Abstract
Background
Lipopolysaccharide (LPS) induces visceral hypersensitivity, and corticotropin-releasing factor (CRF) also modulates visceral sensation. Besides, LPS increases CRF immunoreactivity in rat colon, which raises the possibility of the existence of a link between LPS and the CRF system in modulating visceral sensation. The present study tried to clarify this possibility.
Methods
Visceral sensation was assessed by abdominal muscle contractions induced by colonic balloon distention, i.e., visceromotor response, electrophysiologically in conscious rats. The threshold of visceromotor response was measured before and after administration of drugs.
Results
LPS at a dose of 1 mg/kg subcutaneously (sc) decreased the threshold at 3 h after the administration. Intraperitoneal (ip) administration of anakinra (20 mg/kg), an interleukin-1 (IL-1) receptor antagonist, or interleukin-6 (IL-6) antibody (16.6 µg/kg) blocked this effect. Additionally, IL-1β (10 µg/kg, sc) or IL-6 (10 µg/kg, sc) induced visceral allodynia. Astressin (200 µg/kg, ip), a non-selective CRF receptor antagonist, abolished the effect of LPS, but astressin2-B (200 µg/kg, ip), a CRF receptor type 2 (CRF2) antagonist, did not alter it. Peripheral CRF receptor type 1 (CRF1) stimulation by cortagine (60 µg/kg, ip) exaggerated the effect of LPS, but activation of CRF2 by urocortin 2 (60 µg/kg, ip) abolished it.
Conclusions
LPS induced visceral allodynia possibly through stimulating IL-1 and IL-6 release. In addition, this effect was mediated through peripheral CRF signaling. Since the LPS–cytokine system is thought to contribute to altered visceral sensation in the patients with irritable bowel syndrome, these results may further suggest that CRF plays a crucial role in the pathophysiology of this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress alters gastrointestinal (GI) motility and visceral sensation, and corticotropin-releasing factor (CRF) is involved in these changes [1]. The effects of CRF are mediated through the activation of two receptors, CRF receptor type 1 (CRF1) and type 2 (CRF2) [2, 3]. In addition to CRF, CRF-related peptides, urocotins (Ucns; Ucn1, Ucn2, and Ucn3) bind to CRF receptors, which also mediate visceral stress responses [4, 5].
Irritable bowel syndrome (IBS) is one of the functional GI disorders characterized by the presence of recurrent or chronic abdominal pain or discomfort with altered bowel habits without any organic cause [6]. The pathophysiology remains incompletely understood but disturbed gut motility and altered visceral sensory function play an important role [1]. Since these altered GI functions are reproduced by exogenous CRF or Ucns, CRF signaling is thought to be a key factor in the pathophysiology of IBS [1, 7].
Besides, growing evidence supports that peripheral immune mechanisms could also contribute to the pathophysiology of IBS [8, 9]. Some IBS patients display low-grade gut mucosal inflammation with activated mast cells, enhanced expression of proinflammatory cytokines, and increased gut permeability; this results in abnormal neural responses and leads to altered colonic functions [1, 10]. Incidentally, peripheral administration of lipopolysaccharide (LPS), which is component of gram-negative bacterial cell walls, reproduces these responses [11], and serum LPS is increased in diarrhea-predominant IBS (IBS-D) [12]. Moreover, LPS induces visceral hypersensitivity in humans and rats which is possibly mediated through interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6 [13, 14]. Thus the LPS–cytokine system may be involved in the visceral hypersensitivity observed in IBS.
LPS is also known to increase CRF and Ucns messenger RNA (mRNA) in the rat colon [15, 16]; therefore, there may be a link between LPS–cytokines and CRF systems in modulating visceral sensation.
In the present study, we tried to clarify the mechanisms of LPS-induced visceral hypersensitivity and determine the role of cytokines and peripheral CRF signaling in rats.
Materials and methods
Animals
Experiments were conducted in adult male Sprague–Dawley rats (Charles River Laboratory, Atsugi, Japan) weighing about 300 g. Rats were group housed, 3–4 rats/cage under controlled light/dark conditions (lights on 7 a.m.–7 p.m.) with food (solid rat chow, Oriental Yeast, Tokyo, Japan) and water available ad libitum in a temperature-regulated room (23–25 °C).
