Abstract
Serrated polyps (SPs) are considered the precursor lesions of up to 15–30% of all colorectal carcinomas through the “serrated neoplasia pathway.” Serrated polyposis syndrome (SPS), characterized by the presence large and/or numerous serrated lesions spreading throughout the colorectum, is emerging as one of the most common colorectal cancer polyp syndromes. This condition is associated with an increased personal and familial colorectal cancer risk. Clinical management includes yearly surveillance colonoscopy and surgery. Although the majority of cases occur in patients older than 50 years old with no family history of CRC, several lines of evidence support that a proportion of SPS could be the phenotypic expression of an inherited genetic syndrome, but the genetic basis for SPS remains elusive. Recent studies provided proof of the pathogenicity of RNF43 germline mutation in a small subset of patients. Future research in SPS should be focused on understanding the phenotype and clinical management and on unraveling the pathogenesis of the syndrome.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Clinical Features
1.1 Definition and Cancer Risk
Colorectal cancer (CRC) arises through precursor lesions , called polyps, and the timely detection and removal of these polyps is essential in CRC prevention [1]. Traditionally, conventional adenomas were considered the only precursor lesions that would develop into CRC through the “adenoma-carcinoma” pathway [2]. In contrast, hyperplastic polyps, typically observed in the rectum, were thought to be benign. Over the last 30 years, growing evidence has given rise to an alternative pathway, called “serrated pathway ,” characterized morphologically by the presence of serrated lesions and molecularly by somatic mutations in the BRAF proto-oncogene, hypermethylation of the promoter regions of tumor suppressor genes, and microsatellite instability. This pathway is currently considered the responsible of up to 15–30% of all CRC [3, 4].
Serrated polyps (SPs) are defined as heterogeneous group of lesions morphologically characterized by serrated (“saw-tooth”) architecture of the epithelium that lines the colonic crypts [5]. The World Health Organization (WHO) classifies SPs into three subgroups: hyperplastic polyps (HPs), sessile serrated adenomas/polyps (SSA/Ps) with or without dysplasia, and traditional serrated adenomas/polyps (TSA/Ps) . The main features defining each serrated polyp subtype are reported in Table 15.1.
Hyperplastic polyps (HPs) are common, accounting for 70–90% of all SP. They are characterized by the presence of straight crypts, which extend symmetrically from the surface of the polyp to the muscularis mucosae without significant distortion. Distinct subtypes of HPs have been recognized; basically HPs are subdivided into microvesicular (MVHP) and goblet cell (GCHP) types, based on the characteristics of lining epithelium. MVHPs and GCHPs are well characterized and display considerable differences in molecular and histological features as well as anatomic distribution within the colon. HPs are considered of less clinical importance, especially if they are diminutive and located in the rectosigmoid. It is unclear whether some MVHP can progress to SSA/Ps. The significance of GCHP is poorly understood; some authors have suggested that it may represent the precursor lesion of TSA.
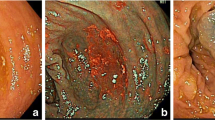
Sessile serrated adenomas (SSAs) are often subtle, appear flat or slightly elevated, and can be covered by yellow mucus. They are typically found in the proximal colon and they are usually larger than 0.5 mm. Histologically, the serrations are more prominent than those of hyperplastic polyps and involve the entire length of the crypt (Fig. 15.1). SSA/Ps, especially when cytological dysplasia is present, are considered the main precursors of serrated colorectal carcinomas [6, 7]. They represent approximately 5–25% of all SPs and are found in 3.3% of average risk population [8].
