Abstract
Background
Due to advances in interferon (IFN) therapy for chronic hepatitis C, most elderly patients, and even many of those with advanced hepatic fibrosis, now achieve a sustained virological response (SVR). However, carcinogenesis remains problematic in these patients. Hence, we aimed to elucidate risk factors for hepatocarcinogenesis in SVR patients and to present an appropriate follow-up protocol for improving outcomes.
Methods
We retrospectively studied 562 consecutive SVR patients for a median observation period of 4.8 years.
Results
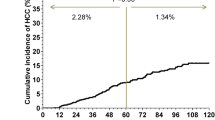
Hepatocellular carcinoma was diagnosed in 31 patients (5.5 %). Respective cumulative incidences were 3.1, 10.1, and 15.9 % at 5, 10, and 15 years after completion of IFN therapy. The proportional hazards model identified moderate or advanced fibrosis stage, advanced age, habitual alcohol consumption, and alpha-fetoprotein elevation as determinants of carcinogenesis, with hazard ratios of 10.7 (p < 0.001), 4.1 (p < 0.01), 3.9 (p < 0.01), and 2.6 (p < 0.05), respectively. Carcinoma was diagnosed in 26 % of patients more than 10 years after completion of IFN therapy. Unexpectedly, F2 fibrosis was detected in 42 % of these patients. The 5-year survival rate was 93 % in the patients who had received periodic cancer screening but only 60 % in those who had not.
Conclusion
We recommend that SVR patients be observed at 6-month intervals, at a minimum, to facilitate diagnosis at an early stage, for as long as possible after completion of therapy even if not at an advanced stage of fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis C virus (HCV) infection is the major cause of chronic liver disease worldwide. It is also a major risk factor for hepatocellular carcinoma (HCC). In Japan, about 70 % of HCC are associated with HCV infection [1].
It is well known that interferon (IFN) therapy reduces the rate of HCC development in patients with HCV infection by reducing hepatic inflammation, thereby attenuating hepatic fibrosis [2, 3]. Multivariate analysis has indicated that IFN therapy reduces the rate of HCC development in patients who show reductions in alanine aminotransferase (ALT) levels in response to IFN therapy [4]. Patients who achieved a sustained virological response (SVR) are considered to show eradication of HCV RNA and normalization of ALT levels [5]. Therefore, the incidence of HCC development is presumed to be lower in patients who achieve SVR than in those who are unresponsive to this therapy. Several studies have demonstrated that SVR patients rarely develop HCC and that they generally have favorable outcomes [4, 6–8].
On the other hand, the development of HCC in SVR patients has been reported [9–15]. Moreover, several reports have shown that HCC can develop more than 10 years after the completion of IFN therapy [16, 17]. In the last 10 years, tremendous progress has been made in the field of HCV therapy, including pegylated interferon (PEG-IFN) and ribavirin plus direct-acting antivirals or IFN-free regimens [18]. SVR was reportedly obtained with these recently developed treatments in approximately 70 % of patients infected with HCV genotype 1 [19, 20], 80–90 % of those infected with genotype 2 or 3 [21], and 50 % of those infected with genotype 4 [22]. As more patients receive treatment, it is anticipated that considerable numbers will achieve SVR. Thus, appropriate follow-up protocols to screen for HCC development in SVR patients will become increasingly important.
We investigated the incidence, risk factors, and characteristics of HCC development in SVR patients and present a follow-up protocol for improving outcomes.
