alterations, CRC derived through adenoma was defined as follows: (1) CRC which is histologically coexistent with adenoma (cancer in/with adenoma); or (2) CRC which has both APC/β-catenin mutation and TP53 mutation/accumulation. On the other hand, the serrated-neoplasia pathway was defined as follows, according to previous reports [1, 27,28,29]: (1) CRC which is histologically coexistent with SSL, TSA, or HP (cancer in/with SSL, TSA or HP); or (2) CRC which has both BRAF mutation and MSI-H/MLH1 loss (serrated-neoplasia pathway) or both BRAF mutation and TP53 mutation/accumulation (alternative serrated-neoplasia pathway). The CRC which does not meet either of the definitions or meets both definitions of adenoma–carcinoma pathway and serrated-neoplasia pathway was classified unknown (unclassifiable) pathway.
Statistical analyses
All data were analyzed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.
Quantitative variables were analyzed using Student’s t test, and qualitative variables were analyzed by Fisher’s exact test with Bonferroni correction, or by Friedman test with Holm correction.
Results
Study flow and patient characteristics
Patient selection was performed as shown in the flowchart (Supplementary Fig. 1). We screened 10,346 patients whose colonoscopy images and corresponding reports were recorded in the endoscopy filing system (SOLEMIO QUEV, Olympus) between January 2009 and November 2021. The patients were screened using the phrase “serrated polyposis syndrome” and “hyperplastic polyposis syndrome” as keywords, and 69 and 11 patients were found, respectively. We also screened using “SSA/P or SSL” and “TSA” as keywords, and found 536 and 98 patients, respectively. Subsequently we evaluated if those selected patients met SPS WHO criteria 2019 using the data in medical records, and identified a total of 47 fulfilling the criteria. Of these, two patients with GAPPS were excluded, and one patient was excluded due to refusal to provide informed consent. We ultimately enrolled 44 SPS patients, including 18 patients with complicated CRC.
The baseline characteristics of patients are summarized in Supplementary Table 1. The median age at diagnosis of SPS was 62 years (IQR 54.8–67), and 14 patients (32%) were female. Of all 44 patients, 25 (56%) fulfilled WHO criterion 1, 11 (25%) fulfilled criterion 2, and the remaining 8 (18%) fulfilled both criteria 1 and 2. The prevalence of CRC was 41% (18/44).
Clinicopathological features of each type of polyps in SPS patients
A total of 956 polyps were analyzed in all 44 SPS patients. Among all the polyps, HP was the most common (67%), followed by SSL (21%), adenoma (11%), and TSA (1%) (Fig. 1a). The prevalence rate of HP, SSL, and TSA in SPS patients was 86% (38/44), 84% (37/44), and 18% (8/44), respectively, whereas the prevalence of adenoma was 73% (32/44) (Fig. 1b). The median number of all SPs was 17 (IQR 8.8–24) per patient; the median numbers of HP, SSL, and TSA were 10.5 (IQR 2.75–23), 4.0 (2.0–6.0), and 0 (0–0), respectively, showing a significant stepwise decrement in this order (HP vs. SSL, p < 0.01; SSL vs. TSA, p < 0.01). While the median number of adenomas was 1 (IQR 0–3.3), which was significantly lower than that of HP and SSL (p < 0.01 and p < 0.01, respectively) and higher than that of TSA (p < 0.01).
Number of colorectal polyps in patients with serrated polyposis syndrome (SPS). a Percentage of each type of polyp (N = 956). b Number (per person) and prevalence of each type of polyp. Statistical analysis was performed by Friedman test with Holm correction. *p < 0.05. c Percentage of each type of polyp in each location. HP hyperplastic polyp, SSL sessile serrated lesion, TSA traditional serrated adenoma
We then examined the distribution of polyps in each region of the colorectum (Fig. 1c). In the proximal colon (from cecum to transverse colon), the majority of polyps were SSL (54%), followed by HP (28%), adenoma (17%), and TSA (1%). In contrast, most of the polyps were HP (97%) in the rectum, followed by adenoma (3%), and SSL (1%). The percentage of SSL decreased from the proximal-to-distal direction step-by-step, whereas the percentage of HP increased step-by-step from the proximal-to-distal direction. Adenoma was distributed throughout the colorectum.
