Abstract
Background
Cold snare polypectomy (CSP) has not undergone sufficient histopathological evaluation. This study aimed to clarify the histopathological features of CSP specimens, including resection depth and layer, as compared with endoscopic mucosal resection (EMR).
Methods
Polyps were recruited retrospectively. Sessile, semi-pedunculated, and 0-IIa polyps of ≤ 9 mm were selected by propensity score matching and classified as either a complete resection or one with an unevaluable/positive (X/+) margin. Resection depth and layer were estimated and the risk factors for an X/+ margin were evaluated.
Results
A total of 1072 polyps were enrolled. After matching, 184 polyp pairs were selected. An X/+ margin was seen in 105/184 (57%) vs. 70/184 (38%) CSP vs. EMR specimens (p < 0.001): specimen damage was 53/184 (29%) vs. 30/184 (16%) (p < 0.01) and vertical margin (VM) X/+ was 11/184 (6%) vs. 2/184 (1%) (p < 0.05). Among 193 completely resected specimens, resection depth from the muscularis mucosae in CSP vs. EMR was 76 vs. 338 µm (p < 0.001) and resection layer was the submucosa in 7/79 (9%) vs. 105/114 (92%) (p < 0.001). In multivariate analysis, CSP was a risk factor for procedure-associated VMX/+ [odds ratio (OR) 6.80, 95% confidence interval (CI) 1.33–34.69, p < 0.05]. Sessile serrated adenoma/polyp (SSA/P) was a risk factor for VMX/+ margin in CSP specimens (OR 58.36, 95% CI 7.45–456.96, p < 0.001).
Conclusions
SSA/P and colorectal cancer may not be suitable for CSP adoption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Japan, colorectal cancer is the leading cause of death by malignancy among women and the third most common cause among men [1]. Although the overall incidence and mortality of colorectal cancer have been rising since the mid 1990s [1, 2], adenomatous polyp resection has been shown to reduce both of these factors [3, 4]. For large polyps, endoscopic resection is also cost-effective than surgery [5]. Various endoscopic techniques are employed to resect colorectal polyps, including polypectomy (cold or hot, snare or forceps), endoscopic mucosal resection (EMR), and endoscopic submucosal dissection. EMR is the most widely used method for polyp removal in Japan. Cold snare polypectomy (CSP), which uses snare resection without submucosal fluid injection or electrocautery [6], has been reported as safe and effective for the removal of small colorectal polyps and is being increasingly adopted for growths of < 10 mm in diameter [7, 8]. The histopathological features of CSP specimens have not, however, been sufficiently evaluated.
In our clinical experience, the frequency of unevaluable or positive margins among CSP specimens appears to be greater than that in samples obtained by EMR. We therefore hypothesized that resection depth in CSP was less than that in EMR. This study clarified histopathological features of CSP specimens as compared with EMR specimens matched by propensity scores to estimate resection layer and depth.
Methods
Selection of participants and polyps
This study was conducted at the Tateiwa Clinic and Shinshu University Hospital in Nagano Prefecture, Japan, between September 2014 and October 2016. Colorectal polyps from consecutive patients were removed by either CSP or EMR. Patients < 20 years of age or with a history of inflammatory bowel disease or polyposis syndrome were excluded. Polyps with a diameter of ≤ 9 mm were included in the study if they were sessile (Is), semi-pedunculated (Isp), or 0–IIa. Polyps were excluded if they were pedunculated (Ip), depressed, ≥ 10 mm in diameter, removed by piecemeal resection, contained a non-epithelial neoplastic lesion on histopathological examination, or were missing endoscopic images.
CSP and EMR procedures
All procedures were performed among 23 endoscopists, consisting of 10 colonoscopy experts (≥ 5000 colonoscopies performed) and 13 non-experts (≥ 1000 colonoscopies performed). A 10-mm Captivator II (Boston Scientific, Marlborough, MA, USA) polypectomy snare was used for CSP, which was performed by ensnaring 1–2 mm of normal mucosa surrounding the polyp under gentle suction to reduce colon wall tension. EMR was conducted by means of the injection and cut technique: normal (0.9%) saline was injected into the submucosa with a 25-gauge needle, and the lifted lesion was resected using an electrosurgical snare (SD-210U-25; Olympus Medical, Tokyo, Japan). Resected polyps were retrieved through the endoscope by suction into a trap. Those unobtainable by suction were taken using a retrieval net or tripod-type grasping forceps. The endoscopist recorded the specimen size, location, and macroscopic type. Resected polyps were later fixed, embedded in paraffin, sectioned into 4 μm slices, and stained with hematoxylin–eosin for further assessment.