Endoscopic and histological appearance of sessile serrated adenoma/polyps. (a) A slightly elevated lesion located at cecum was detected during endoscopic examination in patient with serrated polyposis syndrome. After washing the adherent mucus over the polyp surface its indistinct edges, “cloud-like” surface and color similar to surrounding mucosa could be appreciated. Polyp was removed by endoscopic mucosal resection. (b) Microscopically, the polyp showed a marked serration and dilated, mucus-filled T-shaped (‘anchor’) crypts, corresponding to a sessile serrated adenoma/polyp
Traditional serrated adenomas (TSAs) are less common than other types of SPs (1%). The majority of them are located at the distal colon, they are often >5 mm, and their endoscopic appearance resembles conventional adenomas. The histopathological features of TSA are quite characteristic, often showing a complex and distorted tubulovillous or villous (“filiform”) configuration.
Serrated polyposis syndrome (SPS) is a condition characterized by the combination of large and/or numerous serrated lesions spreading throughout the colorectum with an increased life-time risk of CRC [9,10,11,12,13,14]. Revised World Health Organization (WHO) criteria for SPS are represented in Table 15.2. Although arbitrary, this definition has been useful to standardize the diagnosis and treatment, as well as to prompt research in such a field.
While the prevalence of SPS remains unknown, this syndrome is emerging as one of the most common colorectal cancer polyp syndromes. Endoscopic detection and histopathological characterization is a challenge in clinical practice. Increased awareness of serrated polyps has likely improved the diagnosis of SPS, suggesting that the prevalence is greater than initially reported. Indeed, prevalence of SPS in primary colonoscopy or sigmoidoscopy screening programs was reported to be <0.1%, while recent data suggest a four-time higher prevalence [8]. The prevalence of SPS in preselected screening populations based on a positive fecal immunochemical test (FIT) has been reported to be considerably higher (0.34–0.66%) [15, 16]. Moreover, SPS is often missed during a first screening colonoscopy. The rate of SPS after follow-up, as reported in the FIT-based screening cohort (0.8%) and in primary colonoscopy cohort (0.4%), seems to be more accurate estimates of the true prevalence of SPS.
Initial small series of patients with SPS reported up to 70% rates of CRC [14], a figure that probably traduced an important selection bias, overestimating the perception of CRC in SPS. The prevalence of CRC reported in subsequent studies, with a larger number of patients, showed a lower risk between 7% and 35% [11, 17,18,19,20,21]. Few studies have tried to stratify the CRC risk based on clinical risk factors [20, 22, 23]. Indeed, patients who fulfill both WHO criteria I and III, the presence of advanced adenomas or dysplasia within serrated polyps, and the number of SSA/P are factors that have been associated with an increased risk of CRC in SPS patients. However, cancer risk in patients fulfilling criterion II remains controversial. Since proximal SPs are detected in 4.7–12.2% of the screening population [8], this criterion is probably leading to an overdiagnosis of SPS and should be revised in the near future.
This high prevalence of CRC is far from the rate of incident CRC observed in the two largest and most recent cohorts of patients with SPS. Current observations suggest that once patients with SPS undergo endoscopic surveillance with polyp removal (at least those >3 mm), the CRC risk is very low (5-year-cumulative incidence of CRC around 2%) [22, 23].
1.2 Clinical Characteristics
Characteristics of patients with SPS have been defined mainly based on the publication of series of cases, with significant heterogeneity in the description of the clinical, endoscopic, and histological features. There is no apparent sex predominance and the mean age at diagnosis is between 50 and 60 years. Cigarette smoking history and overweight/obesity have been associated with an increased risk of developing serrated polyps [24], suggesting that environmental factors are involved in the pathogenesis . In this sense, current smoking has been strongly associated with the presence of advanced SP [25]. However, recent studies suggest that the pathogenesis of SPS might be different in smokers and non-smokers [24]. History of smoking (current and former smokers) is significantly associated to fulfillment of WHO criterion III only, compared with non-smokers [23]. Additionally, the CRC risk seems to be smaller in the smokers than in the non-smokers [23]. Future research needs to clarify the role of smoking in SPS and might influence the options for therapy and surveillance for smokers in the near future.