The total number of large SPs (≥ 10 mm) was 154, and they were preferentially located in the proximal colon (119 in the proximal colon, 23 in descending colon, 10 in the sigmoid colon, and 2 in the rectum). In contrast, the number of advanced adenomas was 22, and they were predominantly distributed in the left-side colorectum; 5 in the proximal, 5 in the descending colon, 9 in the sigmoid colon, and 3 in the rectum (Fig. 1c).
Clinicopathological features of colorectal cancer in patients with SPS
A total of 22 CRCs were found in 18 patients with SPS. Clinicopathological features of those cancers are shown in Table 1. Three patients were diagnosed with multiple synchronous CRCs; cases 3 and 14 had double cancers, and case 8 had triple cancers. Twelve of the 18 patients (67%) were male. The median (IQR) ages at diagnosis of SPS and CRC were 62.5 (57.75–67.50) and 62 (52.25–63.75) years, respectively. Of the 18 CRC patients, 6 (33%) had been endoscopically or surgically operated on 4–29 years (median: 5 years) before the SPS diagnosis was made.
The distribution of all 22 CRCs is as follows; 6 (27%) in sigmoid colon, 6 (27%) in rectum, 5 (23%) in ascending colon, 2 (9%) in transverse colon, 1 (5%) in descending colon, 1 (5%) in cecum, and 1 unknown (5%) (Table 1). The median size of CRCs was 16 mm (IQR 10–30). Most of the tumors (19/22, 86%) were diagnosed at an early stage (stage 0, I, and II). Among all 22 CRCs, 12 (55%) were intramucosal carcinomas (stage 0), and cured by endoscopic resection. The pathological diagnosis for all CRCs was adenocarcinoma, low-grade (differentiated type). About half of the CRCs (10/22, 45%) had surrounding benign lesions (2 for SSL, 1 for TSA, 6 for tubular adenoma, 1 for tubulovillous adenoma).
Somatic mutational analysis of CRC to evaluate carcinogenesis pathway
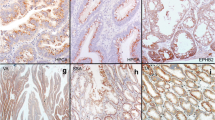
To investigate the mechanism of carcinogenesis in SPS patients, we performed gene mutational analysis of RAS, APC, β-catenin, BRAF, and TP53 using 19 CRC tissues. Figure 2a–d and e–h shows the representative appearance and genetic analysis data of CRC derived from serrated-neoplasia pathway. The type 3 cancer (lesion 14-A; Fig. 2a) in the ascending colon from case 14 (62 y, female) histologically exhibited a differentiated-type tubular adenocarcinoma (Fig. 2b). This cancer showed a BRAF V600E mutation and MSI-H (Fig. 2c, d) but did not have any mutation of the RAS, APC, β-catenin, or TP53 genes. Thus, this cancer was classified as derived through a serrated-neoplasia pathway (BRAF-mutated MMR-deficient carcinoma), according to the definition. The type 3 cancer (lesion 14-B; Fig. 2e) in the same individual's ascending colon (case 14) exhibited histologically differentiated-type tubular adenocarcinoma (Fig. 2f). This cancer showed BRAF V600E (Fig. 2g) and TP53 mutations (R213*) (Fig. 2h), and MSS phenotype, but did not have any mutation of RAS, APC, and β-catenin genes (data not shown); thus, it was classified as derived through an alternative serrated-neoplasia pathway (BRAF-mutated MMR-proficient carcinoma). On the other hand, Fig. 2i–-m shows representative CRC which developed through adenoma–carcinoma pathway. The type 0-Ip cancer (lesion 8-B; Fig. 2i) in the descending colon from case 8 (63 y, male) shows histologically differentiated adenocarcinoma in adenoma (Fig. 2j). This lesion exhibited 2 APC mutations (V1414*, T1459I; Fig. 2k), K-RAS mutation (G13D; Fig. 2l), and TP53 mutation (L252del; Fig. 2m). However, it was negative for BRAF mutation and was an MSS phenotype (data not shown). Thus, it was classified as derived through the adenoma–carcinoma pathway.