Chemicals
LPS obtained from Escherichia coli with the serotype 055:B5, human Ucn2 (Sigma-Aldrich, St. Louis, MO, USA), anakinra (Swedish Orphan Biovitrum, Stockholm, Sweden), IL-1β and IL-6 (Wako Pure Chemical Industries, Osaka, Japan) were dissolved in normal saline. Astressin, astressin2-B (Sigma-Aldrich), and cortagine (PolyPeptide Laboratories, Torrance, CA, USA) were dissolved in double-distilled water. Goat anti-rat IL-6 neutralizing antibody and normal goat IgG (R&D Systems, Minneapolis, MN, USA) were dissolved in sterile phosphate buffered saline. The doses of the chemicals were determined according to the previous reports [13, 17–22]. The volume of injection was 0.2 ml/rat.
Measurement of visceral sensation
Visceral sensation was assessed by abdominal muscle contractions in response to colonic distention (visceromotor response, VMR) using electromyography (EMG) in conscious rats, which was validated as a quantitative measure of visceral nociception [23].
Implantation of electrodes and placement of colonic distention balloon
Under brief ether anesthesia, a small skin incision about 5 mm in length was made in non-fasted rats, and electrodes (Teflon-coated stainless steel, 0.05 mm diameter, MT Giken, Tokyo, Japan) for EMG were inserted approximately 2 mm into left side external oblique musculature through the incision. They were fixed to musculature by cyanoacrylate instant adhesive (Aron Alpha, Toagosei, Tokyo, Japan) together with the incised skin. The electrode leads were externalized directly through this closed incision and threaded through a urethane tube. Next, a distension balloon (6-Fr disposable silicon balloon-urethral catheter, JU-SB0601, Terumo Corporation, Tokyo, Japan) was inserted intra-anally with the distal end positioned 2 cm proximal to the anus. The maximal inflation volume for the balloon was 1.5 ml, and the length of the maximally inflated balloon was 1.2 cm.
Colonic distention and monitoring abdominal muscle contractions
After completing the surgery for the implantation of electrodes and balloon placement, we put the rats in Bollmann cages and allowed them to recover from the anesthesia and adjust to the experimental conditions for 30 min before testing. The animals were trained to the experimental conditions by placing them singly in Bollmann cages for 1 h before the day of experiment. Then electrode leads were connected to an EMG amplifier, and EMG signals were amplified, filtered (3000 Hz), digitized by a PowerLab system (AD Instruments, Colorado Springs, CO, USA), and stored by computer software (LabChart 7, AD Instruments). Colonic distension was performed according to the previous publications [24, 25], namely, the ascending method of limits phasic distension was applied in increments of 0.1 ml for 5 s by manually inflating the balloon with water using a syringe until significant abdominal muscle contractions, i.e., VMR, were induced. The threshold of VMR was defined as the distended balloon volume (ml) inducing VMR. Tang et al. [26] previously demonstrated that the pain threshold induced by colorectal distention (CRD) could be determined as distended balloon volume in rats using a balloon quite similar to ours and also reported that intracolonic pressure was linearly associated with intraballoon volume in the experiments. The threshold was assessed two times (2 min interval) and the mean of the threshold was calculated.
Experimental procedures
First, the basal level of threshold of VMR was determined, and the electrodes and distention balloon were removed. Later, LPS (1 mg/kg) or vehicle was injected subcutaneously (sc) and the rats were returned to the home cages. At 2.5 h after the injection, they underwent the surgery for the electrodes implantation and balloon placement under ether anesthesia and were then put in the Bollmann cages. After 30 min, i.e., at 3 h after the injection, the threshold was determined. This protocol followed the previous study demonstrating that LPS (1 mg/kg) induced visceral allodynia at 3 h after injection in rats [13]. In addition, the thresholds were also determined before and at 3 h after the injection of IL-1β or IL-6.
Next, in order to determine the effects of drugs on LPS-induced allodynia, the drugs were administered 10 min prior to the LPS injection, and the threshold was determined at 3 h later.
Statistical analysis
Data were expressed as means ± standard error. Multiple comparison was performed by one-way analysis of variance (ANOVA) followed by Fisher’s least-significant-difference test. Comparison between two groups was performed using the Student’s t or paired t test. SYSTAT 13 software (Systat Software, Chicago, IL, USA) was used throughout the study.
Ethical considerations
Approval by the Research and Development and Animal Care Committees at Asahikawa Medical University (#15132, approved on April 1, 2015) was obtained for all studies.