Methods and materials
Patients
This was a retrospective cohort study conducted at a single center. We evaluated data from 913 consecutive patients with HCV infection who had received IFN therapy between 1991 and 2011 in the Department of Hepatology, Steel Memorial Yawata Hospital. Chronic hepatitis C (CHC) was diagnosed based on continuous positivity for antibodies to HCV and HCV RNA for more than 6 months before starting IFN therapy. Liver biopsy was performed on almost all patients, the exception being those with clinically diagnosed liver cirrhosis. The histological diagnosis was made according to the classification of Desmet et al. [23]. The screening for HCC development was performed before IFN administration and just after the completion of this therapy. Among the screenings, 562 patients achieved SVR (61.6 %), and were enrolled in this study. They were followed until the end of the analysis (December 2012). Data on the 562 patients were collected from their medical records. We excluded patients who were seropositive for hepatitis B surface antigen, or who had other liver diseases such as autoimmune hepatitis or primary biliary cirrhosis, or who had been diagnosed with HCC, or developed HCC within 12 months after the completion of IFN therapy, because these patients may already have had HCC when IFN therapy was completed. Habitual and excessive alcohol consumptions were defined as an average daily intake of at least 30 or 60 g of pure ethanol for 10 years or more, respectively. The backgrounds of our SVR patients are shown in Table 1. Informed consent was obtained from each patient enrolled in the study, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by our institution’s human research committee.
IFN treatment
IFN therapy was administered once in 490 patients, twice in 58, three times in 12, and four times in 2 patients. IFN monotherapy was administered to 213 patients (37.9 %). IFN or PEG-IFN plus ribavirin combination therapy was administered to 349 patients (62.1 %). Various types of IFN were used: IFN-α (natural or recombinant) in 124 patients (22.1 %), PEG-IFN-α in 385 (68.5 %), and IFN-β in 53 (9.4 %).
SVR was defined as negativity for HCV RNA at least 6 months after the completion of IFN therapy. HCV RNA was measured by the qualitative Amplicor or Taqman HCV assay (Roche Molecular Diagnosis, Tokyo, Japan).
Follow-up and diagnosis of HCC
We performed biochemical examinations, including measurements of alpha-fetoprotein (AFP) and/or protein induced by vitamin K absence or vitamin K antagonist-II (PIVKA-II), and imaging studies using modalities such as ultrasonography (US), computed tomography (CT), or magnetic resonance imaging (MR) every 6 months after the completion of IFN therapy. HCC was diagnosed based on typical findings on dynamic CT or MR, and/or by histology.
At the end of the study, the follow-up rate at our institution or related private hepatology clinics was 80.2 % (452 patients); 7 patients (1.2 %) had died due to non-liver related diseases, and 103 (18.3 %) had been lost to follow-up for various reasons.
Statistical analysis
Continuous variables are shown as medians (range). The cumulative incidence of HCC development was assessed by the Kaplan–Meier method. Differences between carcinogenic curves were tested using the log-rank test. Multivariate analysis of risk factors for the development of HCC was conducted using the Cox proportional hazards model. The correlation between age at the start of IFN therapy and the time elapsed between the end of IFN therapy and HCC development was evaluated using the Pearson correlation coefficient. A p value less than 0.05 was considered statistically significant. All statistical analyses were performed with JMP version 10.0 (SAS Institute, USA).
Results
Overall cumulative rate of HCC development
HCC was diagnosed in 31 of 562 SVR patients (5.5 %) during a median observation period of 4.8 years (1–20.5). The cumulative rates of HCC development were 3.1, 10.1, and 15.9 % at 5, 10, and 15 years after the completion of IFN therapy, respectively (Fig. 1). On the other hand, HCC was diagnosed in 79 of 351 non-SVR patients (22.5 %). The cumulative rates of HCC development were 15.8, 35.5, and 42.3 % at 5, 10, and 15 years after the completion of IFN therapy, respectively.
Risk factors for carcinogenesis
Next, we examined the cumulative incidences of HCC in association with different risk factors estimated by the Kaplan–Meier method and then applied the log-rank test.
Initially, we attempted to divide SVR patients into F0–2 and F3–4 groups, or into F0–3 and F4 groups, by referring to previous studies [13, 14]. However, a large number of our patients with F2 fibrosis developed HCC (Table 2). Therefore, the SVR patients were divided into the F0–1 and F2–4 groups for further analysis. The results of the analysis for fibrosis showed the incidence of HCC to be higher in the F2–4 group than in the F0–1 group (p < 0.001) (Fig. 2a). When patients were divided into 2 groups according to platelet count, which is a convenient and non-invasive index of fibrosis, the incidence of HCC development was higher in the group with platelet counts <140,000/μL than in the group with platelet counts ≥140,000/μL (p < 0.01) (Fig. 2b). Patients who were 50 years or older at the time of starting IFN therapy had a higher incidence of HCC than those starting IFN therapy prior to age 50 years (p < 0.01) (Fig. 2c). As for AFP levels at the start of IFN therapy, the incidence of HCC development was higher in the group with AFP ≥8 ng/mL than in the group with AFP <8 ng/mL (p < 0.01) (Fig. 2d).
