Abstract
Background
The introduction of capsule endoscopy (CE) has facilitated the detection of mucosal changes in the small bowel, and such mucosal changes have been noted in cirrhotic patients with portal hypertension; these changes are described as portal hypertensive enteropathy. The aim of this study was to assess the effects of transjugular intrahepatic portosystemic shunt (TIPS) on the small bowel mucosal changes detected by CE in cirrhotic patients with portal hypertension.
Methods
TIPS was performed in fifteen cirrhotic patients with portal hypertension. All patients underwent CE before and 2 weeks after TIPS. The small bowel mucosal changes were defined as edema, angiodysplasia-like lesions, red spots, and small bowel varices. Changes in the portosystemic pressure gradient (PSG) and CE findings were evaluated.
Results
Before TIPS, small bowel edema was detected in all 15 patients, angiodysplasia-like lesions in 7, and red spots in 14 patients. The PSG decreased significantly, from 21.2 ± 2.6 before TIPS to 8.9 ± 3.3 mmHg (p < 0.001) after the procedure. After TIPS, the small bowel edema was attenuated in 8 of the 15 patients. In two patients with angiodysplasia-like lesions and 4 with red spots, these lesions were attenuated after TIPS. The average score for small bowel edema and the grade of red spots were reduced significantly after TIPS (2.3 ± 0.7–1.8 ± 0.6, p < 0.005 and 1.6 ± 0.9–1.3 ± 0.7, p < 0.05, respectively). Small bowel varices were seen in 4 patients before TIPS and all these varices disappeared after TIPS.
Conclusions
In cirrhotic patients with portal hypertension, small bowel edema, red spots, and small bowel varices were attenuated after TIPS. Portal hypertension may be an important factor in the development of small bowel mucosal changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of capsule endoscopy (CE) has facilitated the detection of mucosal changes in the small bowel [1]. Previous studies have shown that mucosal changes can occur in the small bowel of cirrhotic patients with portal hypertension; these changes are described as portal hypertensive enteropathy (PHE) [2–5]. However, the relationship between CE findings of the small bowel and portal venous pressure in cirrhotic patients with portal hypertension has not been studied.
Transjugular intrahepatic portosystemic shunt (TIPS) is a non-operative technique that reduces portal venous pressure and is used for the treatment of portal hypertension. Accordingly, TIPS has been reported recently to be effective in the treatment of cirrhotic patients with refractory ascites and esophageal or gastric variceal bleeding that is unresponsive to endoscopic and pharmacological treatments [6, 7]. In addition, it has been reported that TIPS is effective for the treatment of portal hypertensive gastropathy (PHG) [8, 9]. However, there has been only one case report describing the treatment of PHE by TIPS [10].
In this study, we evaluated the effects of portal venous pressure decompression brought about by TIPS on the small bowel mucosal changes detected by CE in cirrhotic patients with portal hypertension.
Methods
Patients
Between September 2009 and June 2011, 24 consecutive patients with cirrhosis and portal hypertension underwent TIPS and were considered for inclusion in this study. The diagnosis of cirrhosis was made on the basis of laboratory and ultrasonographic findings or transjugular liver biopsy. Portal hypertension was defined as a hepatic venous pressure gradient of more than 10 mmHg, as defined by the Baveno III criteria [11]. Exclusion criteria were a history of abdominal surgery, episodes of chronic hepatic encephalopathy, hepatocellular carcinoma or other malignancy, portal vein thrombosis, active infection, shunt dysfunction at 2 weeks after TIPS, severe cardiac or pulmonary disease, organic renal disease (urinary protein level >500 mg/24 h, active sediment, or small kidneys on ultrasonography), and failure to obtain consent.
Of the 24 patients, 9 were excluded because of a history of abdominal surgery (n = 2), colon cancer (n = 1), portal vein thrombosis (n = 1), active infection (n = 1), shunt dysfunction at 2 weeks after TIPS (n = 1), failure to obtain consent (n = 2), or death during the evaluation period (n = 1). The remaining 15 patients were included in the study.
