Abstract
Viral infections have the potential to induce carcinogenesis through chronic inflammation. Hepatitis B and hepatitis C (HCV) are two major risk factors for the development of hepatocellular carcinoma (HCC) and are responsible for 70–80 % of cases seen worldwide. The incidence of HCC has been rising in the USA over the last several decades, largely in part to the prevalence of HCV-induced cirrhosis. Curative therapies with resection and orthotopic liver transplantation are only available for individuals with early-stage HCC, and disease recurrence is still common after treatment, highlighting the need for both primary and secondary preventative measures. Despite the low rates of viral eradication with interferon-based regimens, many studies have shown a chemo-protective effect in patients who do achieve a sustained virologic response. The emergence of new direct-acting antiviral agents with cure rates above 95 % has the potential to change the landscape of HCV-induced HCC. This article discusses the current data for HCV therapy as a chemo-preventive agent and HCV treatment strategies for patients with various stages of hepatocellular carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
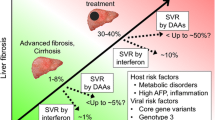
Hepatocellular carcinoma (HCC) is the sixth most common cancer and accounts for 5.6 % of all cancer diagnoses [1]. Both hepatitis B (HBV) and hepatitis C (HCV) are well-documented risk factors for the development of HCC via induction of an inflammatory state that promotes hepatocarcinogenesis [2]. While HBV is the most common cause of HCC worldwide accounting for 53 %, HCV is responsible for 10–20 % of HCC [3]. In the USA, HCV is the most common etiologic factor, seen in 30–60 % of HCC cases. Data derived from cohort studies estimates that patients infected with HCV develop HCC at a rate of 1–3 % over 30 years [4]. Without effective antiviral therapy leading to eradication of viral replication, predictive models have shown that at least 14.4 % of all patients with HCV will develop HCC over 30 years [5]. Fortunately, the ability to treat HCV has changed dramatically over the past 2 years with the emergence of interferon-free direct-acting antiviral agents (DAAs) that produce sustained virologic response (SVR) in over 90 % of treated individuals [6••]. While there have been many advances in the treatment of HCV, curative therapies for HCC are limited to those with early-stage disease and the 5-year overall survival (OS) remains at a dismal 18 % for all stages of disease [7]. As more patients achieve a SVR with highly active antiviral agents, the landscape of HCC would be anticipated to hopefully change. However, there are many uncertainties with regard to management of patients with HCV as it relates to HCC, as most of the studies available are retrospective in nature and utilized the traditional interferon-based regimen associated with significantly lower rates of viral clearance.
Pathogenic Background Behind HCV and HCC
HCV is a single-stranded RNA virus that, in contrast to HBV, remains in the cell cytoplasm and is not capable of integrating into the host genome. This important difference allows for HCV to be potentially curable. There are two proposed mechanisms of how HCV induces carcinogenesis. The most commonly accepted theory is that the virus stimulates a cycle of repetitive hepatocellular injury, cell death, and regeneration that ultimately leads to advanced fibrosis. Through the regeneration process, there are multiple pathways that are susceptible to genetic modification and instability that, if interrupted, can result in malignant transformation of hepatocytes [8, 9]. Evidence supporting the role of inflammation originates from studies showing that patients with persistently elevated aminotransferases are at a higher risk for HCC than those with persistently normal values [10].
The second mechanism of carcinogenesis is related to the unique viral proteins that make up HCV. Proteins such as non-structural (NS) protein-3, NS4B, and NS5A have all been hypothesized to have oncogenic properties [11, 12]. These proteins interact with various pathways such as the Raf/MAPK, PIK3, and WNT/beta-catenin pathways, which are all implicated in the pathogenesis of HCC [13–15]. HCV-associated HCC occurs infrequently in the absence of significant fibrosis or cirrhosis, suggesting that chronic inflammation is largely responsible for carcinogenesis. However, in the HALT-C trial, a large prospective study of 1005 HCV-infected individuals showed that 17 % had HCC in the absence of cirrhosis, implying that these oncogenic proteins likely have a direct effect independent of the presence of advanced fibrosis [16].