Cumulative incidences of hepatocellular carcinoma (HCC) according to liver fibrosis, platelet (PLT) counts, age, and alpha-fetoprotein (AFP) levels. a The incidence of HCC was higher in the F2–4 group than in the F0–1 group (p < 0.001). b The incidence of HCC development was higher in the group with PLT counts <140,000/μL than in the group with PLT counts ≥140,000/μL (p < 0.01). c Patients who were 50 years or older at the time of starting IFN therapy had a higher incidence of HCC than those starting IFN therapy prior to age 50 years (p < 0.01). d The incidence of HCC development was higher in the group with AFP ≥8 ng/mL than in the group with AFP <8 ng/mL (p < 0.01)
Patients with habitual alcohol consumption had a higher incidence of HCC (p < 0.01) (Fig. 3a). The incidence of HCC development was higher in patients with diabetes than in those without (p < 0.01) (Fig. 3b). The cumulative incidence of HCC was higher in the group with γglutamyl transpeptidase (γGTP) ≥40 IU/L than in the group with γGTP <40 IU/L, although the difference did not reach statistical significance (p = 0.056). There were no significant differences in the incidence of HCC development according to gender, ALT level at the start of IFN therapy, body mass index, HCV genotype, the duration of IFN therapy, the type of IFN, or the concomitant use of ribavirin with IFN.
Cumulative incidences of hepatocellular carcinoma (HCC) according to habitual alcohol consumption and diabetes mellitus (DM). a Patients with habitual alcohol consumption had a higher incidence of HCC (p < 0.01). b The incidence of HCC development was higher in patients with diabetes than in those without (p < 0.01).
Next, we performed multivariate analysis using Cox’s proportional hazards model. The independent variables entered in the analysis included gender, which was previously reported to be a risk factor for HCC development, and γGTP at the start of IFN therapy, which did not quite reach statistical significance in the univariate analysis, as well as factors that exhibited significant differences in the univariate analysis using the log-rank test. The latter included age, fibrosis, habitual alcohol consumption, AFP level at the start of IFN therapy, and diabetes. The platelet count was excluded from this analysis because of the possible strong interaction with fibrosis. The multivariate analysis showed progression of liver fibrosis, advanced age, habitual alcohol consumption, and AFP elevation at the start of IFN therapy to be significant risk factors for HCC development. The estimated hazard ratio for HCC development in the F2–4 relative to the F0–1 group was 10.7 (p < 0.001). The ratio in patients ≥50 years of age relative to that of those <50 years of age was 4.1 (p < 0.01); the ratio in patients with habitual alcohol consumption (ethanol consumption ≥30 g/day) relative to those who did not consume alcohol habitually was 3.9 (p < 0.01), and the ratio of patients with AFP levels at the start of IFN therapy ≥8 ng/mL relative to those with levels <8 ng/mL was 2.6 (p < 0.05) (Table 3).
A significant inverse correlation was observed between age at the start of IFN therapy and the time elapsed from the completion of IFN therapy until HCC development (correlation coefficient = −0.43, p < 0.05) (Fig. 4).