Some authors have suggested the existence of various phenotypes within the SPS definition. Some patients display a right-sided phenotype with large SSA/P (i.e., criterion I), some present with a left-sided phenotype with a greater amount of small polyps (i.e., criterion III), and others show a mixed phenotype with shared features of the previous phenotypes [20]. As mentioned, patients who fulfill both WHO criteria I and III seem to be at increased risk to be diagnosed with CRC, compared with patients who fulfill WHO criterion I or WHO criterion III only [23]. Conventional adenomas frequently coexist with serrated polyps in patients with SPS. There is no clear evidence of an increased risk of extracolonic neoplasms in patients with SPS and their relatives [12].
Between 10 and 50% of SPS patients report a family history of CRC [14, 18,19,20,21, 26, 27], and first-degree relatives of SPS patients have an increased risk for both CRC and SPS compared with the general population. In the largest series, the standardized incidence ratio of CRC in first-degree relatives of patients with SPS was approximately five times that of the general population [20].
1.3 Clinical Management
Due to the high risk of CRC in patients with SPS, scientific societies recommend 1-year endoscopic surveillance in all patients with SPS diagnosis [28]. Patients undergoing annual colonoscopy surveillance in experienced centers, with removal of polyps >3 mm, show a low risk of developing CRC (<2% in 5 years) [12, 22]. Ongoing follow-up studies are evaluating whether endoscopic surveillance can be performed at longer time intervals in a subset of patients that do not display CRC risk factors.
Surgical management should be restricted to cases with severe polyposis that is unmanageable endoscopically, unresectable large lesions, or the presence of CRC. The decision for an extended (total colectomy with ileo-rectal anastomosis if the rectum is spared) vs. segmental colectomy needs to be individualized for each patient. After surgery, it is advisable to conduct surveillance of the remaining colorectum every 6–12 months for the risk of metachronous lesions .
First-degree relatives of patients with SPS should undergo CRC screening starting at the age of 35–40 years or 10 years before the age of diagnosis of the youngest affected family member [29, 30].
2 Molecular Features and Pathogenesis
2.1 Serrated Pathway of Carcinogenesis
The serrated pathway has recently emerged as an alternative pathway leading to sporadic CRC. The well-characterized molecular changes associated with serrated pathway are (1) mutations in BRAF and KRAS oncogenes, (2) microsatellite instability (MSI), and (3) CpG island methylator phenotype (CIMP) (Fig. 15.2).
Serrated pathway of colorectal carcinogenesis . Oncogenic BRAF mutation is detected in the earliest serrated lesions, especially in MVHP. MLH1 methylation leads to MSI tumors. In contrast, methylation in other targets (i.e., p16INK4a, IGFBP7, and MGMT) is associated with MSS CRCs. Abbreviations: MSI-H high microsatellite instability, MSS microsatellite stability, CIMP CpG island methylator phenotype
The mitogen-activated protein kinase (MAPK) pathway activation through mutation of the BRAF and KRAS oncogenes leads to uncontrolled cell proliferation. The most distinct molecular alteration associated with the serrated neoplasia pathway is a mutation in the BRAF proto-oncogene [31]. Moreover, aberrant hypermethylation of CpG islands in the promoter region of a gene can result in its silencing. In practice, the CIMP status of a given lesion is determined by the assessment of the promoter methylation status of a panel 1 of five genes, in which hypermethylation of at least three genes is considered to be CIMP high and methylation of one or two genes is considered CIMP low. CIMP-high tumors have been strongly associated with the serrated neoplasia pathway [32, 33]. An example of a tumor suppressor gene that is usually silenced in CIMP tumors is the mismatch repair gene MLH1. The silencing of this gene results in sporadic microsatellite instability, comparable to hereditary microsatellite instability in patients with Lynch syndrome. For this reason, the serrated neoplasia pathway is often referred to as the sporadic microsatellite instability pathway. However, not all CIMP tumors develop microsatellite instability. The silencing of other tumor suppressor genes, such as p16INK4a, IGFBP7, and MGMT, might also have a prominent role in the development of CIMP-high microsatellite stable tumors.