Results
Figure 1a depicts an example electromyogram. The threshold of VMR (ml), i.e., the distended balloon volume inducing significant abdominal muscle contractions, was determined.
a Threshold of visceromotor response (VMR) was determined by the distended balloon volume (ml) inducing apparent sustained abdominal muscle contractions. An example EMG recording is depicted. The threshold of VMR was 0.5 ml in this animal. b LPS (1 mg/kg, subcutaneously) significantly reduced the threshold (ml) from 0.56 ± 0.02 to 0.40 ± 0.03 (p < 0.05), but vehicle did not alter it (basal 0.54 ± 0.04 versus after 0.56 ± 0.05, p > 0.05). ns represents no significant difference. *p < 0.05 (paired t test). c % change threshold was significantly reduced in LPS-treated group (104.4 ± 8.2 for vehicle versus 72.6 ± 6.1 for LPS, p < 0.05). Each column represents the mean ± standard error. Number of rats examined is shown in the parenthesis. *p < 0.05 versus vehicle group (Student’s t test)
LPS (1 mg/kg, sc) significantly reduced the threshold at 3 h after the injection but it was not changed in vehicle-treated rats (Fig. 1b). The % change threshold, i.e., the value of baseline threshold divided by the threshold after drugs and then multiplied by 100 %, was reduced in LPS-treated group as compared to controls (Fig. 1c).
Since LPS was definitely demonstrated to reduce the threshold as described above, the % change threshold was used to assess the effect of drugs on the LPS response.
First, we tested the effect of anakinra, a recombinant human IL-1 receptor antagonist, on LPS-induced visceral allodynia (Fig. 2a). Intraperitoneal (ip) administration of vehicle or anakinra (20 mg/kg) per se did not change the threshold, but the antagonist, 10 min prior to LPS, abolished the LPS-induced allodynia.
Effect of intraperitoneal anakinra or IL-6 antibody on LPS-induced visceral allodynia. a Anakinra (20 mg/kg) itself did not influence the threshold (% change 101.4 ± 8.7 for vehicle + vehicle versus 99.5 ± 5.0 for anakinra + vehicle, p > 0.05) but it abolished the response induced by LPS (77.5 ± 4.0 for vehicle + LPS versus 101.4 ± 5.1 for anakinra + LPS, p < 0.05). b Similarly, IL-6 antibody (16.6 µg/kg) attenuated the response induced by LPS (% change 63.0 ± 3.8 for IgG + LPS versus 89.1 ± 10.0 for IL-6 antibody + LPS, p < 0.05) without modifying the basal threshold (101.5 ± 4.7 for IgG + vehicle versus 100.3 ± 4.7 for IL-6 antibody + vehicle, p > 0.05). Each column represents the mean ± standard error. Number of rats examined is shown in the parenthesis. *p < 0.05 versus vehicle + vehicle or IgG + vehicle group, # p < 0.05 versus vehicle + LPS or IgG + LPS group (one-way ANOVA followed by Fisher’s least-significant-difference test)
Next, in order to clarify the role of IL-6 in the LPS-induced response, vehicle (IgG at a dose of 10 µg/kg) or IL-6 antibody (16.6 µg/kg) was administered ip, 10 min prior to LPS (Fig. 2b). Vehicle or IL-6 antibody per se did not alter the threshold. On the other hand, IL-6 antibody significantly attenuated the LPS-induced allodynia.
Later, in order to determine the role of peripheral CRF signaling, astressin, a non-selective CRF receptor antagonist, astressin2-B, a selective CRF2 antagonist, or vehicle was administered ip at 10 min before LPS or vehicle injection. Astressin (200 µg/kg) per se did not alter the visceral sensation, while the antagonist at both 50 and 200 µg/kg significantly attenuated the LPS-induced allodynia in a dose-responsive manner (Fig. 3a). Meanwhile, astressin2-B (200 µg/kg) neither changed the basal nor the reduced threshold induced by LPS (Fig. 3b).
Effects of intraperitoneal CRF receptor antagonists on LPS-induced visceral allodynia. a Astressin at a dose of 200 µg/kg per se did not alter the threshold (% change 95.0 ± 3.3 for vehicle + vehicle versus 101.7 ± 6.7 for astressin at 200 µg/kg + vehicle, p > 0.05) but the antagonist (50 and 200 µg/kg) inhibited the response induced by LPS in a dose-responsive manner (62.6 ± 3.7 for vehicle + LPS versus 90.8 ± 8.4 for astressin at 50 µg/kg + LPS, or 117.5 ± 13.3 for astressin at 200 µg/kg + LPS, p < 0.05, astressin at 50 µg/kg + LPS versus astressin at 200 µg/kg + LPS, p < 0.05). Ast astressin. b Astressin2-B (200 µg/kg) neither modified basal threshold (% change 97.0 ± 3.2 for vehicle + vehicle versus 107.5 ± 7.5 for astressin2-B + vehicle, p > 0.05) nor LPS-induced allodynia (70.8 ± 5.4 for vehicle + LPS versus 67.9 ± 3.2 for astressin2-B + LPS, p > 0.05). Ast 2 -B astressin2-B. Each column represents the mean ± standard error. Number of rats examined is shown in the parenthesis. *p < 0.05 versus vehicle + vehicle group, # p < 0.05 versus vehicle + LPS group (one-way ANOVA followed by Fisher’s least-significant-difference test)
Next, we determined the effects of peripheral CRF1 or CRF2 activation on LPS-induced allodynia. Cortagine, a selective CRF1 agonist, at a dose of 60 µg/kg ip did not modify the basal threshold, while the agonist injected at 10 min before LPS injection further potentiated the LPS-induced allodynia (Fig. 4a). Ucn2 (60 µg/kg, ip), a selective CRF2 agonist, did not alter the baseline threshold, but abolished the LPS-induced allodynia (Fig. 4b).