No nonsteroidal anti-inflammatory drugs were given during the study. In addition, none of the patients received vasoactive drugs such as beta-blockers, nitrates, calcium-channel blockers, angiotensin II type 1-receptor blockers, or angiotensin-converting enzyme inhibitors. Diuretics were given for the treatment of ascites and pleural effusion.
Study protocol
All patients underwent CE within 3 days prior to and 2 weeks after TIPS. We used the Given PillCam SB2 video capsule system (Given Imaging, Yoqneam, Israel) for CE examination. The CE procedure and methodology for review of images were conducted as described previously [12]. The locations of the duodenum, jejunum, and ileum were estimated with the localization software provided in the CE workstation. The site of the duodenum was judged from estimates of the position of the ligament of Treitz. Based on the transit time of the CE from the last image of the ligament of Treitz to the first image of the cecum, we estimated the jejunum to occupy 40 % of the proximal side, and the ileum 60 % of the distal side of the remaining section of the small bowel. In cases where the CE failed to reach the cecum but reached the terminal ileum, the localization software was used to estimate the position of the last image. Patients whose CE did not reach the terminal ileum were excluded from the study.
The TIPS procedure was performed as described previously [13]. After the TIPS tract was created, an expandable stent (Wallstent; Boston Scientific, Natick, MA, USA) was placed and dilated to obtain a portosystemic pressure gradient (PSG) below 12 mmHg, because variceal hemorrhage and ascites rarely develop below this threshold [14]. To avoid a marked reduction in PSG, which may lead to an increased risk of hepatic encephalopathy [14, 15], the stent was dilated initially to 6 or 8 mm in diameter. If the PSG remained above 12 mmHg, the stent was dilated further to 8 or 10 mm. The PSG was calculated as the portal venous pressure minus the inferior vena cava pressure. A covered stent was not used because such stents were not available in Japan. Color Doppler ultrasonography was performed before and 2 weeks after TIPS to assess shunt function. Angiography was performed if shunt dysfunction was suspected by color Doppler ultrasonography. Shunt dysfunction was defined by angiographic findings of shunt stenosis with an increase in the PSG to greater than 12 mmHg. Patients received lactulose to ensure a few soft bowel movements per day to prevent hepatic encephalopathy.
Measurements of serum total bilirubin, serum albumin, prothrombin time, and serum ammonia were performed before and 2 and 4 weeks after TIPS.
This study was approved by the Nippon Medical School Ethics Committee. Written informed consent was obtained from each patient prior to their undergoing the procedure.
CE findings of small bowel mucosal changes
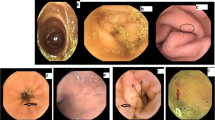
According to a previous report [2], PHE was defined as small bowel mucosal changes with the following findings: edema, angiodysplasia-like lesions, and red spots (Fig. 1). We included isolated erythema in the category of red spots. We also determined the location of lesions in each anatomical area of the small bowel.
The presence of small bowel edema was evaluated by CE and scored for each area of the small bowel. The scoring of small bowel edema was as follows: score of 0, no edema; score of 1, presence of edema. The edema score was the sum of the duodenum, jejunum, and ileum edema scores.
The numbers of angiodysplasia-like lesions and red spots were counted for all areas of the small bowel. The grade of angiodysplasia-like lesions and red spots was assessed as follows: grade 0 (none, score 0), no lesions; grade 1 (mild, score 1), the number of lesions ranged from 1 to 4; grade 2 (moderate, score 2), the number of lesions ranged from 5 to 20; and grade 3 (severe, score 3), the number of lesions was greater than 20.
Small bowel varices were evaluated for their presence or absence, and their location was identified.
Statistical analysis
Data are expressed as means ± standard deviation. The Wilcoxon test was used to compare differences within the patient group. Correlation was analyzed by the Spearman rank correlation test. A two-tailed p value of 0.05 was considered statistically significant.