Other pro-inflammatory conditions, such as non-alcoholic steatohepatitis (NASH), alcohol use, and co-infection with HBV may lead to higher rates of HCC in HCV patients. Two meta-analyses have shown that co-infection with HBV increases the risk of HCC compared to HCV infection alone with odds ratios of 35.7 (95 % CI 26.2–48.5) versus 8.1 (95 % CI 11.5–21.3) respectively [17, 18].
Antiviral Therapy as Primary Prevention of HCC
Primary prevention of HCC through antiviral therapy has been evaluated by several studies. Initial randomized controlled trials (RCTs) compared the effectiveness of IFN in preventing HCC with no therapy, and all but one trial showed that antiviral treatment reduced the risk of HCC [19–23]. The most notable of these studies is that by Nishiguchi et al. which randomized 90 patients with chronic HCV in a 1:1 fashion to receive IFN monotherapy versus no treatment. After a follow-up period of 4–5 years, the IFN-treated subset had significantly lower rates of HCC (relative risk (RR) 0.067; P = .01). Of note, pegylated interferon and ribavirin were not used in any of the RCTs and SVR rates were poor. Only 7 patients achieved SVR in the first randomized study. The anticarcinogenic effects of IFN were attributed largely to its anti-angiogenic and antitumor properties [24]. Despite low rates of SVR, a meta-analysis of these original studies determined that HCV treatment decreased HCC with a RR of 0.39 (95 % CI, 0.26–0.59) [25].
Later studies set out to determine if interferon therapy or viral eradication resulted in fewer cases of HCC. Testing the hypothesis that IFN had anticarcinogenic properties outside of curing viral hepatitis, a few notable randomized trials, including the HALT-C, EPIC, and COPILOT studies, evaluated the role of pegylated interferon (PEG-IFN) in non-responders to prevent HCC. Each study demonstrated that PEG-IFN was ineffective at preventing HCC among patients with HCV-related cirrhosis [16, 26–28]. This supports the idea that viral clearance is key in HCC prevention. A multicenter study from Taiwan also showed that non-responders and untreated patients had similar rates of HCC [29].
Several meta-analyses of observational and cohort studies have examined the role of SVR and development of HCC, confirming a strong association of viral clearance and diminished risk of developing HCC [30–32, 33••]. The meta-analysis by Singal et al. of 26 observational and cohort studies showed that those with SVR had a 79 % (95 % CI 0.73–0.84) risk reduction in the development of HCC in those with HCV-associated cirrhosis. Future studies over the next several years with the new interferon-free DAAs will help solidify our knowledge regarding the protective benefit of achieving SVR.
HCC Risk After Viral Eradication with IFN Therapy
Successful HCV treatment decreases but does not eliminate the risk of HCC. A systematic review of 30 observational studies showed that both cirrhotic and non-cirrhotic patients developed HCC after achieving SVR. In non-cirrhotics, HCC developed in 1.5 % of patients with an SVR compared to 6.2 % without SVR. In cirrhotic patients, HCC developed in 4.2 % of patients with SVR compared to 17.8 % without SVR. Elevated AFP, thrombocytopenia, and advanced fibrosis were identified as risk factors for HCC in those who had achieved SVR [34]. As demonstrated in this review, fibrosis and cirrhosis are the most well-established risk factors for HCC. A multicenter, retrospective cohort study demonstrated that among the 1197 patients who achieved SVR, the 27 who developed HCC had advanced fibrosis histologically prior to initiation of interferon treatment (P = .03) [35].
In non-cirrhotics who achieve SVR, there is limited data about their future risk of HCC. Several studies have attempted to identify biomarkers that may further risk stratify patients who achieve SVR into higher risk surveillance groups. For example, a recent study was conducted in Taiwan of 642 patients, which identified older age and an elevated baseline gamma-glutamyl transferase level as risk factors for HCC in non-cirrhotics. Patients were treated with pegylated IFN/ribavirin, and of the 556 non-cirrhotic patients, 17 (3.1 %) developed HCC compared to 16 (18.6 %) of the 86 cirrhotic patients [36]. Table 1 indicates many similar studies that have identified various risk factors. These studies will need to be validated on a Western population and with the new non-interferon-based therapy.
While those who have achieved SVR have a lower overall risk for HCC, these findings illustrate the necessity of continued screening in patients with advanced fibrosis. NAFLD, co-infection with HBV, and alcohol use are all well-documented risk factors for HCC and should be taken into consideration in the context of each patient. One cohort study showed that diabetes was an independent predictor of HCC in SVR patients without cirrhosis [44]. Although this study was limited by a low overall incidence of HCC in non-cirrhotics with SVR, it describes another potential risk factor that may help further risk stratify patients into screening groups.