Three representative cases of colorectal cancer (CRC) with SPS. a Endoscopic finding of type 3 cancer (lesion 14-A) in the ascending colon. b H&E staining exhibited a differentiated-type tubular adenocarcinoma. Magnification: 100×. c Mutation in codon 600 of the BRAF gene (V600E). d Abnormal electropherogram patterns of tumors compared with normal epithelia in 5 microsatellite markers, representing MSI-H. e Endoscopic finding of type 3 cancer (lesion 14-B) in the ascending colon. f H&E staining showed differentiated-type tubular adenocarcinoma. Magnification: 100×. g Mutation in codon 600 of the BRAF gene (V600E). h Mutation in exon 6 of the TP53 gene (R213*). i Endoscopic finding of type 0-Ip cancer (lesion 8-B) in the descending colon. j H&E staining showed differentiated adenocarcinoma in adenoma (Tis). Magnification: 40x. k Two mutations in exon 15 of the APC gene (V1414*, T1459I). l Mutation in exon 2 of the K-RAS gene (G13D). m Mutation in exon 7 of the TP53 gene (L252del)
We summarized the genetic and immunohistochemical data in 19 CRCs from 15 patients with SPS in Table 2. According to the definition of each pathway, we classified 5 CRCs (3-A, 5, 14-A, 14-B, and 16) as originating through the serrated-neoplasia pathway, and 11 through the adenoma–carcinoma pathway. Of the 5 CRCs via the serrated-neoplasia pathway, only 1 was a well-reported pathway with both BRAF mutation and MSI-H. Three CRCs had both BRAF mutation and TP53 mutation (including p53 protein accumulation), indicative of alternative serrated-neoplasia pathway. The remaining one was thought to develop from TSA. In contrast, among the 11 CRCs derived from adenoma–carcinoma pathway, 10 cancers had both APC/β-catenin mutations and TP53 mutation/accumulation except for one lesion coexistent with tubulovillous adenoma (cancer in tubulovillous adenoma), which was positive for both KRAS mutation and TP53 protein nuclear accumulation, consistent with a previous report [30].
The remaining 3 CRCs (lesions 3-B, 13, 15) were classified as unknown pathway: lesion 3-B, which had both APC mutation and BRAF V600E mutation, and TP 53 nuclear accumulation, met both definitions simultaneously; lesions 13 and 15 did not meet either definition. To investigate more in detail, we performed additional RNF43 mutational analysis on lesions 13 and 15, which were negative for APC mutation. Lesion 13 had only TP53 mutation but not any mutation in APC, β-catenin, RAS, BRAF, and RNF43. Lesion 15 exhibited MSI-H phenotype and RNF43 mutation (R114Q, G659fs) but was also positive for KRAS mutation (Q61H).
Differences in clinicopathological features between carcinogenesis pathways in SPS patients
We assessed differences in clinicopathological features between the serrated-neoplasia and adenoma–carcinoma pathways (Table 3). All patients with CRCs via the serrated-neoplasia pathway met criterion 1, whereas most patients with CRCs via the adenoma–carcinoma pathway met criterion 2. There was a significant correlation between the pathway and the criteria (p = 0.0385). Basically, CRCs via the serrated-neoplasia pathway were located in the proximal colon except for case 16, which developed from TSA. Meanwhile, 9/11 (82%) CRCs via the adenoma–carcinoma pathway were mostly located in the distal to splenic flexure (p = 0.0357). Sex, age and distribution type were not significantly associated with the carcinogenesis pathway.