Results
Patients
The clinical characteristics of the 15 cirrhotic patients with portal hypertension are shown in Table 1. Esophageal and gastric varices were detected in 14 and 4 patients, respectively. Twelve patients received endoscopic sclerotherapy and/or band ligation before TIPS. PHG, as defined by the New Italian Endoscopic Club [16], was seen in 8 patients. Eleven patients had moderate to severe ascites and were treated with diuretics and therapeutic paracentesis with albumin prior to the TIPS treatment. One patient had required diuretics and repeated thoracentesis with albumin for the control of symptoms prior to the TIPS placement. Diuretics were given in 13 patients before TIPS, and the doses of diuretics were decreased in 8 patients and remained unchanged in 5 patients 2 weeks after TIPS. TIPS was performed for refractory ascites, as defined by the International Ascites Club [17], in11 patients, and it was performed for refractory hepatic hydrothorax in 1 patient, esophageal variceal bleeding unresponsive to endoscopic and pharmacological treatments in 2 patients, and bleeding from PHE unresponsive to pharmacological treatments in 1 patient.
TIPS outcomes
The stent diameters were 6, 8, and 10 mm in 3, 11, and 1 patient, respectively. A value of PSG below 12 mmHg after TIPS was achieved in 14 patients. The PSG decreased significantly after TIPS, from 21.2 ± 2.6 to 8.9 ± 3.3 mmHg (p < 0.001) (Fig. 2). One patient experienced an extrahepatic puncture related to TIPS insertion, but this was not serious. The eleven patients with refractory ascites and one patient with refractory hepatic hydrothorax did not require paracentesis and thoracentesis after TIPS. In the 2 patients with esophageal variceal bleeding and the one patient with PHE bleeding, bleeding episodes were not observed after TIPS.
Changes in CE findings
CE reached the terminal ileum in all patients. As shown in Table 2, small bowel edema was seen in all patients before TIPS. The small bowel edema was attenuated in 8 of the 15 patients after TIPS. There was a significant difference in the average edema score between before and after TIPS (2.3 ± 0.7–1.8 ± 0.6, p < 0.005) in 15 patients. In 4 of the 9 patients with PSG less than 10 mmHg after TIPS, small bowel edema was attenuated. There was a significant difference in the average edema score between before and after TIPS (2.3 ± 0.7–1.9 ± 0.6, p < 0.05) in these 9 patients. Angiodysplasia-like lesions were seen in 7 patients before TIPS, and were graded as 1 in 5 patients and 2 in 2 patients. After TIPS, in the 2 patients with grade 2 angiodysplasia-like lesions, the grade was reduced, whereas in the 5 patients with grade 1 angiodysplasia-like lesions the lesions were unchanged. There was no significant difference in the mean grade of angiodysplasia-like lesions between before and after TIPS (0.6 ± 0.7–0.5 ± 0.5, p = 0.157) in the cohort as a whole. In the 9 patients with PSG less than 10 mmHg after TIPS, grade 2 angiodysplasia-like lesions showed a reduced grade in one patient, whereas the grades of angiodysplasia-like lesions in the other patients were unchanged after TIPS. There was no significant difference in the mean grade of angiodysplasia-like lesions between before and after TIPS (0.7 ± 0.7–0.6 ± 0.5, p = 0.319) in these 9 patients. Red spots were seen before TIPS in 14 patients and were graded as 1, 2, and 3 in 7, 4, and 3 patients, respectively. The grade improved after TIPS in 2 patients with grade 2 red spots and 2 patients with grade 3 red spots, whereas the grades of red spots in the other patients were unchanged. There was a significant difference in the mean grade of red spots between before and after TIPS (1.6 ± 0.9–1.3 ± 0.7, p < 0.05) in 15 patients. In the 9 patients with PSG less than 10 mmHg after TIPS, the grade of red spots improved after TIPS in one patient with grade 3 red spots, whereas the grades of red spots in the other patients were unchanged. There was no significant difference in the mean grade of red spots between before and after TIPS (1.6 ± 1.0–1.4 ± 0.9, p = 0.319) in these 9 patients. Small bowel varices were seen in 4 patients (all ileal varices) before TIPS and all varices disappeared after TIPS. Two of the 4 patients with small bowel varices showed a decreased PSG to less than 10 mmHg after TIPS. There were no significant correlations between the changes in edema score, grade of angiodysplasia-like lesions, grade of red spots, or grade of small bowel varices and the changes in PSG. In one patient (patient 1, numbered in Table 2) with repeated bleeding owing to red spots in the small bowel unresponsive to pharmacological treatments, the hemorrhage stopped after TIPS (Fig. 3). The small bowel mucosal changes did not worsen in any patients after TIPS.