As non-IFN-based therapy becomes widely available, increasing numbers of patients will achieve SVR in the upcoming years. The challenge will be identifying patients with advanced fibrosis and enrolling them in surveillance programs. Surveillance programs currently have poor compliance rates, with studies reporting that less than 20 % of cirrhotics who develop HCC had participated in a surveillance program [45]. While surveillance is not universally recommended due to limited evidence, the American Association for the Study of Liver Diseases (AASLD) HCV guidance document recommends continued surveillance with ultrasonography every 6 months for patients with a METAVIR fibrosis stage of F3–F4 [6••]. This is in contrast to the older AASLD HCC guidelines that recommended HCC surveillance only among cirrhotic patients. With the advent of non-invasive measurements to measure fibrosis, such as transient elastography, liver biopsies are being performed less frequently. Transient elastography may help risk stratify patients, yet there is no clear consensus as to what degree of liver stiffness (LS) correlates with a heightened risk of HCC. One study of 190 patients who achieved SVR with pegylated IFN/ribavirin therapy showed that the 8 patients who developed HCC had a LS measurement ≥7 kPa [46]. This study was limited by the small sample size and short follow-up time of 43 months. Another study also reported HCC risk being related to LS as well. Among all patients irrespective of whether they had SVR, independent predictors of HCC development included age >68 and a LS measurement >10.8 kPa [47].
Secondary Prevention of HCC
The prognosis of a patient with HCC depends on its stage at diagnosis and the degree of underlying cirrhosis. Local resection, orthotopic liver transplantation (OLT), or liver-directed ablative therapies offer the greatest chance of curative therapy, but are associated with different recurrence rates.
OLT has the highest recurrence-free survival rate at over 70 %, in those with early HCC defined as meeting Milan criteria, which is defined as a single lesion ≤5 cm or a maximum of three lesions each <3 cm. Some centers have reported survival rates as high as 80 % [48, 49]. The advantage of liver transplantation is that it replaces the underlying field defect in a cirrhotic liver in addition to treating the HCC. The limited supply of organs and wait list mortality have led to concomitant bridging with liver-directed therapy while awaiting OLT, specifically if the anticipated wait list exceeds 6 months. With the institution of the revised MELD upgrade for patients with HCC meeting Milan criteria on October 8, 2015, it is likely that most patients will indeed wait at least 6 months [50].
Patients eligible for hepatic resection and local ablative therapies are at risk of HCC recurrence, and the 5-year recurrence rates documented range from 50 to 70 % [51]. Early recurrence (<2 years after treatment) is felt to be from intrahepatic spread of the original lesion, while late recurrence (>2 years) is felt to be from a de novo HCC lesion. In the latter scenario, the risk of HCC occurrence is felt to be associated with persistent HCV viremia. Thus, viral eradication would be expected to have the potential to prevent de novo HCC lesions [52].
There is a significant amount of data describing the benefits of HCV eradication in the secondary prevention of HCC post treatment with resection or radio-ablative therapy. Even without achieving SVR, a study by Shindoh et al. showed that patients with lower viral loads had better recurrence-free survival (RFS) after undergoing hepatic resection. The 1-, 3-, and 5-year RFS were 66.1, 37.4, and 36.1 % in the low viral load group compared to 60.2, 25.8, and 14.9 % in the high viral load group respectively (P < .001 [53]). Similar results post resection have been seen in patients with low HBV who have low viral loads and is likely attributable to reduced inflammation at lower viral loads [54].
Five randomized controlled trials have supported that achieving SVR reduces the risk of HCC recurrence after resection or ablative therapies; however, these trials had small sample sizes and were not powered to show significant differences between the groups [55–59]. Risk reduction was primarily observed in the prevention of late recurrence or de novo lesions. Mazzefaro et al. found no effect of treatment on early recurrences, but did find a benefit in preventing late recurrence (HR 0.3; 95 % CI 0.09–0.9; P = .04). The dosing protocols of interferon varied and achievement of SVR was low across all studies. A systematic review of ten studies of patients treated with interferon showed a benefit of IFN over no therapy (OR 0.26 (0.15–0.45); P < .00001). Those who obtained SVR had a greater benefit than those who were non-responders, with an improved survival benefit with an odds ratio of 0.31 (0.11–0.90) [60•].