Effect of intraperitoneal CRF receptor agonists on LPS-induced visceral allodynia. a Cortagine (60 µg/kg) itself did not change the threshold (% change 103.0 ± 4.7 for vehicle + vehicle versus 106.1 ± 7.2 for cortagine + vehicle, p > 0.05) but further enhanced the LPS-induced allodynia (74.5 ± 6.6 for vehicle + LPS versus 54.0 ± 5.8 for cortagine + LPS, p < 0.05). b Urocortin 2 (60 µg/kg) itself did not modify the threshold (104.3 ± 6.0 for vehicle + vehicle versus 101.3 ± 10.3 for urocortin 2 + vehicle, p > 0.05) but abolished the response induced by LPS (77.0 ± 4.3 for vehicle + LPS versus 113.0 ± 5.4 for urocortin 2 + LPS, p < 0.05). Ucn2 urocortin 2. Each column represents the mean ± standard error. Number of rats examined is shown in the parenthesis. *p < 0.05 versus vehicle + vehicle group, # p < 0.05 versus vehicle + LPS group (one-way ANOVA followed by Fisher’s least-significant-difference test)
IL-1β at a dose of 10 µg/kg sc significantly reduced the threshold (ml) at 3 h after the injection, indicating that IL-1β induced visceral allodynia (Fig. 5a). Similarly, IL-6 (10 µg/kg sc) also reduced the threshold (Fig. 5b).
a IL-1β (10 µg/kg, subcutaneously) significantly reduced the visceromotor response (VMR) threshold (ml) from 0.52 ± 0.02 to 0.33 ± 0.03 (p < 0.05) at 3 h after the injection, while vehicle did not change it (basal 0.53 ± 0.03 versus after 0.50 ± 0.05, p > 0.05). b Similarly, IL-6 (10 µg/kg, subcutaneously) also reduced the threshold (ml) from 0.54 ± 0.03 to 0.42 ± 0.03 (p < 0.05), but vehicle did not alter it (basal 0.52 ± 0.02 versus after 0.52 ± 0.02, p > 0.05). ns represents no significant difference. *p < 0.05 (paired t test). c The effect of intraperitoneal astressin on IL-1β-induced visceral allodynia. Astressin (200 µg/kg) did not alter this effect by IL-1β (% change 101.3 ± 7.7 for vehicle + vehicle versus 61.3 ± 5.6 for vehicle + IL-1β, p < 0.05; 66.0 ± 4.1 for astressin + IL-1β versus vehicle + IL-1β, p > 0.05). *p < 0.05 versus vehicle + vehicle group (one-way ANOVA followed by Fisher’s least-significant-difference test). Each column represents the mean ± standard error. Number of rats examined is shown in the parenthesis
Then the role of peripheral CRF signaling in IL-1β-induced allodynia was determined. Astressin (200 µg/kg, ip) at 10 min prior to IL-1β did not modify the response (Fig. 5c).
Discussion
The present study provides new insights regarding the effects of LPS on visceral sensation. LPS induced visceral allodynia, which was mediated through IL-1 and IL-6 pathways. Moreover, this response induced by LPS was also modulated by peripheral CRF signaling. Activating CRF1 further enhanced the allodynia induced by LPS but it was abolished by activation of CRF2.
There have been several studies demonstrating that LPS induces visceral hypersensitivity in rats so far [13, 27], and Coelho et al. [13] demonstrated that LPS-induced visceral allodynia was mediated through IL-1β and TNF-α release. We showed that anakinra blocked the response induced by LPS and, moreover, IL-1β reproduced this response; these observations are consistent with the above evidence.