The median number of SPs in patients with CRC via the adenoma–carcinoma pathway (23 [IQR 19.5–33.5]) was significantly higher than that via the serrated-neoplasia pathway (16 [16–18]); p = 0.0402). The same tendency could be shown for HP; 23 (14.5–31.5) for the adenoma–carcinoma pathway vs. 9 (9–12) for the serrated-neoplasia pathway (p = 0.0307). The median number of advanced adenomas in patients with CRC via the adenoma–carcinoma pathway (1 [0.5–6]) was significantly higher than via the serrated-neoplasia pathway (0 [0–0]; p = 0.0169). Similarly, the prevalence of advanced adenoma in patients with CRC via the adenoma–carcinoma pathway was significantly higher than via the serrated-neoplasia pathway [73% (8/11) vs. 0% (0/5); p = 0.0256].
Risk factors for colorectal cancer in SPS patients
We performed a univariate analysis of possible risk factors for CRC in SPS patients (Table 4). No significant differences in sex ratio, age at diagnosis of SPS, WHO criteria, BMI, smoking habit, incidence of other organ malignancy, and family history of CRC were observed. Although the patients with CRC had more SPs, particularly HPs, no statistically significant difference was observed. However, the prevalence of adenoma in patients with CRC was significantly higher than in patients without CRC [94% (17/18) vs. 62% (16/26); p = 0.0157]. Similarly, the number of advanced adenomas tended to be higher in the former than in the latter. These results suggest an important role of adenoma in colorectal carcinogenesis in SPS.
Discussion
This study demonstrated that more than half of CRCs in patients with SPS developed from an adenoma through the adenoma–carcinoma pathway. Specifically, of the 19 CRCs examined in SPS patients, 11 (58%) were considered to develop from adenoma through the adenoma–carcinoma pathway, whereas 5 (26%) developed from SPs via the serrated-neoplasia pathway, and the remaining 3 (16%) were classified as an unknown pathway. We also revealed that the carcinogenesis pathway was strongly associated with the location of CRC, i.e., most of the CRCs located in the ascending colon originated from the serrated-neoplasia pathway, and the patients bearing those CRCs met with WHO criterion 1 and were characterized by a large SSL. On the other hand, most CRCs located in the distal to splenic flexure developed through the adenoma–carcinoma pathway, and patients bearing those CRCs met with criterion 2 and were characterized by a high number of HPs and advanced adenomas. To our knowledge, this is the first report demonstrating the importance of the adenoma–carcinoma pathway as a carcinogenesis pathway in SPS patients. This result is also supported by the data showing that the presence of adenoma was a risk factor for CRC in SPS patients.
The gender ratio (male/female) of SPS patients in this study was relatively high (30/14). Since the ratio in previous studies was roughly equivalent [2, 3], it should be re-evaluated in a larger scale cohort in the future. The incidence of CRC including intramucosal carcinoma in this study was 41%, which was roughly comparable with previous reports ranging 15.8% to 41.9% [2, 3, 7]. The population and distribution of each type of polyp was also mostly consistent with previous reports [2]; the most common type of polyp was HP, followed by SSL, adenoma, and TSA. SSL distributed predominantly in the proximal region, whereas HP distributed predominantly in the distal regions. The adenomas were distributed throughout the colorectum, which was mostly similar to the distribution in the general population [31, 32]. However, the incidence of adenomas (73%) in SPS patients was higher than in the general population.