Other Benefits of HCV Therapy
Achieving SVR has benefits beyond attenuating a patient’s HCC risk. Studies investigating long-term clinical outcomes have shown that SVR is associated with a decrease in mortality, progression of fibrosis, the need for transplantation, and extrahepatic complications [61]. An observational cohort study from the Veterans Affairs HCV registry showed that of the 4 % of patients who achieved SVR, the unadjusted death rates were 6.8 per 1000 person-years in the SVR group compared to 21.8 per 1000 person-years in those with continued viremia [62].
Patients who achieved SVR also had improvements in their fibrosis scores. In another study, 344 patients were followed a median of 3 years, of whom 126 had pre- and post-treatment liver biopsies available for analysis. Fibrosis improved in 56 %, remained stable in 32 %, and deteriorated in 12 %. Regression of cirrhosis was also observed in 9 out of 14 patients (64 %). None of the patients developed clinical decompensation during the course of the study [63].
Without continued inflammation in the liver, compensated cirrhotics who achieve SVR are unlikely to develop portal hypertension. This was demonstrated in a study of 920 patients in Italy which showed that the 124 patients who had undetectable HCV-RNA after treatment with interferon monotherapy had 0 liver-related complications per 100 person-years compared to 1.88 liver-related complications per 100 person-years in the non-SVR group. Ascites and esophageal varices were the most common complications in unsuccessfully treated patients [64]. Another study showing the benefits of treating HCV was the HALT-C trial, where patients were prospectively followed for 7.5 years. Patients who achieved SVR had a lower rate of death or liver transplant compared to the non-responders (2.2 vs 21.3 % respectively, P < .001) [65•]. Of note, one study by Lens et al. indicated that patients with severe portal hypertension, defined as a HVPG ≥10, may be at a continued risk for decompensated disease within the first 5 years of treatment despite achieving SVR [66]. Therefore, patients with known portal hypertension should continue to be monitored closely with regular variceal screening.
Lastly, a recent study also showed an association between SVR and an improvement in extrahepatic outcomes such as end-stage renal disease (HR 0.15; 95 % CI 0.07–0.31), acute coronary syndrome (HR 0.77; 95 % CI 0.62–0.97), and ischemic stroke (HR 0.62; 95 % CI 0.46–0.83), all of which were less prevalent in patients with SVR [67]. In aggregate, the positive clinical impact of SVR beyond cancer risk would be expected to decrease the need for OLT in the future. Among those that require transplant for HCC, they could be deemed more favorable candidates for transplant due to fewer comorbidities.
Recommendations for HCV Treatment in Patients with HCC
The current AASLD recommendations for the treatment of HCV in patients with HCC are not specific as to who and when to treat. They state that it is safe to treat patients who are awaiting liver transplantation who are within Milan criteria. The recommended regimen for genotypes 1 and 4 is daily ledipasvir 90 mg/sofosbuvir 400 mg in combination with ribavirin 600 mg as tolerated for a total of 12 weeks. Those with genotypes 2 and 3 should be treated with sofosbuvir 400 mg and weight-based ribavirin for up to 48 weeks [68].
The European Association for the Study of the Liver (EASL) guidelines recommend treatment for all patients with the new oral therapies as they extrapolate from the interferon studies that achieving SVR will improve outcomes. Since these medications are well tolerated, the primary barrier to treatment is cost. The next sections will discuss special considerations for certain patient populations.
Treatment of HCV in Patients Awaiting OLT for HCC
Some controversy exists over the appropriate time to treat HCV in patients awaiting OLT. Left untreated, HCV will have a 100 % recurrence rate if a patient is viremic at the time of surgery, with cirrhosis seen in 20–30 % of patients within 5 years of transplant [69]. Some studies have also shown a higher rate of biliary complications in HCV-positive patients [70]. In the past, patients with HCC listed for transplant were not routinely treated due to the side effects of interferon-based regimens. A randomized controlled trial done by the Adult-to-Adult Living Donor Transplant Cohort Study randomized HCV-positive patients listed for OLT with a potential living donor or HCC MELD exception points to receive PEG-IFN and ribavirin or no treatment. Results showed that while successful treatment was possible for some individuals, treated patients had a higher rate of serious adverse events [71].