Clinical characteristics of 31 patients who developed HCC after SVR
The patient population consisted of 24 men and 7 women. The median age at the start of IFN therapy was 58 (range 38–75) years, and the median age at the time of HCC development was 66 (range 54–80) years. The median time elapsed from the completion of IFN therapy until HCC development was 7.4 (range 1–17.8) years. Among a total of 31 patients, 8 developed HCC 10 years or more after the completion of IFN therapy, with the longest interval being 17.8 years. Thirteen patients (41.9 %) had habitually consumed alcohol, and 8 (25.8 %) had diabetes. A majority of patients had advanced fibrosis at the start of IFN therapy; F3 was found in 10 (32.3 %) and F4 in 7 (22.6 %), while 13 patients (41.9 %) had relatively mild F2 fibrosis. HCC was detected by US in 21 patients (67.7 %), by CT in 5 (16.1 %), based on subjective symptoms in 3 (9.7 %), and by increased levels of tumor markers in 2 (6.5 %). As for tumor markers, 12 patients (38.7 %) were positive for AFP, 16 (51.6 %) for PIVKA-II, and 22 (71.0 %) for one of these two markers, whereas 9 (29.0 %) were negative for both markers. The median ALT level at the time of HCC diagnosis was 19 U/L, and most patients had ALT levels below 30 U/L, the exception being patients diagnosed at advanced stages.
According to the Barcelona-Clinic Liver Cancer staging classification, 26 patients (83.9 %) had early stage HCC (single nodule, or up to 3 nodules <3 cm) [24]. Twenty-eight patients (90.3 %) had good liver functional reserve, with Child-Pugh grade A, and 25 (80.6 %) underwent curative treatment including hepatectomy and percutaneous radiofrequency ablation. The initial treatment response was complete remission in 26 (83.9 %) patients. Among these patients, 10 had recurrence of HCC, and 8 were able to receive curative treatment for recurrent HCC. This might have been because good liver functional reserve was maintained by successful eradication of HCV with IFN therapy. Histopathological examination of non-cancerous margins was performed in 19 (61.3 %) patients, and liver fibrosis was reduced in 9 (47.4 %), while 9 (47.4 %) showed no change. Only 1 (5.3 %) exhibited exacerbation. Most patients showed no histological progression of liver fibrosis. Patients who developed HCC after achieving SVR had a good 5-year survival rate of 83 %, whereas 4 patients who were diagnosed at far advanced stages died within 6 months. Since these 4 patients had received neither periodic examinations nor adequate liver cancer screening, HCC was detected at far advanced stages based on subjective symptoms.
Next, we compared the patients who had undergone liver cancer screening every 6 months with those who had not. The former and latter groups consisted of 23 and 8 patients, respectively. The mean tumor numbers were 1.2 and 3.3; the mean maximum tumor sizes were 2.4 and 7.0 cm. The proportions of patients receiving curative treatment for HCC were 96 and 38 %, and the 5-year survival rates were 94 and 50 % in the former and latter groups, respectively. Based on these results, screening for HCC development every 6 months after SVR is especially important for improving outcomes.
Discussion
Advances in IFN therapy may increase the number of patients at high risk for HCC development who achieve SVR, including elderly patients and those with advanced fibrosis. Thus, appropriate follow-up is especially important after SVR.
In this study involving 562 patients achieving SVR, 31 (5.5 %) developed HCC during the median observation period of 4.8 (range 1–20.5) years. A recent meta-analysis determined the incidence of HCC after SVR to be 0.0–3.2 % [25], indicating a rather high incidence in our study. Possible explanations for our high incidence include differences in the clinical backgrounds of the patients such as more advanced age at the start of IFN therapy, a larger number of patients with advanced fibrosis, and the longer observation period in our study. Of the 31 patients who developed HCC, 8 were found to have HCC at 10 years or more after achieving SVR, with the longest interval being 17.8 years. As our results show, hepatocarcinogenesis can occur as long as 10 years, or even more, after the completion of IFN therapy.