There are only a few published reports on molecular analysis of SPS-derived CRC. In particular, specific carcinogenic pathways other than the serrated-neoplasia pathway in SPS patients have not been thoroughly investigated. We defined the serrated-neoplasia pathway and adenoma–carcinoma pathway in the Methods section and evaluated whether CRC in SPS patients originated from any of those pathways. Out of 19 CRCs, 5 (26%) were classified as originating from the serrated-neoplasia pathway. The rate of BRAF mutation, an important gene mutation in the serrated-neoplasia pathway, was 26% (5/19) among all CRCs in this study, which was slightly lower than previously reported (49–53% of BRAF mutations) [13, 14]. In the Japanese population, BRAF mutations are estimated to occur in 4.5% of CRCs [33], which may account for the difference in the rate of BRAF mutations. In addition, MSI-H or MLH1 downregulation, which are also important gene alterations in the serrated-neoplasia pathway, were identified in two patients (11%; 2/19) in our study, and this rate is apparently lower than the rate of 32–53% reported from Western countries [13, 14]. Interestingly, this discrepancy also might be associated with racial or geographical differences, evidenced by the fact that MSI-H was found in 5.9% of CRCs in a Japanese cohort [34], which was a lower rate than that in a European cohort (12–16%) [35, 36]. On the other hand, 11 CRCs (58%) were classified as arising from the adenoma–carcinoma pathway. Among all 19 CRCs, the APC mutation, specific for adenoma–carcinoma pathway, was detected in 53% (10/19), and RAS mutation in 37% (7/19), and they were higher than in previous reports (i.e., 11% for APC mutation and 3–5% for RAS mutation) [13, 14]. The higher rate of RAS mutations found here might be due to the higher sensitivity of the sequencing method we used, compared to that of previous reports, in which several codons of RAS other than KRAS codons 12 and 13 were examined [37]. Thus, although the low rates of BRAF mutation and MSI-H may affect the incidence of CRC via the serrated-neoplasia pathway, a more than half of CRCs developed from adenoma via the adenoma–carcinoma pathway. This is quite paradoxical, because the SPS patients had many precancerous SPs in the colorectum; however, CRC did not develop from those SPs but rather from adenoma, which is much less frequent than SPs in those SPS patients.
Three CRCs (16%) were categorized as arising from an unknown pathway in this study. One lesion (3-B), which was located in the cecum, had BRAF and APC mutation and TP53 nuclear accumulation. It is plausible that the SSL with BRAF mutation, or adenoma with APC mutation, had additional APC or BRAF mutations incidentally, and finally developed into cancer via TP53 mutation. However, considering the fact that patient 3 had another CRC in the ascending colon (lesion 3-A) which was histologically surrounded by SSL, near lesion 3-B, the lesion 3-B seemed to have developed through a similar pathway to lesion 3-A. Lesion 13, located in the rectum, had only TP53 abnormality without any mutation of the BRAF, RAS, APC, and β-catenin genes. A rare type of mutation in these genes, which was not detected by our methods, might have been positive, otherwise a mutation in the other rare genes such as axin and GSK3 might have been positive. Lesion 15, located in the ascending colon, exhibited RAS mutation and MSI-H (MLH1 loss), which is an uncommon combination. This CRC was positive for MSI-H and RNF43 mutation, and patient 15 had 6 large SSLs but no adenomas. Since KRAS mutation was reported to be positive in 7–10% of SSLs [38], this CRC more likely developed through the serrated-neoplasia pathway.
It is quite impressive that all CRCs in the ascending colon were derived from the serrated-neoplasia pathway, whereas all the distal CRCs and the transverse CRC were derived from the adenoma–carcinoma pathway, except for carcinoma with TSA, regardless of the diagnostic criteria (1 and 2) (Fig. 3). Moreover, patients who fulfilled criterion 2, characterized by a high number of HP, developed CRC through the adenoma–carcinoma pathway mainly in the distal colorectum, whereas patients who fulfilled criterion 1, characterized by large SSLs, developed CRC not only in the proximal colon through the serrated-neoplasia pathway, but also in the distal colorectum via the adenoma–carcinoma pathway. This may be partly explained by the current results showing that large SSLs were predominantly located in the proximal colon, whereas advanced adenomas were predominantly observed in the distal colorectum in SPS patients (Fig. 1c). This is also consistent with our data showing that patients with CRCs from the adenoma–carcinoma pathway had a greater number of advanced adenomas (Table 3). It is well documented that the adenoma–carcinoma pathway, which is mainly initiated by APC mutation, is likely to occur in the distal colorectum [39, 40], whereas SSL, which mainly occurs via BRAF mutation, is predominantly located in the proximal colon in the general population [12, 28]. However, the reason why the incidence of adenoma in SPS patients is higher than in the general population is currently unknown.