Given the minimal side effects of the new DAAs, the risk of HCV-associated graft complications, and the possibility of lower recurrence rates of de novo HCC based upon the interferon data, HCV treatment should be highly considered prior to transplantation.
The downside of treating prior to transplant is that it would exclude these patients from receiving HCV+ grafts. Due to the long wait list times from a limited supply of organs, transplantation of HCV+ grafts became an option for HCV+ recipients. There have been no differences in graft survival or post-transplant outcomes using these HCV+ grafts [72]. The use of extended donor criteria, such as HCV+ grafts, has been shown to increase patient access to grafts and decrease wait list dropout due to death [73].
The AASLD guidelines state that it is acceptable to treat while patients are awaiting transplantation. The only study specifically looking at HCV-infected patients with HCC who were treated with the new antivirals while awaiting transplant was conducted as a phase 2 open-label study in 61 patients of any genotype and Child-Turcotte-Pugh score ≤7. All patients met Milan criteria and were listed for transplant. They received up to 48 weeks of sofosbuvir 400 mg and ribavirin. Out of the 43 transplanted patients who achieved SVR prior to transplant, 30 patients (70 %) had undetectable HCV RNA at 12 weeks post transplant and 10 patients (23 %) had HCV relapse. HCV relapse was inversely related to the number of days with undetectable HCV RNA prior to transplantation [74••].
Treatment of HCV in Patients with Advanced HCC
Limited data exists for the use of antiviral therapy in patients with advanced HCC. Patients with metastatic disease are often excluded from most drug trials, and the AASLD does not offer guidelines as to what stage of HCC should be considered for treatment. From a cost perspective, the new DAAs are very expensive with the current list price of Harvoni documented around $1125.00 per pill. Cost-effective analyses have shown that treatment at this price may be cost-effective when balanced with the quality of life gained and the cost of future hospitalizations for HCV-related complications [75]. As most patients with metastatic HCC have a life expectancy of less than a year, it is not currently cost-effective to treat HCV in this patient subset. This mindset, however, raises ethical considerations for those suffering from complications of portal hypertension, in addition to their underlying malignancy. Treatment of HCV may improve these complications and synthetic function, therefore potentially allowing more aggressive therapy for HCC. Case reports have also documented regression of metastatic HCC after HCV eradication. One patient with genotype 1B HCV who underwent liver transplantation was subsequently found to have metastatic disease to the lung. He was treated with sofosbuvir and ribavirin for 24 weeks. His end-of-treatment viral load was less than 43 IU/mL and his largest lung metastasis decreased in size [76]. Ribavirin inhibits MAPK phosphorylation, which may result in an antitumor effect [77].
Future Directions in HCC Therapy
Recent research for new HCC therapies has led to the discovery of a cell receptor called programmed death-1 (PD-1). PD-1 is an immunoinhibitor receptor that belongs to the CD28 family and is expressed on many different types of inflammatory cells. Its presence on T cells facilitates the persistence of chronic infection with HBV and HCV. Its link to HCC was first seen in patients with HBV who had higher PD-1 expression that correlated with elevated viral loads. Those patients were also found to have a 6.29-fold increase in HCC risk [43]. Overexpression of PD-1 has also been associated with poor outcomes in HCC. Given that persistent viremia is associated with higher PD-1 expression, this provides further evidence to support treating HCV in this patient population.
Currently, a phase I/II study with a PD-1 inhibitor, nivolumab, may be a promising treatment for HCC. Forty-seven patients were included in the study and a complete response was reported in two patients (5 %) and a partial response in six patients (14 %). Unlike other therapies, the responses thus far are of prolonged duration and researchers are projecting 1-year overall survival of around 62 % [78].
Conclusions
After years of research, there is finally a safe and effective treatment for HCV. Until the same can be said about HCC, we must continue to focus our efforts on prevention of HCC in this patient population through viral eradication. As this treatment becomes more affordable and widely available, this will hopefully lead to a decrease in cases of HCV cirrhosis and ideally HCC in future generations. As these medications have been initially prioritized by insurance for patients with advanced fibrosis, future research as to the benefits of primary and secondary prevention with the new generation of antivirals will further substantiate the utility of these medications as a chemo-preventive tool. The true challenge will be ensuring that patients with advanced fibrosis who are successfully treated with the new antivirals continue to undergo surveillance for HCC. Most importantly, the controversy over whether HCC patients should be treated has ended with the arrival of safe and effective antiviral therapy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, et al. GLOBOCAN 2012. Cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon: International Agency for Research on Cancer; 2013.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38.