A 57-year-old man (patient 1) showed tarry stool and anemia requiring multiple blood transfusions. Upper gastrointestinal endoscopy showed diffuse red spots in the duodenum but no esophagogastric varices and no portal hypertensive gastropathy. Colonoscopy revealed small rectal varices. However, there was no evidence of active bleeding from these lesions. To rule out any other cause of anemia, we performed a capsule endoscopy (CE) that showed active oozing of blood from the red spots on the jejunal mucosa. Because of the patient’s peripheral vascular disease, we could not use beta-blockers and performed transjugular intrahepatic portosystemic shunt (TIPS). The portosystemic pressure gradient decreased from 21 to 6 mmHg after TIPS. Anemia and bleeding episodes were not observed after TIPS and no further blood transfusion was necessary. a CE showed diffuse red spots on the duodenal and jejunal mucosa with active oozing of blood before TIPS. b CE showed a decrease in the diffuse red spots 14 days after TIPS
Changes in liver function test results
Differences in liver function test results before and after TIPS are shown in Table 3. Serum total bilirubin had increased significantly 4 weeks after TIPS, whereas the prothrombin time had increased significantly 2 and 4 weeks after TIPS. There was no significant difference in the concentrations of serum albumin before and after TIPS. No patients had hepatic encephalopathy before TIPS. Although serum ammonia showed a significant increase after TIPS, no patients had hepatic encephalopathy 2 and 4 weeks after TIPS. Deterioration of these liver function test results did not affect small bowel mucosal changes.
Discussion
This is the first study demonstrating the effects of portal venous pressure decompression by TIPS on the small bowel mucosal changes detected by CE in cirrhotic patients with portal hypertension.
In this study, TIPS decreased portal venous pressure, and small bowel edema was attenuated in 8 of the 15 (53 %) patients after TIPS. It has been suggested that the presence of small bowel edema is strongly correlated with high portal venous pressure [18]. Higaki et al. [19] reported that small bowel edema, which was described in their study as a ‘herring roe’ appearance, was seen by double-balloon endoscopy in 8 (38 %) patients. The biopsy specimens they obtained from small bowel edema had edema of the lamina propria and submucosa. They also showed that serum albumin (which was unchanged after TIPS in our study) was not associated with small bowel edema, but that the degree of portal hypertension may be associated with the presence of this lesion. These findings indicate that portal hypertension may be related to the development of small bowel edema. As shown in Table 2, small bowel edema was attenuated in 8 of our 14 (57 %) patients with PSG less than 12 mmHg after TIPS. A PSG value below 12 mmHg may be necessary to attenuate small bowel edema in cirrhotic patients with portal hypertension.
There are controversial reports in the literature linking the presence of angiodysplasia-like lesions and/or red spots and portal hypertension. One case report showed that these lesions were a complication of portal hypertension and were attenuated by TIPS [10]. In contrast, another study did not find a correlation between the presence of angiodysplasia-like lesions and/or red spots and high portal venous pressure [18]. In our study, 2 of the 7 (29 %) patients with angiodysplasia-like lesions and 4 of the 14 (29 %) patients with red spots showed improved findings after TIPS. However, there was a no significant reduction in the mean grade of angiodysplasia-like lesions between before and after TIPS. We think that portal hypertension may be partly related to the development of these lesions in the small bowel.
In our study, 53 % of the patients with small bowel edema and 29 % of the patients with angiodysplasia-like lesions and red spots showed improved findings after TIPS. Although there was a significant reduction of the portal venous pressure after TIPS, the rate of favorable mucosal changes seems to be relatively low. One possible explanation for this may be the evaluation time after TIPS. There have been no reports about the evaluation time after TIPS for PHE. In previous studies that demonstrated the efficacy of TIPS for PHG, PHG was evaluated 2–4 weeks after TIPS [8, 9]. We think that assessing the efficacy of TIPS on small bowel mucosal changes should not be done too soon after TIPS. Therefore, the evaluation of small bowel mucosal changes was performed 2 weeks after TIPS in the present study. It may be necessary to evaluate the CE findings with a more extensive follow up. Another possible explanation for the relatively low rate of favorable mucosal changes seen in our study may be the contribution of factors other than portal venous pressure. In our study, there were no significant correlations between the changes in CE findings and the changes in portal venous pressure. It has been suggested that factors independent of portal hypertension, such as gastric mucosal blood flow and other local factors (overproduction of nitric oxide, oxygen free radicals, endothelin-1, tumor necrosis factor alpha, and prostaglandins) may also be involved in the pathogenesis of PHG [20].