Distribution of CRCs and the number of each type of polyp in patients with SPS. A blue circle represents a cancer from the serrated-neoplasia pathway via an SSL. A red circle represents a cancer from the serrated-neoplasia pathway via a TSA. A green circle represents a cancer from the adenoma–carcinoma pathway. Criteria 1/2 indicate WHO criteria 1/2 for SPS; criterion 1 defined as ≥ 5 serrated lesions/polyps proximal to the rectum, all being ≥ 5 mm in size, with ≥ 2 being ≥ 10 mm in size; or criterion 2 defined as > 20 serrated lesions/polyps of any size distributed throughout the large bowel, with ≥ 5 being proximal to the rectum. The bar graph indicates the number of each type of polyp. Yellow, HP; blue, SSL; red, TSA; and green, adenoma. The dark color represents a larger (≥ 10 mm) polyp
Several recent studies suggest that CRC risk depends on patient-specific risk factors, such as a history of SP with dysplasia, advanced adenomas, or satisfying both WHO 2010 criteria 1 and 3 [2, 3]. In this study, no significant risk of CRC could be detected among clinical, endoscopic, and histopathological parameters except for the presence of conventional adenoma. Buchanan et al. reported that the presence of adenoma was approximately 4-times higher risk for CRC among the patients with multiple SPs (≥ 5) [41]. Our result is consistent with their findings, and also suggest the importance of advanced adenomas, which were predominantly located in the left-side colorectum, in carcinogenesis of SPS patients.
The guideline for SPS recommends periodic colonoscopy to detect and remove proximal SSLs and HPs, particularly those lesions ≥ 10 mm, but does not mention conventional adenomas [42]. However, in this study, more than half of CRCs in SPS patients developed from adenoma through the adenoma–carcinoma pathway. Moreover, in patients fulfilling criterion 2 alone, all the CRCs developed through the adenoma–carcinoma pathway in the distal colorectum. Therefore, we should perform colonoscopy and carefully detect adenoma in the distal colorectum to remove it. While for patients who meet criterion 1, we should search not only for SSL in the proximal colon but also for adenoma in the distal colorectum, as well as the transverse colon, to remove them.
The major limitation of the present study was that it had a limited sample size and was performed at a single institution, thus warranting further evaluation in a larger multicenter cohort. However, since SPS is a rare disease and SPS with CRCs is even rarer, our cohort was of an acceptable size for the genetic analysis. Moreover, we did not perform comprehensive whole genomic and epigenetic analysis in CRCs, since the analysis of variants outside the coding regions of the genome (e.g., promoters, deep intronic regions), aberrant DNA methylation, and structural rearrangements was beyond the scope of this study.
In conclusion, our data suggest that CRC in the left-side colorectum was originated from the adenoma–carcinoma pathway, whereas CRC in the right-side (ascending) colon was derived from the serrated-neoplasia pathway in SPS patients. More than half of the CRCs was supposedly derived from the adenoma–carcinoma pathway in SPS patients. Adenoma was a risk factor for CRC in SPS patients, suggesting an important role of adenoma in SPS.
References
Pai RK (Rish) MM, Rosty C. Colorectal serrated lesions and polyps. In: the WHO Classification of Tumours Editorial Board, editor. WHO classification of tumours of the digestive system. 5th ed. Lyon: IARC press; 2019. pp. 163–9.