IL-1 and TNF-α receptors are located in the neurons in rat dorsal root ganglia (DRG) and these cytokines act through these receptors to activate sensory neurons [28–30]. Moreover, proinflammatory cytokines induced by LPS facilitate the release of proalgesic mediators such as prostaglandins, which are well known to be a stimulator of sensory afferents [31]. Thus IL-1β and TNF-α evoke visceral hypersensitivity through possibly directly and/or indirectly activating sensory afferents.
IL-6 is also known to be one of the major cytokines induced by LPS, and we tested the role of IL-6 in LPS-induced visceral response. IL-6 antibody (16.6 µg/kg) attenuated the LPS-induced allodynia. This dose of antibody was based on the work of Tuna et al. [20] studying blood flow in the central nervous system and the study by Toth et al. [21] determining the role of IL-6 in trauma-hemorrhagic shock-induced liver injury in rats. Moreover, the administration of IL-6 reproduced the response induced by LPS.
IL-6 binds either to a membrane-bound IL-6 receptor or a soluble IL-6 receptor, and these IL-6-receptor complexes bind to the transmembrane signal-transducing subunit gp130, thereby generating signal transduction [32]. DRG neurons express gp130 [33], and the injection of IL-6 into a normal knee causes a long-lasting sensitization of nociceptive C-fibers for mechanical stimuli applied to the joint [34]. Like this study, the role of IL-6 in somatic hypersensitivity is well demonstrated [35], but there are only a few studies regarding IL-6 and visceral pain. Buckley et al. [36] reported that monoclonal anti-IL-6 receptor antibodies administered ip attenuated visceral hyperalgesia in response to CRD in a WKY rat model of IBS. These lines of evidence together with our data suggest that IL-6 may activate DRG neurons, which may be one of the mechanisms by which LPS induces visceral hypersensitivity.
In addition, peripheral cytokines can affect neural processing of ascending visceral input by directly reaching the brain through the brain area with incomplete blood–brain barrier, leading to altering neural activity [37], which may also be involved in the LPS response.
Incidentally, since peripheral administration of LPS also increases proinflammatory cytokine mRNAs in the brain [38], the cytokines produced in the brain may also be involved. Actually, delayed visceral hypersensitivity at 12 h after LPS injection has been shown to be linked to the central release of IL-1β and/or TNF-α [27]. However, since our study determined the threshold at 3 h after LPS injection, this mechanism might not significantly contribute to our results.
The most important point of our study is that ip administration of astressin, a non-selective CRF antagonist, blocked the LPS-induced allodynia, indicating that peripheral CRF signaling mediated the LPS response. The peripheral cellular origins of the CRF and Ucns have not been determined definitely so far. However, recent studies demonstrated the expression of CRF receptors and ligands in the colon in various cells such as neuronal (enteric nervous system), enterochromaffin, epithelial, and immune cells (macrophage, mast cells, lymphocytes) in rodents and humans [39]. Therefore immune challenge by LPS may stimulate the secretion of these peptides from these cells to activate CRF signaling.
Classically, peripheral CRF1 signaling was thought to exclusively contribute to stress or CRF-induced enhanced colonic motility and visceral hypersensitivity [7]. However, recent reports including ours demonstrated that peripheral CRF2 signaling suppressed these CRF1-triggered altered colonic functions [18, 22, 40, 41]. In this context, we recently proposed the balance theory of peripheral CRF signaling as follows [18]. Colonic motor and sensation may be determined by the state of the intensity of CRF1 signaling. CRF2 signaling may inhibit the intensity of CRF1 signaling, and the activity balance of peripheral CRF1 and CRF2 signaling possibly determines the functional colonic changes.
In the present study, increased intensity of CRF1 signaling by cortagine further enhanced the allodynia induced by LPS and CRF2 activation by Ucn2 abolished it; these observations are consistent with the concept of balance theory. Additionally, Ucn2 itself did not alter the basal threshold, which may also support the adequacy of the theory, because CRF2 activation itself does not change the colonic functions because of a lack of activated CRF1 signaling which is inhibited by CRF2 [18]. These results suggest that modulation of the LPS-induced altered visceral sensation by peripheral CRF receptors may follow the balance theory.