In our study, small bowel varices (all ileal varices) were seen in 4 patients before TIPS and all had disappeared after TIPS. TIPS has been reported to be effective for the treatment of bleeding from small bowel varices [21, 22], although none of our patients had bleeding from their small bowel varices. Therefore, we think that portal hypertension may be an important factor in the development and bleeding of small bowel varices.
Before TIPS, we detected small bowel edema in all (100 %) of our patients, and angiodysplasia-like lesions were detected in 7 (47 %), red spots in 14 (93 %), and small bowel varices in 4 (27 %). It has been reported that, based on findings from CE, cirrhotic patients with portal hypertension had mucosal inflammatory-like abnormalities, including small bowel edema in 22–42 %, angiodysplasia-like lesions in 24–52 %, red spots in 55–62 %, and small bowel varices in 8–16 % [2, 4]. The high portal venous pressure in our study may be associated with the high rate of small bowel edema, because a previous study reported a correlation between the presence of small bowel edema and high portal venous pressure [18]. The reason for the high proportion of patients with red spots in our study is not clear. Two studies reported that cirrhotic patients with small bowel mucosal lesions had a high Child-Pugh score [2, 4]. The percentage of Child-Pugh class C patients (40 %) in our study was higher than the percentages in those two studies (3–27 %) [2, 4]. Therefore, the high Child-Pugh score in our study may be associated with the proportion of patients with small bowel mucosal lesions.
In the present study, one patient had repeated bleeding owing to small bowel mucosal changes. Akyuz et al. [5] recently reported that 21 of 444 (5 %) patients with portal hypertension had obscure bleeding that could not be explained by other portal hypertensive bleeding. Of these 21 patients, only one had active bleeding from small bowel mucosal changes. Although such bleeding seems to be rare, these lesions are a possible cause of bleeding.
We had one patient with PHE bleeding and the hemorrhage stopped after TIPS. To the best of our knowledge, there has been only one case report showing that TIPS was effective for the treatment of PHE bleeding unresponsive to pharmacological treatments [10]. Although there is not enough evidence to support the use of TIPS in PHE bleeding, TIPS may become one of the treatment options for refractory PHE bleeding.
Unfortunately, classification of the CE appearance of the small bowel mucosal changes in patients with portal hypertension is poor and consensus is generally lacking, resulting in a wide range of reported prevalence and making comparisons between studies difficult. De Palma et al. [2] proposed that PHE should be classified into two grades: mucosal inflammatory-like abnormalities (edema, erythema, granularity, and friability) and vascular lesions (cherry-red spots, telangiectasias or angiodysplasia-like lesions, and varices). However, we think that PHE should not include small bowel varices because PHG does not include gastric varices. Therefore, we defined PHE as small bowel mucosal changes with edema, angiodysplasia-like lesions, and red spots (excluding varices) based on their classification. It is necessary to develop a definitive classification of the CE appearance of PHE.
In conclusion, in cirrhotic patients with portal hypertension, small bowel edema, red spots, and small bowel varices were attenuated after TIPS. Portal hypertension may be an important factor in the development of small bowel mucosal changes. Because TIPS reduces portal venous pressure, it may become accepted as one of the treatment options for refractory PHE bleeding. Further studies should be carried out in larger numbers of patients to clarify the efficacy of this treatment on small bowel mucosal changes.
References
Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417.
De Palma GD, Rega M, Masone S, Persico F, Siciliano S, Patrone F, et al. Mucosal abnormalities of the small bowel in patients with cirrhosis and portal hypertension: a capsule endoscopy study. Gastrointest Endosc. 2005;62:529–34.