Several studies have demonstrated male gender, advanced age, progression of liver fibrosis, and low platelet count to be major risk factors for HCC development after SVR [11–15]. In previous reports, it was found that liver fibrosis at advanced stages (F3 or liver cirrhosis) often leads to HCC development, while mild/moderate fibrosis rarely results in HCC [12, 14, 15]. However, there is also a study reporting HCC development in patients with mild fibrosis [13]. Unexpectedly, a large number of our patients with F2 developed HCC (13/31 patients, 41.9 %). The respective cumulative rates of HCC development were 3.5, 14.7, and 17.2 % at 5, 10, and 15 years after the completion of IFN therapy in patients who had F2 fibrosis. The rate of carcinogenesis in patients with F2 fibrosis was not low as compared to those in patients with F3 and F4 fibrosis (Table 2). Therefore, we attempted to elucidate the risk factors for HCC development from F2 fibrosis. We performed a multivariate analysis using Cox’s proportional hazards model. The independent variables entered into the model for analysis included gender, age, habitual alcohol consumption, AFP level at the start of IFN therapy, and diabetes. The proportional hazards model identified advanced age (≥50 years), AFP elevation (≥8 ng/mL), and habitual alcohol consumption (ethanol consumption ≥30 g/day) as determinants of carcinogenesis, with hazard ratios of 5.9 (p < 0.05), 3.7 (p < 0.05), and 3.7 (p < 0.05), respectively (data not shown).
Even though male gender was found to be a significant risk factor for HCC development in previous studies [13, 14], no significant gender differences were noted in the univariate and multivariate analyses in our study. In fact, 7 female patients who developed HCC had more advanced fibrosis at the start of IFN therapy (F4, 3 cases; F3, 3; F2, 1), as compared with 24 male patients who developed HCC (F4, 4 cases; F3, 7; F2, 12; F0, 1). Of the total of 13 patients with F2 who developed HCC, however, 12 were male, indicating that males may develop HCC from not only advanced fibrosis but also moderate fibrosis. Therefore, careful monitoring is necessary.
Excessive alcohol consumption has been identified as an important risk factor for liver fibrosis progression and cancer development in patients with hepatitis C [26, 27]. Unlike previous studies, no statistically significant association with excessive alcohol consumption (≥60 g/day) was detected in the univariate analysis of HCC development in our study (p = 0.49). However, habitual alcohol consumption (≥30 g/day) was determined to be a significant risk factor in our multivariate analysis (hazard ratio 3.9, p < 0.01). Tokita et al. [12] also found habitual alcohol consumption (≥27 g/day) to be a significant risk factor on univariate analysis in their study involving patients who developed HCC after SVR, although they did not perform multivariate analyses due to the limited number of patients. In addition, Khan et al. [28] reported 1.5-fold to 2.5-fold increases in the incidence of hepatocarcinogenesis for hepatitis C patients with excessive or moderate consumption of alcohol, as compared to those abstaining from alcohol. Further studies are needed to determine whether even moderate (i.e., not excessive) consumption of alcohol is a risk factor for HCC development.
One of the other risk factors for HCC development is AFP elevation since a positive correlation is known to exist between progression of liver fibrosis and AFP levels [29]. Although elevated AFP (>20 ng/mL) is reportedly an independent risk factor for hepatocarcinogenesis [30], recent studies showed that even lower AFP levels (>5–6 ng/mL) may also be an independent risk factor [29, 31]. In this study, we also found that an AFP level before the start of IFN therapy ≥8 ng/mL was an independent risk factor for hepatocarcinogenesis (hazard ratio 2.6, p < 0.05).
Several studies have demonstrated an association between hepatocarcinogenesis and diabetes, or insulin resistance [32, 33]. In this study, the univariate analysis showed a significantly higher incidence of HCC development in patients with diabetes. However, no significant difference was detected by our multivariate analysis (p = 0.19), possibly due to the strong correlations between diabetes and other risk factors, especially liver fibrosis [the prevalence of diabetes was 3.9 % (9/231) in patients with F0–1 fibrosis and 14.3 % (46/322) in those with F2–4 fibrosis].