However, we also had the conflicting result that astressin2-B, a selective CRF2 antagonist, did not alter the LPS response. Since LPS is thought to activate not only peripheral CRF1 but also CRF2 by stimulating the release of Ucns [16], the blocking CRF2 would further enhance the intensity of CRF1 signaling activated by LPS and augment the sensitization according to the balance theory. This discrepancy might be explained by the following reason. It was demonstrated that LPS decreased CRF2b mRNA in the rat colon, which is the most common functional CRF2 isoform in the periphery [42], suggesting that LPS may activate CRF1 more strongly than CRF2. In this condition, inhibiting CRF2 might not have enough power to further increase the intensity of CRF1 signaling. The signaling balance might be changed by the expression profile of CRF1 and CRF2, which is dependent on the stress sensitivity of the animals and the nature of loaded stress [43].
Our study also showed the interesting result that cortagine (60 µg/kg) per se did not alter visceral sensation. Since cortagine at this dose induces visceral hyperalgesia through activating CRF1 in rodents [18], this result is thought to be in conflict with the balance theory. However, allodynia (pain due to a stimulus that does not usually provoke pain) and hyperalgesia (increased pain from a stimulus that usually provokes pain) are different phenomena [44]. Thus the sensory neurons contributing to hyperalgesia and allodynia might be different. It is possible to think that activating peripheral CRF1 signaling induces exclusively visceral hyperalgesia but does not evoke allodynia. Another possibility is the difference of timing of cortagine injection and measuring the threshold. Cortagine-induced hyperalgesia was detected at 15 or 30 min after injection [18, 45], which was a much shorter time compared to that of the present study, i.e., 3 h and 10 min. The effect of cortagine per se in modulating visceral sensation might therefore have missed in the present study design.
Since both cytokines and peripheral CRF signaling modulated the LPS response, some interaction between two systems may be expected. We showed that IL-1β-induced allodynia was not reversed by astressin, indicating that the IL-1 system might be more downstream signaling to modulate the effect of LPS. In this context, there is a possibility that CRF signaling modulates the cytokines release induced by LPS. There are several lines of evidence supporting this possibility. Yuan et al. [16] very recently demonstrated that peripheral CRF2 signaling dampened the increased expression of proinflammatory cytokines in the rat colonic wall induced by LPS. On the other hand, CRF intensifies the response of macrophages to LPS, i.e., augmenting their synthesis of the proinflammatory cytokines through CRF1 [46]. These results may not only support the possibility but also suggest that CRF signaling may have both pro- and anti-inflammatory effects through CRF1 and CRF2, respectively, which agrees well with the balance theory.
The present study has several limitations. We did not test the effect of CRF1 antagonist because all currently available selective CRF1 antagonists have been designed to cross the blood–brain barrier [47]. Therefore the role of peripheral CRF1 cannot be clarified directly at present. Central CRF signaling is also known to be involved in visceral hypersensitivity [7], and peripheral LPS activates brain CRF neurons, and increases CRF mRNA [48]. Thus brain CRF is also thought to be involved in the LPS-induced allodynia. Moreover, we did not determine the source of cytokines which were related to the LPS-induced allodynia. Further studies are needed to clarify these issues.
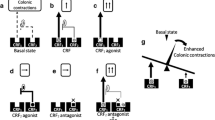
Finally, on the basis of the evidence which has been demonstrated so far, together with our present results, let us discuss the pathophysiology of IBS in terms of LPS, cytokines, and peripheral CRF (Fig. 6a). Circulatory LPS and proinflammatory cytokines are increased in IBS [12, 49]. LPS may acts on immune cells to stimulate the release of proinflammatory cytokines, which possibly activates visceral afferents, thereby inducing visceral hypersensitivity. It is also known that these released cytokines increase the colonic permeability [50] and induce bacterial translocation and mucosal inflammation [11], which further increase LPS and the cytokines. All these changes are observed in IBS patients [1, 10].
Schematic illustration of our hypothesis in terms of LPS, proinflammatory cytokines, and peripheral CRF in the visceral hypersensitivity observed in IBS. a LPS acts on immune cells to stimulate the release of proinflammatory cytokines, which possibly activates visceral afferents, thereby inducing visceral hypersensitivity. These released cytokines increase the colonic permeability [50] and induce bacterial translocation and mucosal inflammation [11], which further increases LPS and the cytokines. Incidentally, LPS activates peripheral CRF signaling [15, 16], and it facilitates or inhibits the release of proinflammatory cytokines through CRF1 or CRF2, respectively [16, 46]. Additionally, CRF per se facilitates or inhibits visceral hypersensitivity [18]. Moreover, CRF has the capability to increase gut permeability through CRF1 [1], which may also result in increased LPS and the cytokines. b CRF signaling balance may be abnormally shifted toward CRF1 in IBS [7]. Increased CRF1 signaling in IBS may enhance the cytokine response to LPS, thereby potentiating visceral hypersensitivity. Moreover, it also increases gut permeability, resulting in increased circulatory level of LPS and the cytokines. This hypothesis may explain the fact the visceral hypersensitivity and increased circulatory levels of LPS and the cytokines observed in IBS [12, 49]
Besides, LPS activates peripheral CRF signaling [15, 16], and it may modulate the immune response induced by LPS, i.e., facilitating or inhibiting the release of proinflammatory cytokines through CRF1 or CRF2, respectively [16, 46], thereby modulating visceral sensation indirectly. Additionally, CRF per se also facilitates or inhibits visceral hypersensitivity, which is not through an LPS–cytokine-related cascade [18]. Moreover, CRF has the capability to increase gut permeability through CRF1 [1], which may also result in increased LPS and the cytokines.