Serrated pathway of colorectal carcinogenesis is represented in Fig. 15.2. Oncogenic BRAF (V600E) mutation seems to be the earliest event in the “classical serrated pathway” that proposes a progression from MVHP → SSA/P → SSA/P with cytological dysplasia → CRC. This sequence occurs most commonly in the proximal colon, leading to tumors that show CIMP high.
An alternative serrated pathway has been described, characterized by KRAS instead of BRAF mutation as earliest event, CIMP low, and progression to TSA from GCHP. However, this alternate pathway remains poorly understood [34].
Despite the association between SSA/Ps and serrated adenocarcinomas, up to 50% of patients with SPS develop CRC at the rectosigmoid or left colon, and BRAF mutation is observed only in 33% of CRC [35]. These data suggest that, in the setting of SPS, a considerable proportion of CRCs may arise from an adenoma rather than serrated polyps. Further prospective studies are needed to clarify the relationship between histopathology and CRC development in SPS [22, 23].
2.2 Pathogenesis
Given that most patients with SPS are diagnosed in the 50s, with no family history of polyposis, and a strong association with environmental factors (i.e., smoking) [21, 23], it has been suggested that, overall, SPS is not an inherited genetic syndrome and rather behaves as a complex disorder where disease appears as a consequence of the interaction of genetic susceptibility and environment.
Nevertheless, several lines of evidence support that a small proportion of SPS could be the phenotypic expression of an inherited genetic syndrome: first-degree relatives of patients with SPS appear to have an increased risk for both CRC and SPS [26, 36]. Also, the multiplicity of lesions and the unrelenting and sometimes rapid development of colorectal neoplasia in affected individuals suggest that, for a minority of cases, a genetic basis is yet to be discovered. Both autosomal and recessive patterns of inheritance have been described. Biallelic MUTYH mutations have been reported in some patients fulfilling the WHO criteria of SPS usually in the context of a concomitant attenuated form of adenomatous polyposis [37]. Additionally, studies in individual families have reported linkage to loci on chromosomes 1p and 2q [37]. Despite of these findings, the genetic basis of SPS remains largely unknown.
3 RNF43-Associated Serrated Polyposis
BRAF or KRAS mutations that are associated with serrated polyps are alone insufficient to induce intestinal tumorigenesis. After a short period of hyperproliferation , crypt cells undergo growth arrest due to metabolic and replicative stress, a process termed oncogene-induced senescence . Recently, a whole-exome sequencing study of 20 unrelated subjects with multiple SSA/Ps identified mutations in several putative genes (ATM, PIF1, TELO2, XAF1, RBL1, and RNF43) functionally related to oncogene-induced senescence [37].
RNF43 is an E3 ubiquitin ligase expressed in colon stem cells that acts as a Wnt inhibitor by targeting Wnt receptors for degradation. It regulates Wnt signal strength through the R-spondin/LGR5/RNF43 module, with its effect antagonized by the Wnt amplifier R-spondin [38, 39]. In the mentioned study, two patients shared the same germline nonsense mutation in RNF43 (p.R113X), indicating that it is also associated with multiple serrated polyps (odds ratio, 460; 95% confidence interval, 23.1–16.384; p = 6.8 × 10−5).
Another study reported a family with two siblings carrying germline nonsense RNF43 mutation (p.R132X) and numerous serrated polyps at a young age, one of whom developed a CRC with microsatellite instability (MSI) [40].