We further investigated the effects of aging on hepatocarcinogenesis after SVR. This issue has two aspects; one is the possibility that relatively young patients achieving SVR may develop liver cancer when they reach an advanced age at which they are at greater risk for HCC development, and the other is HCC development in elderly patients achieving SVR. With regard to the first possibility, Hamada et al. [34] performed an analysis on hepatocarcinogenesis in patients who had received only one transfusion, i.e., who knew when they had become infected with HCV, and found a significant negative correlation between the duration from HCV infection until the development of HCC and the age of the patient at the time of infection. They also showed that 92 % of HCC patients were diagnosed at age 60 years or older, regardless of the duration of HCV infection. Their findings indicate that patient age may play a more significant role in hepatocarcinogenesis than the duration of HCV infection in those with transfusion-transmitted HCV infection. In this study, we also found a weak but significant inverse correlation between age at the start of IFN therapy and the time elapsed from the completion of IFN therapy until HCC development. This raises the possibility that elderly patients, i.e., those ≥60 years of age, develop HCC sooner after achieving SVR while relatively young patients with F2 or more advanced fibrosis who achieved SVR develop HCC when they reach age ≥60 years after 10 or more years since achieving SVR.
As for the second aforementioned possibility, the recent aging of patients with hepatitis C has been accompanied by aging of those with hepatitis C-related HCC in Japan [35]. Aging of the hepatitis C patient population would present problems not only in Japan but also in other countries where HCV dissemination occurred later [36]. Elderly patients with hepatitis C may develop HCC even if fibrosis has not reached an advanced stage. Miki et al. [37] demonstrated that among 133 patients with CHC-related HCC who underwent hepatic resection, fibrosis stages F0/1, F2, F3, and F4 were identified in 4, 14, 27, and 55 % of the younger group (<70 years), and in 5, 38, 22, and 35 % of the elderly group (≥70 years), respectively. They concluded that the elderly patients developed HCC more often, despite their lower grade of fibrosis, than the younger patients. Honda et al. [38] compared an elderly group of HCC patients (≥75 years old) with a younger group (<75 years old) and reported that there were significantly fewer who developed liver cirrhosis and significantly more who developed HCC in the elderly group with normal liver functions. They suggested telomere length reduction with aging and aberrant DNA methylation as possible causes of HCC development in the absence of potent risk factors for hepatocarcinogenesis, including fibrosis and viral infection. There is also a study showing that immune abnormalities occurring in the livers of elderly patients are involved in hepatocarcinogenesis [39]. Although the effects of aging on hepatocarcinogenesis are clinically apparent, further studies are needed to elucidate the underlying mechanisms in detail.
To examine involvement of chronic hepatitis B virus (HBV) infection in HCC development after SVR, we measured antibody levels in these patients and found that 51.7 % (15/29) who developed HCC were positive for antibody against hepatitis B core antigen. This positivity rate was approximately the same as the 51.5 % (105/204) observed in hepatitis C patients ≥60 years of age undergoing IFN therapy in our hospital after 2004 who did not develop HCC (data not shown). Based on these results, it is suggested that a history of HBV infection does not affect hepatocarcinogenesis after achieving SVR.
In this study, we did not analyze the risk factors for HCC development after SVR. This is a limitation of our study. Further studies are needed to elucidate the risk factors for HCC development after SVR.
In the present study involving 562 patients achieving SVR, the respective cumulative incidences of HCC at 5, 10, and 15 years were 6.2, 18.9, and 27.8 % for patients at risk for HCC development, i.e., who had F2 or more advanced fibrosis and were ≥50 years of age at the completion of IFN therapy (n = 255), while the incidences at 5, 10, and 15 years were 0.5, 1.4, and 3.6 % in the remaining patients who had F0–1 fibrosis or were <50 years of age (n = 307), indicating a higher incidence of HCC development in high-risk patients (data not shown). Therefore, lifelong screening for HCC development, every 6 months at a minimum, is especially important in these high-risk patients.
Patients tend to think that they have been cured when they achieve SVR with IFN therapy and, consequently, may discontinue visits to the outpatient clinic. Thus, medical professionals need to thoroughly explain the possibility of HCC development after SVR to patients. Our assessment of outcomes in 562 patients after SVR revealed no liver disease-related deaths attributable to conditions, including hepatic failure and rupture of esophageal varices, other than HCC. This indicates that early diagnosis and treatment of HCC with adequate follow-up after SVR can reduce liver disease-related deaths to virtually zero in patients achieving SVR.