Data collection
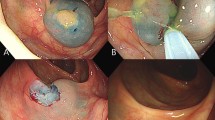
Data on patient sex, age, size, location, macroscopic type, and histopathological type of polyps, and adverse events were isolated from electronic records, endoscopy and histopathology reports. Delayed bleeding was defined as hemorrhage requiring endoscopic intervention within 2 weeks after polypectomy. The cut ends of each case were recorded as being either a complete resection or having an unevaluable/positive (X/+) margin based on histopathological examination. Complete resection was defined as an endoscopic en bloc resection of the entire lesion with histopathologically tumor-free margins horizontally (more than two directions) and vertically (Fig. 1a, b). Cases of X/+ margins were further subdivided into one of four histopathological groups: (1) destruction of the specimen due to retrieval (specimen damage) (Fig. 1c), (2) inadequate sectioning of the specimen (inadequate sectioning) (Fig. 1d, e), (3) tumor invasion or damage to the horizontal margin by a burn effect or mechanical process (HMX/+), or (4) tumor invasion or damage to the vertical margin by a burn effect or mechanical process (VMX/+). All specimens were reviewed independently by a pathologist (HO) and a gastroenterologist (AI) who were blinded to the patient details. Inter-observer variation was resolved by re-evaluation under a multihead microscope and discussion to reach a consensus in the case of a discrepancy.
Histopathological evaluation of cut ends. a Schematic of an adequately sectioned specimen. b Example of a completely resected specimen obtained by cold snare polypectomy using the approach in Fig. 1a. c Example of specimen damage of a specimen obtained by cold snare polypectomy. d Schematic of an inadequately sectioned specimen. e Example of an incompletely resected specimen obtained by endoscopic mucosal resection using the inadequate approach in Fig. 1d
Completely resected specimens were adopted to evaluate resection layer based on the presence or absence of the submucosa or muscularis mucosae and resection depth using a virtual pathology system (Nanozoomer digital pathology, Hamamatsu photonics, Hamamatsu, Japan). Resection depth was determined using the calculated areas of the submucosa and muscularis mucosae and the length of the muscularis mucosae obtained from the virtual pathology system (Fig. 2).
Formula for the calculation of resection depth and measurement location. Resection depth = (area of muscularis mucosae + submucosa)/(length of muscularis mucosae) (resection depth = 667 μm in the specimen shown). a Completely resected specimen obtained by endoscopic mucosal resection. b Highlighted section indicates the area of the muscularis mucosae + submucosa (13.0 mm2); c Superimposed line shows the length of the muscularis mucosae (19.5 mm)
Sample size calculations
The primary outcome of interest was the VMX/+ rate as assessed by the histopathological examination of CSP specimens. We hypothesized that the residual polyps after CSP were mainly caused by VMX/+ due to shallower resection depth. A previous study reported that the residual polyp rate for small polyps (6–9 mm) by CSP and EMR was 8.5 and 1.5%, respectively [9], based on additional biopsy at the CSP or EMR sites. In contrast, we presumed that VMX/+ rarely contributed to residual polyps in EMR. Although our search of the literature revealed no data for VMX/+ for polyps ≤ 9 mm resected by CSP or EMR, the above study’s value was used as the basis for our sample size estimation. At an α value of 0.05, a sample size of 179 polyps per study arm was required to confirm a higher VMX/+ rate in the CSP group than in the EMR group with 80% power.
Statistical analysis
Data were described as the median (interquartile range [IQR], range) for continuous variables and percentages for categorical variables. The Fisher’s exact, Pearson’s Chi-squared, and Mann–Whitney U tests were used as appropriate. The odds ratio (OR) and 95% confidence interval (CI) were calculated using logistic regression models to identify risk factors associated with X/+ margins. Propensity score matching was conducted to reduce bias in polyp selection [10], in which logistic regression was used to generate a propensity score using seven variables considered most likely to influence histopathological classification (age, sex, macroscopic type, location, lesion size, histopathological type, and endoscopist experience). Propensity score matching was applied at a 1:1 CSP-to-EMR ratio using the nearest-neighbor matching method without replacement using a 0.1 caliper width [11]. Statistical analyses were performed with IBM SPSS Statistics software for Windows, v23.0 (IBM Corp., Armonk, NY, USA). A p value of < 0.05 was considered statistically significant.