More recently, Yan et al. [41] reported the results from a combination of whole-exome sequencing and target gene Sanger sequencing to study SPS families, sporadic SPs, and CRCs. In one out four SPS families, exome sequencing identified a germline likely pathogenic mutation in RNF43 (c.953-1G > A; c.953_954delAG; p.E318fs). This mutation was detected in two siblings who fulfilled WHO criteria I and/or III for SPS and also in a third sibling with one SP proximal to the sigmoid (criterion II of WHO) and a rectal cancer diagnosed at 49 years old. Several SPs at right colon were also detected during screening colonoscopy in the two children of this last case (both confirmed gene carriers). One gene carrier could not be screened, and the other four family members that did not carry the germline mutation had normal colonoscopies. In addition, RNF43 second hit by loss of heterozygosity or somatic mutation was observed in all serrated polyps (n = 16), adenomas (n = 5), and CRCs (n = 1) arising from germline RNF43 mutation carriers. Concurrently, somatic RNF43 mutations were identified in 34% of sporadic SSAs/TSAs, but 0% of HPs. Another recent study also reported frequent RNF43 somatic mutations in SPs [42].
The results reported by Yan et al. suggest that germline RNF43 mutations are responsible for a subgroup of SPS patients, and that should become part of the routine germline testing in SPS patients.
4 Unexplained Serrated Polyposis
After Yan et al. publication, the results of two genetic screens of a large cohort of individuals with SPS have been published [43]. The 295 individuals of these cohorts were recruited from the Genetics of Colorectal Polyposis Study. The first screen comprised 74 individuals with SPS selected based on early age at diagnosis, high numbers of SPs throughout the colon, and having a first-degree relative with SPS or CRC. By performing whole-exome or whole-genome sequencing , no pathogenic variants were identified; however, two uncommon non-synonymous variants predicted to be damaging were detected in a single carrier each (RNF43 NM_017763; exon6, c.C640G; p.L214 V and exon4, c.C443G; p.A148G). A second targeted genetic screen was performed specifically testing for the RNF43 p.R113X and p.R132X variants to determine their prevalence in individuals with SPS (n = 221). None of the tested individuals with SPS were carriers of either of these two RNF43 germline pathogenic variants.
The scarcity of RNF43 germline pathogenic variants in these 295 patients with SPS indicates that mutations in RNF43 may account for only a small proportion of SPS suggesting that additional genetic risk factors for SPS are yet to be identified. Given these results, it is likely that the underlying genetic cause of SPS is genetically complex and heterogeneous.
4.1 Future Directions
Serrated polyposis syndrome is an emerging disease associated with an increased CRC risk. Although a great body of evidence has emerged in the last decade, many challenges remain ahead:
-
Reassessment of the WHO definition criteria. Based on current knowledge, some aspects of the current WHO guidelines for the diagnosis of SPS could be challenged. Current diagnostic criteria exclude lesions based on their location in the rectosigmoid. This seems mainly due to the fact that diminutive HPs in the rectosigmoid should probably not be taken into account for the diagnosis of SPS. However, up to 50% of CRC in SPS occur in this location. Accordingly, it would seem reasonable to reassess the WHO criteria and not exclude lesions purely on their location without taking into account their size and histopathology. Also, as mentioned above, WHO criterion II should be probably removed from the definition of SPS. These adjustments to the current WHO guidelines could help in defining those patients who are at risk of developing CRC.
-
CRC risk stratification. Which patients benefit most from surveillance colonoscopy or prophylactic surgery? Future studies should mainly focus on the safety and feasibility of personalized treatment and surveillance for patients with SPS according to CRC risk factors in order to decrease the colonoscopy burden as well as the incidence of colonoscopy interval CRCs.
-
Improving colonoscopy diagnosis. The detection rate of serrated polyps is widely variable among endoscopists [44].The role of ancillary techniques (narrow band imaging, chromoendoscopy) needs to be defined in SPS.
-
Unraveling the genetic cause of SPS. Although it is likely that the majority of the highly penetrant familial CRC genes have been already discovered, it is likely that SPS displays genetic heterogeneity and new candidate genes wait to be discovered. Moreover, the role of low- or moderate-penetrance genes that interact with other genetic variants and/or environmental factors (i.e., smoking) will need to be clarified.
References
Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–81. https://doi.org/10.1056/NEJM199312303292701.
Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36(6):2251–70.
Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138(6):2088–100. S0016-5085(10)00172-1 [pii]. https://doi.org/10.1053/j.gastro.2009.12.066.
Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42(1):1–10. S0046-8177(10)00206-6 [pii]. https://doi.org/10.1016/j.humpath.2010.06.002.
Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107(9):1315–29; quiz 4, 30. doi:ajg2012161 [pii]. https://doi.org/10.1038/ajg.2012.161.
Oono Y, Fu K, Nakamura H, Iriguchi Y, Yamamura A, Tomino Y, et al. Progression of a sessile serrated adenoma to an early invasive cancer within 8 months. Dig Dis Sci. 2009;54(4):906–9. https://doi.org/10.1007/s10620-008-0407-7.
Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63(8):681–6. jcp.2010.075507 [pii]. https://doi.org/10.1136/jcp.2010.075507.
IJspeert J, Bevan R, Senore C, Kaminski MF, Kuipers EJ, Mroz A, et al. Detection rate of serrated polyps and serrated polyposis syndrome in colorectal cancer screening cohorts: a European overview. Gut. 2016. gutjnl-2015-310784 [pii]. https://doi.org/10.1136/gutjnl-2015-310784.
Lockett MJ, Atkin WS. Hyperplastic polyposis: prevalence and cancer risk. Gut. 2001;48(Supl 1):A4.
Hyman NH, Anderson P, Blasyk H. Hyperplastic polyposis and the risk of colorectal cancer. Dis Colon Rectum. 2004;47(12):2101–4. https://doi.org/10.1007/s10350-004-0709-6.
Boparai KS, Mathus-Vliegen EM, Koornstra JJ, Nagengast FM, van Leerdam M, van Noesel CJ, et al. Increased colorectal cancer risk during follow-up in patients with hyperplastic polyposis syndrome: a multicentre cohort study. Gut. 2010;59(8):1094–100. gut.2009.185884 [pii]. https://doi.org/10.1136/gut.2009.185884.
Hazewinkel Y, Tytgat KM, van Eeden S, Bastiaansen B, Tanis PJ, Boparai KS, et al. Incidence of colonic neoplasia in patients with serrated polyposis syndrome who undergo annual endoscopic surveillance. Gastroenterology. 2014;147(1):88–95. S0016-5085(14)00364-3 [pii]. https://doi.org/10.1053/j.gastro.2014.03.015.
Orlowska J. Hyperplastic polyposis syndrome and the risk of colorectal cancer. Gut. 2012;61(3):470–1.; author reply 1–2. gutjnl-2011-300141 [pii]. https://doi.org/10.1136/gutjnl-2011-300141.
Rubio CA, Stemme S, Jaramillo E, Lindblom A. Hyperplastic polyposis coli syndrome and colorectal carcinoma. Endoscopy. 2006;38(3):266–70. https://doi.org/10.1055/s-2006-925026.
Moreira L, Pellise M, Carballal S, Bessa X, Ocana T, Serradesanferm A, et al. High prevalence of serrated polyposis syndrome in FIT-based colorectal cancer screening programmes. Gut. 2013;62(3):476–7. gutjnl-2012-303496 [pii]. https://doi.org/10.1136/gutjnl-2012-303496.
Biswas S, Ellis AJ, Guy R, Savage H, Madronal K, East JE. High prevalence of hyperplastic polyposis syndrome (serrated polyposis) in the NHS bowel cancer screening programme. Gut. 2013;62(3):475. gutjnl-2012-303233 [pii]. https://doi.org/10.1136/gutjnl-2012-303233.