In this study, the 5-year survival rate was 93 % in the patients who had received periodic cancer screening, every 6 months at a minimum, but was only 60 % in those who had not. Adequate follow-up, no less frequently than every 6 months, by imaging modalities such as US and CT and tumor marker measurements is important for detecting HCC at an early stage.
In conclusion, we recommend that SVR patients be observed at 6-month intervals, at a minimum, to facilitate HCC diagnosis at an early stage, for as long as possible, even if not at an advanced stage of fibrosis, i.e. F2 fibrosis.
Abbreviations
- HCV:
-
Hepatitis C virus
- HCC:
-
Hepatocellular carcinoma
- IFN:
-
Interferon
- ALT:
-
Alanine aminotransferase
- SVR:
-
Sustained virological response
- PEG-IFN:
-
Pegylated interferon
- CHC:
-
Chronic hepatitis C
- AFP:
-
Alpha-fetoprotein
- PIVKA-II:
-
Protein induced by vitamin K absence or vitamin K antagonist-II
- US:
-
Ultrasonography
- CT:
-
Computed tomography
- MR:
-
Magnetic resonance imaging
- γGTP:
-
γGlutamyl transpeptidase
- HBV:
-
Hepatitis B virus
References
Uemura T, Ichijo T, Yoshizawa K, et al. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol. 2009;44(suppl 19):102–7.
Nishiguchi S, Kuroki T, Nakatani S, et al. Randomized trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–5.
Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT study group. Inhibition of hepatocarcinogenesis by interferon therapy. Ann Intern Med. 1999;131:174–81.
Ikeda K, Saitoh S, Arase Y, et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: a long-term observation study of 1643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124–30.
Marcellin P, Boyer N, Gervais A, et al. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-alpha therapy. Ann Intern Med. 1997;127:875–81.
Okanoue T, Itoh Y, Minami M, et al. Interferon therapy lowers the rate of progression to hepatocellular carcinoma in chronic hepatitis C but not significantly in an advanced stage: a retrospective study in 1148 patients. J Hepatol. 1999;30:653–9.
Nishiguchi S, Shiomi S, Nakatani S, et al. Prevention of hepatocellular carcinoma in patients with chronic active hepatitis C and cirrhosis. Lancet. 2001;357:196–7.
Cardoso AC, Moucari R, Figueiredo-Mendes C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C with advanced fibrosis. J Hepatol. 2010;52:652–7.
Toyoda H, Kumada T, Tokuda A, et al. Long-term follow-up of sustained responders to interferon therapy in patients with chronic hepatitis C. J Viral Hepat. 2000;7:414–9.
Enokimura N, Shiraki K, Kawakita T, et al. Hepatocellular carcinoma development in sustained viral responders to interferon therapy in patients with chronic hepatitis C. Anticancer Res. 2003;23:593–6.
Ikeda M, Fujiyama S, Tanaka M, et al. Risk factors for development of hepatocellular carcinoma in patients with chronic hepatitis C after sustained response to interferon. J Gastroenterol. 2005;40:148–56.
Tokita H, Fukui H, Tanaka A, et al. Risk factors for the development of hepatocellular carcinoma among patients with chronic hepatitis C who achieved a sustained virological response to interferon therapy. J Gastroenterol Hepatol. 2005;20:752–8.
Kobayashi S, Takeda T, Enomoto M, et al. Development of hepatocellular carcinoma in patients with chronic hepatitis C who had a sustained virological response to interferon therapy: a multicenter, retrospective cohort study of 1124 patients. Liver Int. 2007;27:186–91.
Hirakawa M, Ikeda K, Arase Y, et al. Hepatocarcinogenesis following HCV RNA eradication by interferon in chronic hepatitis patients. Intern Med. 2008;47:1637–43.
Chang KC, Hung CH, Lu SN, et al. A novel predictive score for hepatocellular carcinoma development in patients with chronic hepatitis C after sustained response to pegylated interferon and ribavirin combination therapy. J Antimicrob Chemother. 2012;67:2766–72.