El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60:1767–75.
Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66–72.
Panel AIHG. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–54. These are the current guidelines for HCV treatment with the new direct-acting antivirals. These guidelines also identify which patients need continued surveillance for HCC after successful treatment.
Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology. 2012;55:476–82.
Hsu YC, Wu CY, Lin JT. Hepatitis C virus infection, antiviral therapy, and risk of hepatocellular carcinoma. Semin Oncol. 2015;42:329–38.
Lemon SM, McGivern DR. Is hepatitis C virus carcinogenic? Gastroenterology. 2012;142:1274–8.
Benvegnu L, Alberti A. Risk factors and prevention of hepatocellular carcinoma in HCV infection. Dig Dis Sci. 1996;41:49S–55.
Zemel R, Gerechet S, Greif H, Bachmatove L, Birk Y, Golan-Goldhirsh A, et al. Cell transformation induced by hepatitis C virus NS3 serine protease. J Viral Hepat. 2001;8:96–102.
McGivern DR, Lemon SM. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene. 2011;30:1969–83.
Street A, Macdonald A, McCormick C, Harris M. Hepatitis C virus NS5A-mediated activation of phosphoinositide 3-kinase results in stabilization of cellular beta-catenin and stimulation of beta-catenin-responsive transcription. J Virol. 2005;79:5006–16.
Milward A, Mankouri J, Harris M. Hepatitis C virus NS5A protein interacts with beta-catenin and stimulates its transcriptional activity in a phosphoinositide-3 kinase-dependent fashion. J Gen Virol. 2010;91:373–81.
Aoki H, Hayashi J, Moriyama M, Arakawa Y, Hino O. Hepatitis C virus core protein interacts with 14-3-3 protein and activates the kinase Raf-1. J Virol. 2000;74:1736–41.
Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–48.
Shi J, Zhu L, Liu S, Xie WF. A meta-analysis of case–control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607–12.
Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347–54.
Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–5.
Valla DC, Chevallier M, Marcellin P, Payen JL, Trepo C, Fonck M, et al. Treatment of hepatitis C virus-related cirrhosis: a randomized, controlled trial of interferon alfa-2b versus no treatment. Hepatology. 1999;29:1870–5.
Bernardinello E, Cavalletto L, Chemello L, Mezzocolli I, Donada C, Benvegnu L, et al. Long-term clinical outcome after beta-interferon therapy in cirrhotic patients with chronic hepatitis C. TVVH Study Group. Hepatogastroenterology. 1999;46:3216–22.
Azzaroli F, Accogli E, Nigro G, Trere D, Giovanelli S, Miracolo A, et al. Interferon plus ribavirin and interferon alone in preventing hepatocellular carcinoma: a prospective study on patients with HCV related cirrhosis. World J Gastroenterol. 2004;10:3099–102.
Soga K, Shibasaki K, Aoyagi Y. Effect of interferon on incidence of hepatocellular carcinoma in patients with chronic hepatitis C. Hepatogastroenterology. 2005;52:1154–8.
Singh RK, Gutman M, Bucana CD, Sanchez R, Llansa N, Fidler IJ. Interferons alpha and beta down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc Natl Acad Sci U S A. 1995;92:4562–6.
Zhang CH, Xu GL, Jia WD, Li JS, Ma JL, Ge YS. Effects of interferon treatment on development and progression of hepatocellular carcinoma in patients with chronic virus infection: a meta-analysis of randomized controlled trials. Int J Cancer. 2011;129:1254–64.
Bruix J, Poynard T, Colombo M, Schiff E, Burak K, Heathcote EJ, et al. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140:1990–9.
Afdhal NH LR, Brown RJ, et al. Colchicine versus peg-interferon alfa 2b long-term therapy: results of the 4 year COPILOT trial. In: 43rd Annual Meeting of the European Association for the Study of the Liver; 2008; Milan, Italy; 2008.
Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–41.