Figueiredo P, Almeida N, Lerias C, Lopes S, Gouveia H, Leitao MC, et al. Effect of portal hypertension in the small bowel: an endoscopic approach. Dig Dis Sci. 2008;53:2144–50.
Abdelaal UM, Morita E, Nouda S, Kuramoto T, Miyaji K, Fukui H, et al. Evaluation of portal hypertensive enteropathy by scoring with capsule endoscopy: is transient elastography of clinical impact? J Clin Biochem Nutr. 2010;47:37–44.
Akyuz F, Pinarbasi B, Ermis F, Uyanikoglu A, Demir K, Ozdil S, et al. Is portal hypertensive enteropathy an important additional cause of blood loss in portal hypertensive patients? Scand J Gastroenterol. 2010;45:1497–502.
Boyer TD, Haskal ZJ. American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386–400.
Garcia-Tsao G. Portal hypertension. Curr Opin Gastroenterol. 2006;22:254–62.
Urata J, Yamashita Y, Tsuchigame T, Hatanaka Y, Matsukawa T, Sumi S, et al. The effects of transjugular intrahepatic portosystemic shunt on portal hypertensive gastropathy. J Gastroenterol Hepatol. 1998;13:1061–7.
Mezawa S, Homma H, Ohta H, Masuko E, Doi T, Miyanishi K, et al. Effect of transjugular intrahepatic portosystemic shunt formation on portal hypertensive gastropathy and gastric circulation. Am J Gastroenterol. 2001;96:1155–9.
Pezzoli A, Fusetti N, Simone L, Zelante A, Cifala V, Carella A, et al. Portal hypertensive enteropathy diagnosed by capsule endoscopy and demonstration of the ileal changes after transjugular intrahepatic portosystemic shunt placement: a case report. J Med Case Rep. 2011;5:90.
de Franchis R. Updating consensus in portal hypertension: report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol. 2000;33:846–52.
Delvaux M, Gay G. Capsule endoscopy: technique and indications. Best Pract Res Clin Gastroenterol. 2008;22:813–37.
Rösch J, Keller FS. Transjugular intrahepatic portosystemic shunt: present status, comparison with endoscopic therapy and shunt surgery, and future prospectives. World J Surg. 2001;25:337–45.
Casado M, Bosch J, Garcia-Pagan JC, Bru C, Banares R, Bandi JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296–303.
Thalheimer U, Leandro G, Samonakis DN, Triantos CK, Senzolo M, Fung K, et al. TIPS for refractory ascites: a single-centre experience. J Gastroenterol. 2009;44:1089–95.
Spina GP, Arcidiacono R, Bosch J, Pagliaro L, Burroughs AK, Santambrogio R, et al. Gastric endoscopic features in portal hypertension: final report of a consensus conference, Milan, Italy, September 19, 1992. J Hepatol. 1994;21:461–7.
Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–66.
Takahashi Y, Fujimori S, Narahara Y, Gudis K, Ensaka Y, Kosugi Y, et al. Small intestinal edema had the strongest correlation with portal venous pressure amongst capsule endoscopy findings. Digestion. 2012;86:48–54.
Higaki N, Matsui H, Imaoka H, Ikeda Y, Murakami H, Hiasa Y, et al. Characteristic endoscopic features of portal hypertensive enteropathy. J Gastroenterol. 2008;43:327–31.
Cubillas R, Rockey DC. Portal hypertensive gastropathy: a review. Liver Int. 2010;30:1094–102.
Vidal V, Joly L, Perreault P, Bouchard L, Lafortune M, Pomier-Layrargues G. Usefulness of transjugular intrahepatic portosystemic shunt in the management of bleeding ectopic varices in cirrhotic patients. Cardiovasc Intervent Radiol. 2006;29:216–9.
Haskal ZJ, Scott M, Rubin RA, Cope C. Intestinal varices: treatment with the transjugular intrahepatic portosystemic shunt. Radiology. 1994;191:183–7.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsushita, Y., Narahara, Y., Fujimori, S. et al. Effects of transjugular intrahepatic portosystemic shunt on changes in the small bowel mucosa of cirrhotic patients with portal hypertension. J Gastroenterol 48, 633–639 (2013). https://doi.org/10.1007/s00535-012-0660-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-012-0660-6