Ito Y, Yamamoto N, Nakata R, et al. Delayed development of hepatocellular carcinoma during long-term follow-up after eradication of hepatitis C virus by interferon therapy. World J Gastroenterol. 2005;11:7218–21.
Mashitani T, Yoshiji H, Yamazaki M, et al. Development of hepatocellular carcinoma in a patient 13 years after sustained virological response to interferon against chronic hepatitis C: a case report. Cases J. 2009;2:18.
Conteduca V, Sansonno D, Russi S, et al. Therapy of chronic hepatitis C virus infection in the era of direct-acting and host-targeting antiviral agents. J Infect. 2013. doi:10.1016/j.jinf.2013.08.019.
Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16.
Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: The randomized PILLAR study. Hepatology. 2013;58:1918–29.
Zeuzem S, Hultcrantz R, Bourliere M, et al. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotype 2 or 3. J Hepatol. 2004;40:993–9.
Urquijo JJ, Diago M, Boadas J, et al. Safety and efficacy of treatment with pegylated interferon alpha-2a with ribavirin in chronic hepatitis C genotype 4. Ann Hepatol. 2013;12(1):30–5.
Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading, and staging. Hepatology. 1994;19:1513–20.
Llovet JM, Fuster J, Bruix J, Barcelona-Clinic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(2 suppl 1):S115–20.
Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–37.
Colombo M. Natural history and pathogenesis of hepatitis C virus-related hepatocellular carcinoma. J Hepatol. 1999;31:25–30.
Ikeda K, Saitoh S, Suzuki Y, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930–8.
Khan KN, Yatsuhashi H. Effect of alcohol consumption on the progression of hepatitis C virus infection and risk of hepatocellular carcinoma in Japanese patients. Alcohol. 2000;35:286–95.
Tateyama M, Yatsuhashi H, Taura N, et al. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011;46:92–100.
Sangiovanni A, Colombo E, Radaelli F, et al. Hepatocyte proliferation and risk of hepatocellular carcinoma in cirrhotic patients. Am J Gastroenterol. 2001;96:1575–80.
Taura N, Fukuda S, Ichikawa T, et al. Relationship of α-fetoprotein levels and development of hepatocellular carcinoma in hepatitis C patients with liver cirrhosis. Exp Ther Med. 2012;4:972–6.
Hung CH, Lee CM, Wang JH, et al. Impact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapy. Int J Cancer. 2011;128:2344–52.
Khattab MA, Eslam M, Mousa YI, et al. Association between metabolic abnormalities and hepatitis C-related hepatocellular carcinoma. Ann Hepatol. 2012;11:487–94.
Hamada H, Yatsuhashi H, Yano K, et al. Impact of aging on the development of hepatocellular carcinoma in patients with posttransfusion chronic hepatitis C. Cancer. 2002;95:331–9.
Ohishi W, Kitamoto M, Aikata H, et al. Impact of aging on the development of hepatocellular carcinoma in patients with hepatitis C virus infection in Japan. Scand J Gastroenterol. 2003;8:894–900.
Tanaka Y, Hanada K, Mizokami M, et al. A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci U S A. 2002;99:15584–9.
Miki D, Aikata H, Uka K, et al. Clinicopathological features of elderly patients with hepatitis C virus-related hepatocellular carcinoma. J Gastroenterol. 2008;43:550–7.
Honda T, Miyaaki H, Ichikawa T, et al. Clinical characteristics of hepatocellular carcinoma in elderly patients. Oncol Lett. 2011;2:851–4.
Singh P, Coskun ZZ, Goode C, et al. Lymphoid neogenesis and immune infiltration in aged liver. Hepatology. 2008;47:1680–90.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, N., Ohho, A., Yamasaki, A. et al. Hepatocarcinogenesis in chronic hepatitis C patients achieving a sustained virological response to interferon: significance of lifelong periodic cancer screening for improving outcomes. J Gastroenterol 49, 1504–1513 (2014). https://doi.org/10.1007/s00535-013-0921-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-013-0921-z