Ethical considerations
This study was approved by the Ethics Committee of Shinshu University School of Medicine (reference number: 3369). Written informed consent for endoscopic resection was obtained from every patient prior to the investigation. Further informed consent for the retrospective study was not necessary.
Results
Participant and polyp characteristics
A total of 1490 colorectal polyps were resected from 643 individuals who met the participant inclusion criteria. Of these, 418 (28%) specimens did not meet the polyp inclusion criteria and were excluded (Fig. 3). Of the remaining 1072 polyps from 462 participants, 373 (35%; 126 participants) were resected by CSP and 699 (65%; 408 participants) by EMR. After matching by propensity scores, 368/1072 (34%) polyps were ultimately selected for analysis [184/373 (49%) CSP, 184/699 (26%) EMR] (Fig. 3).
The overall median age was 69 (IQR: 61–75, range: 31–90) years and 346 (65%) participants were male. Among the 1072 polyps, significant differences were noted between the CSP and EMR groups for median polyp size (4 vs. 5 mm; p < 0.001) and macroscopic polyp type distribution (p < 0.001) (Table 1). No remarkable differences were detected between the groups after propensity score matching (Table 1).
Cut end histopathology
On histopathological examination, X/+ margins were found in 105/184 (57%) polyps in the CSP group and 70/184 (38%) polyps in the EMR group (p < 0.001) (Table 2). Polyps with X/+ margins in the CSP group were significantly more likely to have specimen damage or VMX/+ margins than those in the EMR group (29 vs. 16% and 6 vs. 1%; p < 0.01 and p < 0.05, respectively).
Resection depth and layer
Resection depth and layer were assessed in 193 completely resected specimens. The median resection depth from the muscularis mucosae was 76 (IQR: 44–127, range: 7–290) μm in the CSP group and 338 (IQR: 237–468, range: 49–1017) μm in the EMR group (p < 0.001). The respective median resection depths by non-experts and experts were 87 and 68 μm in the CSP group (p = 0.583) and 293 μm and 425 μm in the EMR group (p < 0.01). The resected layer was mostly the muscularis mucosae [72/79 (91%)] in the CSP group and predominantly the submucosa [105/114 (92%)] in the EMR group (p < 0.001).
Risk factors for X/+ margins
CSP was a significant risk factor for procedure-associated specimen damage and VMX/+ in CSP and EMR specimens (OR 2.02, 95% CI 1.21–3.35, p < 0.01 and OR 6.80, 95% CI 1.33–34.69, p < 0.05, respectively) (Table 3). SSA/P was found to be a significant risk factor for VMX/+ in CSP specimens (OR 58.36, 95% CI 7.45–456.96, p < 0.001) (Table 4).
Adverse events
There were no complications of perforation in either group. Delayed bleeding was observed in two cases [2/373 (1%)] in the CSP group and 19 cases [19/699 (3%)] in the EMR group (p < 0.05), all of which were managed successfully by clips. There were no polyps clipped just after CSP.
Discussion
In the present study, a higher proportion of specimens obtained by CSP had X/+ margins than did propensity score-matched polyps taken by EMR, likely due to specimen damage and VMX/+ . The median resection depth of CSP specimens was shallower than that of EMR specimens regardless of endoscopist experience, with most layers being in the muscularis mucosae. In multivariate analysis, CSP was an independent risk factor for procedure-associated VMX/+ , and SSA/P was a significant risk factor for VMX/+ in CSP specimens. To our knowledge, this is the first study to clarify the details of X/+ margins in CSP and compare resection depth with those of EMR specimens.