CRF-CRF1 signaling is thought to be a key factor in the pathophysiology of IBS, and CRF1 signaling might be increased in these patients [1, 7]. In other words, CRF signaling balance is abnormally shifted toward CRF1 in IBS according to the balance theory (Fig. 6b) [7]. Interestingly, LPS-induced stimulation of cytokine release from peripheral blood mononuclear cells is enhanced, and higher symptom severity such as urgency, diarrhea, etc. is associated with a higher cytokine response induced by LPS in IBS [51]. These lines of evidence suggest that increased CRF1 signaling in IBS may enhance the immune response to LPS, thereby potentiating visceral hypersensitivity, which may be another mechanism by which CRF contributes to the pathophysiology of IBS.
In summary, we demonstrated that LPS-induced visceral allodynia was mediated through IL-1 and IL-6 pathways. It is furthermore speculated that the activity balance of peripheral CRF1 and CRF2 signaling may contribute to the LPS-induced visceral allodynia. Since the LPS-cytokine system may be associated with the symptoms of IBS patients, CRF may contribute to the pathophysiology of IBS through modulating the effect of LPS.
References
Taché Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2009;11:270–7.
Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–86.
Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci. 1999;885:312–28.
Martínez V, Wang L, Million M, et al. Urocortins and the regulation of gastrointestinal motor function and visceral pain. Peptides. 2004;25:1733–44.
Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27.
Talley NJ, Spiller R. Irritable bowel syndrome: a little understood organic bowel disease? Lancet. 2002;360:555–64.
Nozu T, Okumura T. Corticotropin-releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome. J Gastroenterol. 2015;50:819–30.
Elsenbruch S. Abdominal pain in irritable bowel syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386–94.
Bercik P, Verdu EF, Collins SM. Is irritable bowel syndrome a low-grade inflammatory bowel disease? Gastroenterol Clin N Am. 2005;34:235–45.
Barbara G, Zecchi L, Barbaro R, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46(Suppl):S52–5.
Moriez R, Salvador-Cartier C, Theodorou V, et al. Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am J Pathol. 2005;167:1071–9.
Dlugosz A, Nowak P, D’Amato M, et al. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:1747–54.
Coelho AM, Fioramonti J, Bueno L. Systemic lipopolysaccharide influences rectal sensitivity in rats: role of mast cells, cytokines, and vagus nerve. Am J Physiol Gastrointest Liver Physiol. 2000;279:G781–90.
Benson S, Kattoor J, Wegner A, et al. Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain. 2012;153:794–9.
Yuan PQ, Wu SV, Wang L, et al. Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides. 2010;31:322–31.
Yuan PQ, Wu SV, Pothoulakis C, et al. Urocortins and CRF receptor type 2 variants in the male rat colon: gene expression and regulation by endotoxin and anti-inflammatory effect. Am J Physiol Gastrointest Liver Physiol. 2016;. doi:10.1152/ajpgi.00337.2015.
Tsuchiya Y, Nozu T, Kumei S, et al. IL-1 receptor antagonist blocks the lipopolysaccharide-induced inhibition of gastric motility in freely moving conscious rats. Dig Dis Sci. 2012;57:2555–61.
Nozu T, Takakusaki K, Okumura T. A balance theory of peripheral corticotropin-releasing factor receptor type 1 and type 2 signaling to induce colonic contractions and visceral hyperalgesia in rats. Endocrinology. 2014;155:4655–64.
Nozu T, Kumei S, Miyagishi S, et al. Colorectal distention induces acute and delayed visceral hypersensitivity: role of peripheral corticotropin-releasing factor and interleukin-1 in rats. J Gastroenterol. 2015;50:1153–61.
Tuna M, Erman T, Ildan F, et al. Effect of neutralization of rat IL-6 bioactivity on collateral blood supply from retrograde flow via cortical anastomoses in the rat central nervous system. Neurol Res. 2002;24:405–8.