Carballal S, Rodríguez-Alcalde D, Moreira L, et al. Colorectal cancer risk factors in patients with serrated polyposis syndrome: a large multicentre study. Gut. 2016;65:1829–37.
Ijspeert JEG, Rana SAQ, Atkinson NSS, et al. Clinical risk factors of colorectal cancer in patients with serrated polyposis syndrome: a multicentre cohort analysis. Gut. 2017;66:278–84.
Quintana I, Mejías-Luque R, Terradas M, et al. Evidence suggests that germline RNF43 mutations are a rare cause of serrated polyposis. Gut. 2018;67:2230–2.
Buchanan DD, Clendenning M, Zhuoer L, et al. Lack of evidence for germline RNF43 mutations in patients with serrated polyposis syndrome from a large multinational study. Gut. 2017;66:1170–2.
Rosty CBL, Dekker E, Nagtegaal ID. Serrated polyposis. In: the WHO Classification of Tumours Editorial Board, editor. WHO classification of tumours of the digestive system. 5th ed. Lyon: IARC press; 2019. pp. 532–4.
Rosty C, Buchanan DD, Walsh MD, et al. Phenotype and polyp landscape in serrated polyposis syndrome: a series of 100 patients from genetics clinics. Am J Surg Pathol. 2012;36:876–82.
Bleijenberg AG, JE IJ, van Herwaarden YJ, et al. Personalised surveillance for serrated polyposis syndrome: results from a prospective 5-year international cohort study. Gut. 2020;69:112–21.
Boparai KS, Mathus-Vliegen EM, Koornstra JJ, et al. Increased colorectal cancer risk during follow-up in patients with hyperplastic polyposis syndrome: a multicentre cohort study. Gut. 2010;59:1094–100.
Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32.
Rosty C, Hewett DG, Brown IS, et al. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48:287–302.
Crockett SD, Nagtegaal ID. Terminology, molecular features, epidemiology, and management of serrated colorectal neoplasia. Gastroenterology. 2019;157:949–66.
He EY, Wyld L, Sloane MA, et al. The molecular characteristics of colonic neoplasms in serrated polyposis: a systematic review and meta-analysis. J Pathol Clin Res. 2016;2:127–37.
Boparai KS, Dekker E, Polak MM, et al. A serrated colorectal cancer pathway predominates over the classic WNT pathway in patients with hyperplastic polyposis syndrome. Am J Pathol. 2011;178:2700–7.
Nagtegaal AM, Odze RD, Lam AK. Tumours of the colon and rectum. In: the WHO Classification of Tumours Editorial Board, editor. WHO classification of tumours of the digestive system. 5th ed. Lyon: IARC press; 2019. pp. 157–91.
Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42.
Taniguchi H, Okamoto W, Muro K, et al. Clinical validation of newly developed multiplex kit using luminex xMAP technology for detecting simultaneous RAS and BRAF mutations in colorectal cancer: results of the RASKET-B Study. Neoplasia. 2018;20:1219–26.
Nagasaka T, Sasamoto H, Notohara K, et al. Colorectal cancer with mutation in BRAF, KRAS, and wild-type with respect to both oncogenes showing different patterns of DNA methylation. J Clin Oncol. 2004;22:4584–94.
Miyoshi Y, Ando H, Nagase H, et al. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci U S A. 1992;89:4452–6.
Song G, Yuan Y, Zheng F, et al. Novel insertion mutation p.Asp610GlyfsX23 in APC gene causes familial adenomatous polyposis in Chinese families. Gene. 2013;516:204–8.
Chang XY, Wu Y, Jiang Y, et al. RNF43 Mutations in IPMN Cases: A Potential Prognostic Factor. Gastroenterol Res Pract. 2020. https://doi.org/10.1155/2020/1457452.
Murphy KM, Zhang S, Geiger T, et al. Comparison of the microsatellite instability analysis system and the Bethesda panel for the determination of microsatellite instability in colorectal cancers. J Mol Diagn. 2006;8:305–11.