From the lumen outwards, the intestinal walls are composed of four layers: the mucosa (mucosal epithelium, lamina propria and muscularis mucosae), submucosa, muscularis propria and serosa/adventitia. The lamina propria and submucosa are connective tissue composed mostly of collagen fibers [12, 13]. Both the muscularis mucosae and muscularis propria are characterized by outer longitudinal and inner circular muscle layers [14]. Thus, the muscularis mucosae is supported by bidirectional collagen fibers (lamina propria and submucosa), with possible fragility between the longitudinal and circular muscle layers. As the snaring approach employed in CSP is often conducted longitudinally along the intestinal tract, there is a chance for a polyp to be physically resected from a fragile area. One study found that the submucosa was resected by CSP in only 1/59 (2%) polyps [15], and another suggested that protrusions within the cold snare defect represent incomplete mucosal layer resection [16]. Our results were consistent with these reports.
CSP is widely believed to be a safe and easy procedure for the removal of colorectal polyps [7, 8]. Recent studies have found that the complete resection rate of CSP by experts was 93.8–98.5% based on the absence rate of residual polyp tissue in additional EMR or biopsy performed at the CSP site [9, 15, 17], i.e., the residual tissue rate after CSP was several percent. CSP is indeed a clinically useful procedure, but we believe that its adoption will be controversial. The most remarkable results were a higher proportion of VMX/+ in CSP specimens than in EMR specimens (Table 2). Furthermore, SSA/P was an independent risk factor for VMX/+ in CSP specimens (Table 4). Immediately after CSP, it is important to sufficiently assess endoscopic resection sites for the presence of a residual polyp, although bleeding renders it difficult to evaluate for residual polyps of VM endoscopically. Since CSP was found to be a significant risk factor for procedure-associated VMX/+ in multivariate analysis (Table 3), it will be important to evaluate histopathological VM in CSP specimens.
Earlier studies have indicated a higher proportion of HMX/+ in SSA/P in specimens obtained by EMR than TA [18], although VM was not noted. A proportion of VMX/+ in EMR specimens might have been due to the resection layer including the submucosa. SSA/P was an independent risk factor for VMX/+ in CSP specimens in our study for two possible reasons. First, the muscularis mucosae in SSA/P specimens were thin; those resected by EMR were significantly thinner than TA specimens (Supplemental Table 1). If a fragile area between the longitudinal and circular muscle layers is removed, the resection depth in CSP seems to become shallower in SSA/P. Therefore, VM may be easily influenced by peristalsis and the position of the polyp. Second, SSA/P with epithelial misplacement may be related to this finding. Epithelial misplacement is a phenomenon caused by crypts located within the muscularis mucosae or submucosa of the polyp [19, 20]. The incidence of epithelial misplacement for SSA/P and hyperplastic polyps was 8.8 and 5.7%, respectively [20]. If CSP is performed for those lesions, the possibility of VMX/+ appears to be high. Thus, we believe that SSA/P cannot easily be resected by CSP.
In Japan, early colorectal cancer with an invasion depth of < 1000 μm is considered an indication for endoscopic resection due to no probability of lymph node metastasis [21]. In our results, CSP specimens could frequently not resect enough of the submucosa, and there were only a few CSP specimens for which histopathological curability could be estimated. The incidence rate of colorectal cancer was 0.46% in polyps of ≤ 5 mm in diameter and 3.3% in polyps with a diameter of 6–9 mm [22]. Submucosal invasive colorectal cancer from polyps of ≤ 10 mm is low [23]. In cases of slightly invasive colorectal cancer, additional surgical resection would be necessary if histological vertical margin evaluation in the CSP specimen was positive. As there are no clear guidelines for when the histopathological evaluation is X/+ , colorectal cancer may be not suitable for resection by CSP.
This study had several limitations. First, it was retrospective and conducted at only two institutions. We used propensity score matching analysis to minimize selection bias. Second, the study did not include a systematic evaluation of recurrence after polyp resection, so it was unknown which method minimized recurrence. Third, the selection criteria for CSP and EMR for colorectal polyps may have differed slightly among the endoscopists, which could not be clarified in this retrospective study. Although we believe that polyps of ≤ 5 mm in diameter are not suitable for EMR, many such cases were resected with polyps of > 6 mm. Polyps of ≤ 3 mm in diameter are a good indication for cold forceps polypectomy (CFP) [24]. Fourth, we were unable to examine for risk factors for HMX/+ in CSP specimens due to our limited sample size (2/182). Prospective, multicenter studies involving larger numbers of individuals are needed to further investigate this issue.