Toth B, Yokoyama Y, Schwacha MG, et al. Insights into the role of interleukin-6 in the induction of hepatic injury after trauma-hemorrhagic shock. J Appl Physiol. 1985;2004(97):2184–9.
Million M, Wang L, Wang Y, et al. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–81.
Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–69.
Okumura T, Nozu T, Kumei S, et al. Antinociceptive action against colonic distension by brain orexin in conscious rats. Brain Res. 2015;1598:12–7.
Nozu T, Miyagishi S, Nozu R, et al. Water avoidance stress induces visceral hyposensitivity through peripheral corticotropin releasing factor receptor type 2 and central dopamine D2 receptor in rats. Neurogastroenterol Motil. 2015. doi:10.1111/nmo.12747.
Tang QL, Lai ML, Zhong YF, et al. Antinociceptive effect of berberine on visceral hypersensitivity in rats. World J Gastroenterol. 2013;19:4582–9.
Coelho A, Fioramonti J, Bueno L. Brain interleukin-1beta and tumor necrosis factor-alpha are involved in lipopolysaccharide-induced delayed rectal allodynia in awake rats. Brain Res Bull. 2000;52:223–8.
Obreja O, Rathee PK, Lips KS, et al. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16:1497–503.
Schafers M, Sorkin LS, Geis C, et al. Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci Lett. 2003;347:179–82.
Leo M, Argalski S, Schafers M, et al. Modulation of voltage-gated sodium channels by activation of tumor necrosis factor receptor-1 and receptor-2 in small DRG neurons of rats. Mediat Inflamm. 2015. doi:10.1155/2015/124942.
Cunha TM, Verri WA Jr, Silva JS, et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci. 2005;102:1755–60.
Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819.
von Banchet GS, Kiehl M, Schaible HG. Acute and long-term effects of IL-6 on cultured dorsal root ganglion neurones from adult rat. J Neurochem. 2005;94:238–48.
Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 2007;56:351–9.
Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther. 2014;16:470.
Buckley MM, O’Halloran KD, Rae MG, et al. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol. 2014;592:5235–50.
Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: implications for cancer and cancer treatment. Brain Behav Immun. 2003;17(Suppl 1):S125–31.
Laye S, Parnet P, Goujon E, et al. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–62.
Larauche M, Kiank C, Taché Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60(Suppl 7):33–46.
Gourcerol G, Wu SV, Yuan PQ, et al. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology. 2011;140(1586–96):e6.
Million M, Maillot C, Adelson DA, et al. Peripheral injection of sauvagine prevents repeated colorectal distension-induced visceral pain in female rats. Peptides. 2005;26:1188–95.
Yuan PQ, Wu SV, Taché Y. Urocortins and CRF type 2 receptor isoforms expression in the rat stomach are regulated by endotoxin: role in the modulation of delayed gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2012;303:G20–31.
O’malley D, Julio-Pieper M, Gibney SM, et al. Differential stress-induced alterations of colonic corticotropin-releasing factor receptors in the Wistar Kyoto rat. Neurogastroenterol Motil. 2010;22:301–11.
Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13:924–35.
Larauche M, Gourcerol G, Wang L, et al. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am J Physiol Gastrointest Liver Physiol. 2009;297:G215–27.
Agelaki S, Tsatsanis C, Gravanis A, et al. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 2002;70:6068–74.
Heinrichs SC, De Souza EB, Schulteis G, et al. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202.
Rorato R, Reis WL, de Carvalho Borges B, et al. Cannabinoid CB1 receptor restrains accentuated activity of hypothalamic corticotropin-releasing factor and brainstem tyrosine hydroxylase neurons in endotoxemia-induced hypophagia in rats. Neuropharmacology. 2012;63:154–60.
Ortiz-Lucas M, Saz-Peiro P, Sebastian-Domingo JJ. Irritable bowel syndrome immune hypothesis. Part two: the role of cytokines. Rev Esp Enferm Dig. 2010;102:711–7.
Bruewer M, Luegering A, Kucharzik T, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–72.
Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–20.
Acknowledgments
This work was supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [C-26460287 (TN) and C-26460955 (TO)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nozu, T., Miyagishi, S., Nozu, R. et al. Lipopolysaccharide induces visceral hypersensitivity: role of interleukin-1, interleukin-6, and peripheral corticotropin-releasing factor in rats. J Gastroenterol 52, 72–80 (2017). https://doi.org/10.1007/s00535-016-1208-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1208-y