Miyamoto Y, Muguruma N, Fujimoto S, et al. Epidermal growth factor receptor-targeted molecular imaging of colorectal tumors: Detection and treatment evaluation of tumors in animal models. Cancer Sci. 2019;110:1921–30.
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67.
Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90.
Takayama T, Miyanishi K, Hayashi T, et al. Colorectal cancer: genetics of development and metastasis. J Gastroenterol. 2006;41:185–92.
Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–100.
Bettington M, Walker N, Rosty C, et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66:97–106.
Bettington ML, Walker NI, Rosty C, et al. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod Pathol. 2015;28:414–27.
Tanaka Y, Eizuka M, Uesugi N, et al. Traditional serrated adenoma has two distinct genetic pathways for molecular tumorigenesis with potential neoplastic progression. J Gastroenterol. 2020;55:846–57.
Ikeda Y, Mori M, Yoshizumi T, et al. Cancer and adenomatous polyp distribution in the colorectum. Am J Gastroenterol. 1999;94:191–3.
Bai Y, Gao J, Zou DW, et al. Distribution trends of colorectal adenoma and cancer: a colonoscopy database analysis of 11,025 Chinese patients. J Gastroenterol Hepatol. 2010;25:1668–73.
Yokota T, Ura T, Shibata N, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104:856–62.
Asaka S, Arai Y, Nishimura Y, et al. Microsatellite instability-low colorectal cancer acquires a KRAS mutation during the progression from Dukes’ A to Dukes’ B. Carcinogenesis. 2009;30:494–9.
Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–7.
Peltomäki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21:1174–9.
Yoshino T, Muro K, Yamaguchi K, et al. Clinical validation of a multiplex kit for RAS mutations in colorectal cancer: results of the RASKET (RAS KEy testing) prospective. Multicent Study EBioMed. 2015;2:317–23.
Hashimoto T, Yamashita S, Yoshida H, et al. WNT pathway gene mutations are associated with the presence of dysplasia in colorectal sessile serrated adenoma/polyps. Am J Surg Pathol. 2017;41:1188–97.
Lee GH, Malietzis G, Askari A, et al. Is right-sided colon cancer different to left-sided colorectal cancer? a systematic review. Eur J Surg Oncol. 2015;41:300–8.
Sugai T, Habano W, Jiao YF, et al. Analysis of molecular alterations in left- and right-sided colorectal carcinomas reveals distinct pathways of carcinogenesis: proposal for new molecular profile of colorectal carcinomas. J Mol Diagn. 2006;8:193–201.
Buchanan DD, Sweet K, Drini M, et al. Risk factors for colorectal cancer in patients with multiple serrated polyps: a cross-sectional case series from genetics clinics. PLoS ONE. 2010;5:e11636.
East JE, Atkin WS, Bateman AC, et al. British society of gastroenterology position statement on serrated polyps in the colon and rectum. Gut. 2017;66:1181–96.
Acknowledgements
We are grateful to Misato Hirata and Masahiro Bando (Department of Gastroenterology and Oncology, Tokushima University, Tokushima) for their technical assistance. We also appreciate professor Yoshimi Bando (Division of Pathology, Tokushima University Hospital, Tokushima) for helping us by consultation for pathological diagnosis.
Funding
This work was partly supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS; grant number 19K08471).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2022_1858_MOESM1_ESM.pptx
Flow diagram. SSA/P, sessile serrated adenoma/polyp; IBD, inflammatory bowel disease; FAP, familial adenomatous polyposis; GAPPS, gastric adenocarcinoma and proximal polyposis syndrome mentary
Rights and permissions
About this article
Cite this article
Nakamura, F., Sato, Y., Okamoto, K. et al. Colorectal carcinoma occurring via the adenoma–carcinoma pathway in patients with serrated polyposis syndrome. J Gastroenterol 57, 286–299 (2022). https://doi.org/10.1007/s00535-022-01858-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-022-01858-8