Ferrandez A, Samowitz W, DiSario JA, Burt RW. Phenotypic characteristics and risk of cancer development in hyperplastic polyposis: case series and literature review. Am J Gastroenterol. 2004;99(10):2012–8. https://doi.org/10.1111/j.1572-0241.2004.30021.x. AJG30021 [pii]
Lage P, Cravo M, Sousa R, Chaves P, Salazar M, Fonseca R, et al. Management of Portuguese patients with hyperplastic polyposis and screening of at-risk first-degree relatives: a contribution for future guidelines based on a clinical study. Am J Gastroenterol. 2004;99(9):1779–84. https://doi.org/10.1111/j.1572-0241.2004.30178.x. AJG30178 [pii]
Chow E, Lipton L, Lynch E, D'Souza R, Aragona C, Hodgkin L, et al. Hyperplastic polyposis syndrome: phenotypic presentations and the role of MBD4 and MYH. Gastroenterology. 2006;131(1):30–9. S0016-5085(06)00713-X [pii]. https://doi.org/10.1053/j.gastro.2006.03.046.
Kalady MF, Jarrar A, Leach B, LaGuardia L, O'Malley M, Eng C, et al. Defining phenotypes and cancer risk in hyperplastic polyposis syndrome. Dis Colon Rectum. 2011;54(2):164–70. https://doi.org/10.1007/DCR.0b013e3181fd4c15. 00003453-201102000-00007 [pii]
Navarro M, Gonzalez S, Iglesias S, Capella G, Rodriguez-Moranta F, Blanco I. Hyperplastic polyposis syndrome: phenotypic diversity and association to colorectal cancer. Med Clin (Barc). 2013;141(2):62–6. S0025-7753(12)00512-X [pii]. https://doi.org/10.1016/j.medcli.2012.04.024.
Carballal S, Rodriguez-Alcalde D, Moreira L, Hernandez L, Rodriguez L, Rodriguez-Moranta F, et al. Colorectal cancer risk factors in patients with serrated polyposis syndrome: a large multicentre study. Gut. 2015. gutjnl-2015-309647 [pii]. https://doi.org/10.1136/gutjnl-2015-309647.
Ijspeert J, Rana SA, Atkinson NS, van Herwaarden YJ, Bastiaansen BA, van Leerdam ME, et al. Clinical risk factors of colorectal cancer in patients with serrated polyposis syndrome: a multicentre cohort analysis. Gut. 2015. gutjnl-2015-310630 [pii]. https://doi.org/10.1136/gutjnl-2015-310630.
Buchanan DD, Sweet K, Drini M, Jenkins MA, Win AK, Gattas M, et al. Phenotypic diversity in patients with multiple serrated polyps: a genetics clinic study. Int J Color Dis. 2010;25(6):703–12. https://doi.org/10.1007/s00384-010-0907-8.
IJspeert J, Bossuyt PM, Kuipers EJ, Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Smoking status informs about the risk of advanced serrated polyps in a screening population. Endosc Int Open. 2016;4(1):E73–8. https://doi.org/10.1055/s-0034-1393361.
Boparai KS, Reitsma JB, Lemmens V, van Os TA, Mathus-Vliegen EM, Koornstra JJ, et al. Increased colorectal cancer risk in first-degree relatives of patients with hyperplastic polyposis syndrome. Gut. 2010;59(9):1222–5. gut.2009.200741 [pii]. https://doi.org/10.1136/gut.2009.200741.
Oquinena S, Guerra A, Pueyo A, Eguaras J, Montes M, Razquin S, et al. Serrated polyposis: prospective study of first-degree relatives. Eur J Gastroenterol Hepatol. 2013;25(1):28–32. https://doi.org/10.1097/MEG.0b013e3283598506.
Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–57. S0016-5085(12)00812-8 [pii]. https://doi.org/10.1053/j.gastro.2012.06.001.
Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110(2):223–62.; quiz 63. ajg2014435 [pii]. https://doi.org/10.1038/ajg.2014.435.
Stefanius K, Ylitalo L, Tuomisto A, Kuivila R, Kantola T, Sirnio P, et al. Frequent mutations of KRAS in addition to BRAF in colorectal serrated adenocarcinoma. Histopathology. 2011;58(5):679–92. https://doi.org/10.1111/j.1365-2559.2011.03821.x.
Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4(12):937–47. nrc1503 [pii]. https://doi.org/10.1038/nrc1503.
Kim KM, Lee EJ, Ha S, Kang SY, Jang KT, Park CK, et al. Molecular features of colorectal hyperplastic polyps and sessile serrated adenoma/polyps from Korea. Am J Surg Pathol. 2011;35(9):1274–86. https://doi.org/10.1097/PAS.0b013e318224cd2e.
Moran A, Ortega P, de Juan C, Fernandez-Marcelo T, Frias C, Sanchez-Pernaute A, et al. Differential colorectal carcinogenesis: molecular basis and clinical relevance. World J Gastrointest Oncol. 2010;2(3):151–8. https://doi.org/10.4251/wjgo.v2.i3.151.
Yamane L, Scapulatempo-Neto C, Reis RM, Guimaraes DP. Serrated pathway in colorectal carcinogenesis. World J Gastroenterol. 2014;20(10):2634–40. https://doi.org/10.3748/wjg.v20.i10.2634.
Rosty C, Parry S, Young JP. Serrated polyposis: an enigmatic model of colorectal cancer predisposition. Pathol Res Int. 2011;2011:157073. https://doi.org/10.4061/2011/157073.
Win AK, Walters RJ, Buchanan DD, Jenkins MA, Sweet K, Frankel WL, et al. Cancer risks for relatives of patients with serrated polyposis. Am J Gastroenterol. 2012;107(5):770–8. ajg201252 [pii]. https://doi.org/10.1038/ajg.2012.52.
Gala MK, Mizukami Y, Le LP, Moriichi K, Austin T, Yamamoto M, et al. Germline mutations in oncogene-induced senescence pathways are associated with multiple sessile serrated adenomas. Gastroenterology. 2014;146(2):520–9. S0016-5085(13)01519-9 [pii]. https://doi.org/10.1053/j.gastro.2013.10.045.
de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28(4):305–16. 28/4/305 [pii]. https://doi.org/10.1101/gad.235473.113.
Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–9. nature11308 [pii]. https://doi.org/10.1038/nature11308.
Taupin D, Lam W, Rangiah D, McCallum L, Whittle B, Zhang Y, et al. A deleterious RNF43 germline mutation in a severely affected serrated polyposis kindred. Hum Genome Var. 2015;2:15013. https://doi.org/10.1038/hgv.2015.13.
Yan HH, Lai JC, Ho SL, Leung WK, Law WL, Lee JF, et al. RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut. 2016. gutjnl-2016-311849 [pii]. https://doi.org/10.1136/gutjnl-2016-311849.
Sekine S, Yamashita S, Tanabe T, Hashimoto T, Yoshida H, Taniguchi H, et al. Frequent PTPRK-RSPO3 fusions and RNF43 mutations in colorectal traditional serrated adenoma. J Pathol. 2016;239(2):133–8. https://doi.org/10.1002/path.4709.
Buchanan DD, Clendenning M, Zhuoer L, Stewart JR, Joseland S, Woodall S, et al. Lack of evidence for germline RNF43 mutations in patients with serrated polyposis syndrome from a large multinational study. Gut. 2016. gutjnl-2016-312773 [pii]. https://doi.org/10.1136/gutjnl-2016-312773.
IJspeert J, van Doorn SC, van der Brug YM, Bastiaansen BA, Fockens P, Dekker E. The proximal serrated polyp detection rate is an easy-to-measure proxy for the detection rate of clinically relevant serrated polyps. Gastrointest Endosc. 2015;82(5):870–7. S0016-5107(15)00216-3 [pii]. https://doi.org/10.1016/j.gie.2015.02.044.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Carballal, S., Balaguer, F., Castells, A. (2018). Serrated Polyposis Syndrome. In: Valle, L., Gruber, S., Capellá, G. (eds) Hereditary Colorectal Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-74259-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-74259-5_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74258-8
Online ISBN: 978-3-319-74259-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)