In conclusion, our findings suggest that histopathological type may be an important factor associated with VMX/+ or histopathological curability in CSP specimens. We believe that CSP may eventually become the standard treatment for non-pedunculated polyps of ≤ 9 mm, but recommend that SSA/P and colorectal cancer might be excluded from CSP adoption. Endoscopists should perform careful endoscopic diagnosis prior to resection by CSP, including magnified endoscopy and image-enhanced endoscopy.
Abbreviations
- CSP:
-
Cold snare polypectomy
- EMR:
-
Endoscopic mucosal resection
- X/+:
-
Unevaluable/positive
- VM:
-
Vertical margin
- HM:
-
Horizontal margin
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- FOBT:
-
Fecal occult blood test
- SSA/P:
-
Sessile serrated adenoma/polyp
- CFP:
-
Cold forceps polypectomy
References
Katanoda K, Hori M, Matsuda T, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn J Clin Oncol. 2015;45:390–401.
Park H-M, Woo H, Jung SJ, et al. Colorectal cancer incidence in 5 Asian countries by subsite: an analysis of cancer incidence in five continents (1998–2007). Cancer Epidemiol. 2016;45:65–70.
Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–81.
Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96.
Law R, Das A, Gregory D, et al. Endoscopic resection is cost-effective compared with laparoscopic resection in the management of complex colon polyps: an economic analysis. Gastrointest Endosc. 2016;83:1248–57.
Tappero G, Gaia E, De Giuli P, et al. Cold snare excision of small colorectal polyps. Gastrointest Endosc. 1992;38:310–3.
Repici A, Hassan C, Vitetta E, et al. Safety of cold polypectomy for < 10 mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44:27–31.
Horiuchi A, Nakayama Y, Kajiyama M, et al. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc. 2014;79:417–23.
Zhang Q, Gao P, Han B, et al. Polypectomy for complete endoscopic resection of small colorectal polyps. Gastrointest Endosc. 2017;87:733–40.
d’Agostino RB. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
Komuro T, Hashimoto Y. Three-dimensional structure of the rat intestinal wall (mucosa and submucosa). Arch Histol Cytol. 1990;53:1–21.
Takahashi-Iwanaga H, Fujita T. Lamina propria of intestinal mucosa as a typical reticular tissue. A scanning electron-microscopic study of the rat jejunum. Cell Tissue Res. 1985;242:57–66.
Fawcett DW. Bloom and DW Fawcett: a textbook of histology. New York: Chapman & Hall. p; 1994. p. 636–8.
Lee CK, Shim J-J, Jang JY. Cold snare polypectomy vs. cold forceps polypectomy using double-biopsy technique for removal of diminutive colorectal polyps: a prospective randomized study. Am J Gastroenterol. 2013;108:1593–600.
Tutticci N, Burgess NG, Pellise M, et al. Characterization and significance of protrusions in the mucosal defect after cold snare polypectomy. Gastrointest Endosc. 2015;82:523–8.
Kim JS, Lee B-I, Choi H, et al. Cold snare polypectomy versus cold forceps polypectomy for diminutive and small colorectal polyps: a randomized controlled trial. Gastrointest Endosc. 2015;81:741–7.
Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy—results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144(74–80):e1.
Sobin LH. Inverted hyperplastic polyps of the colon. Am J Surg Pathol. 1985;9:265–72.
Kawasaki K, Kurahara K, Oshiro Y, et al. Clinicopathologic features of inverted serrated lesions of the large bowel. Digestion. 2016;93:280–7.
Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1–29.
Sakamoto T, Matsuda T, Nakajima T, et al. Clinicopathological features of colorectal polyps: evaluation of the ‘predict, resect and discard’strategies. Colorectal Dis. 2013;15:e295–300.
Oka S, Tanaka S, Nakadoi K, et al. Endoscopic features and management of diminutive colorectal submucosal invasive carcinoma. Dig Endosc. 2014;26:78–83.
Uraoka T, Ramberan H, Matsuda T, et al. Cold polypectomy techniques for diminutive polyps in the colorectum. Dig Endosc. 2014;26:98–103.
Acknowledgements
The manuscript was edited for clarity, consistency, and English language usage by Aaron S Karat and Trevor Ralph.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ito, A., Suga, T., Ota, H. et al. Resection depth and layer of cold snare polypectomy versus endoscopic mucosal resection. J Gastroenterol 53, 1171–1178 (2018). https://doi.org/10.1007/s00535-018-1446-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-1446-2