Yu ML, Lin SM, Chuang WL, Dai CY, Wang JH, Lu SN, et al. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther. 2006;11:985–94.
Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–8. 288 e281.
Ng V, Saab S. Effects of a sustained virologic response on outcomes of patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2011;9:923–30.
Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889–900.
Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–37. This is one of the more recent meta-analyses looking at the effect of primary prevention of HCC through viral eradication of HCV.
Chang KC, Hung CH, Lu SN, Wang JH, Lee CM, Chen CH, et al. A novel predictive score for hepatocellular carcinoma development in patients with chronic hepatitis C after sustained response to pegylated interferon and ribavirin combination therapy. J Antimicrob Chemother. 2012;67:2766–72.
Makiyama A, Itoh Y, Kasahara A, Imai Y, Kawata S, Yoshioka K, et al. Characteristics of patients with chronic hepatitis C who develop hepatocellular carcinoma after a sustained response to interferon therapy. Cancer. 2004;101:1616–22.
Huang CF, Yeh ML, Tsai PC, Hsieh MH, Yang HL, Hsieh MY, et al. Baseline gamma-glutamyl transferase levels strongly correlate with hepatocellular carcinoma development in non-cirrhotic patients with successful hepatitis C virus eradication. J Hepatol. 2014;61:67–74.
Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121:2874–82.
Ikeda M, Fujiyama S, Tanaka M, Sata M, Ide T, Yatsuhashi H, et al. Risk factors for development of hepatocellular carcinoma in patients with chronic hepatitis C after sustained response to interferon. J Gastroenterol. 2005;40:148–56.
Arase Y, Kobayashi M, Suzuki F, Suzuki Y, Kawamura Y, Akuta N, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57:964–73.
Oze T, Hiramatsu N, Yakushijin T, Miyazaki M, Yamada A, Oshita M, et al. Post-treatment levels of alpha-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol. 2014;12:1186–95.
Yamashita N, Ohho A, Yamasaki A, Kurokawa M, Kotoh K, Kajiwara E. Hepatocarcinogenesis in chronic hepatitis C patients achieving a sustained virological response to interferon: significance of lifelong periodic cancer screening for improving outcomes. J Gastroenterol. 2014;49:1504–13.
Toyoda H, Kumada T, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, et al. Risk factors of hepatocellular carcinoma development in non-cirrhotic patients with sustained virologic response for chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2015;30:1183–9. This study showed that patients who achieve SVR are less likely to have sequelae of portal hypertension.
Chang KC, Tseng PL, Wu YY, Hung HC, Huang CM, Lu SN, et al. A polymorphism in interferon L3 is an independent risk factor for development of hepatocellular carcinoma after treatment of hepatitis C virus infection. Clin Gastroenterol Hepatol. 2015;13:1017–24.
Hung CH, Lee CM, Wang JH, Hu TH, Chen CH, Lin CY, et al. Impact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapy. Int J Cancer. 2011;128:2344–52.
Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–41.
Lee HW, Chon YE, Kim SU, Kim BK, Park JY, Kim DY, et al. Predicting liver-related events using transient elastography in chronic hepatitis C patients with sustained virological response. Gut Liver. 2015. doi:10.5009/gnl15021.
Taniki NEH, et al. The usefulness of non-invasive liver stiffness measurement by Fibroscan® for risk management of HCC in chronic hepatitis C patients who achieved sustained virological response (SVR). In: Boston, MA: AASLD; 2014.
Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44–57.
Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–96.
OPTN. Revised liver policy regarding HCC exception scores. 2015. https://optn.transplant.hrsa.gov/news/revised-liver-policy-regarding-hcc-exception-scores/.
Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–82.
Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7.
Shindoh J, Hasegawa K, Matsuyama Y, Inoue Y, Ishizawa T, Aoki T, et al. Low hepatitis C viral load predicts better long-term outcomes in patients undergoing resection of hepatocellular carcinoma irrespective of serologic eradication of hepatitis C virus. J Clin Oncol. 2013;31:766–73.
Liu WR, Tian MX, Jin L, Yang LX, Ding ZB, Shen YH, et al. High levels of hepatitis B surface antigen are associated with poorer survival and early recurrence of hepatocellular carcinoma in patients with low hepatitis B viral loads. Ann Surg Oncol. 2015;22:843–50.
Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Kinoshita H. Randomized clinical trial of long-term outcome after resection of hepatitis C virus-related hepatocellular carcinoma by postoperative interferon therapy. Br J Surg. 2002;89:418–22.
Ikeda K, Arase Y, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, et al. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor—a prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228–32.
Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–54.
Shiratori Y, Shiina S, Teratani T, Imamura M, Obi S, Sato S, et al. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299–306.
Ishikawa T, Higuchi K, Kubota T, Seki K, Honma T, Yoshida T, et al. Combination PEG-IFN a-2b/ribavirin therapy following treatment of hepatitis C virus-associated hepatocellular carcinoma is capable of improving hepatic functional reserve and survival. Hepatogastroenterology. 2012;59:529–32.
Singal AK, Freeman Jr DH, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;32:851–8. This meta-analysis was the most recent to look at secondary prevention of HCC. It looked at patients who received definitive treatment for HCC with either ablation or resection, showing that those who achieve SVR have fewer recurrences.
van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93.
McCombs J, Matsuda T, Tonnu-Mihara I, Saab S, Hines P, L’Italien G, et al. The risk of long-term morbidity and mortality in patients with chronic hepatitis C: results from an analysis of data from a Department of Veterans Affairs Clinical Registry. JAMA Intern Med. 2014;174:204–12.
Maylin S, Martinot-Peignoux M, Moucari R, Boyer N, Ripault MP, Cazals-Hatem D, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology. 2008;135:821–9.
Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnu L, Mazzella G, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–87.
Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–44. This study evaluated patients in the HALT-C trial and showed that those who achieved SVR had a lower rate of liver transplantation and overall mortality.
Lens S, Rincon D, Garcia-Retortillo M, Albillos A, Calleja JL, Banares R, et al. Association between severe portal hypertension and risk of liver decompensation in patients with hepatitis C, regardless of response to antiviral therapy. Clin Gastroenterol Hepatol. 2015;13:1846–53. e1841.
Hsu YC, Ho HJ, Huang YT, Wang HH, Wu MS, Lin JT, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495–503.
AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed January 12, 2016.
Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815–20.
Horster S, Bauerlein FJ, Mandel P, Raziorrouh B, Hopf C, Stemmler HJ, et al. Influence of hepatitis C virus infection and high virus serum load on biliary complications in liver transplantation. Transpl Infect Dis. 2013;15:306–13.
Everson GT, Terrault NA, Lok AS, del Rodrigo R, Brown Jr RS, Saab S, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57:1752–62.
Vargas HE, Laskus T, Wang LF, Lee R, Radkowski M, Dodson F, et al. Outcome of liver transplantation in hepatitis C virus-infected patients who received hepatitis C virus-infected grafts. Gastroenterology. 1999;117:149–53.
Renz JF, Kin C, Kinkhabwala M, Jan D, Varadarajan R, Goldstein M, et al. Utilization of extended donor criteria liver allografts maximizes donor use and patient access to liver transplantation. Ann Surg. 2005;242:556–63. discussion 563–555.
Curry MP, Forns X, Chung RT, Terrault NA, Brown Jr R, Fenkel JM, et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2015;148:100–7. This study is the only study looking at the effectiveness of the new direct-acting antiviral in patients with hepatocellular carcinoma.
Najafzadeh M, Andersson K, Shrank WH, Krumme AA, Matlin OS, Brennan T, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407–19.
Verla-Tebit E, Rahma OE. Regression of hepatocellular carcinoma after treatment of hepatitis C: a case report. J Gastrointest Oncol. 2015;6:E52–4.
Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer. 2013;4:342–59.
ASCO. Nivolumab in HCC and pembrolizumab in tumors with mismatch repair deficiency. In: ASCO Daily News; 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Stacey Prenner declares that she has no conflicts of interest. Laura Kulik reports advisory board membership with Gilead.
Human and Animal Rights and Informed Consent
With regard to the authors’ research cited in this paper, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. In addition, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
This article is part of the Topical Collection on Management of the Cirrhotic Patient
Rights and permissions
About this article
Cite this article
Prenner, S., Kulik, L. HCC: Where Does HCV Therapy Play a Role?. Curr Hepatology Rep 15, 17–25 (2016). https://doi.org/10.1007/s11901-016-0292-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-016-0292-z