Abstract
Background
Metabolic syndrome, which includes obesity, hyperglycemia, dyslipidemia, and hypertension, is a major risk factor for the development of nonalcoholic fatty liver disease (NAFLD). Cigarette smoking is a well-known risk factor for metabolic syndrome, but the epidemiological impact of cigarette smoking on development of NAFLD is unclear.
Methods
In this retrospective study, 2,029 subjects underwent a complete medical health checkup in 1998 and again in 2008. Those who were positive for hepatitis B surface antigen or hepatitis C virus antibody, or had an alcohol intake of >20 g/day as assessed by questionnaire, were excluded. Fatty liver was diagnosed by abdominal ultrasonography. Independent risk factors associated with the development of NAFLD were determined by multiple logistic regression analysis. Smoking status was expressed using the Brinkman index (BI), which was calculated as the number of cigarettes smoked per day multiplied by the number of years of smoking.
Results
Of 1,560 subjects without NAFLD in 1998, 266 (17.1%) were newly diagnosed with NAFLD in 2008. Multiple logistic analysis identified age [adjusted odds ratio (AOR) 0.95, 95% confidence interval (95% CI) 0.94–0.97], male sex (AOR 1.46, 95% CI 1.01–2.10), body mass index ≥25 (AOR 3.08, 95% CI 2.20–4.32), dyslipidemia (AOR 1.79, 95% CI 1.25–2.58) and cigarette smoking (AOR 1.91, 95% CI 1.34–2.72) as risk factors associated with the development of NAFLD. Smoking status at baseline was also associated with the development of NAFLD (BI 1–399: AOR 1.77, 95% CI 1.02–3.07, BI ≥400: AOR 2.04, 95% CI 1.37–3.03).
Conclusion
Cigarette smoking is an independent risk factor for onset of NAFLD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a liver disorder characterized by fatty changes of the liver with no apparent history of habitual alcohol intake. NAFLD was initially considered to be a reversible chronic liver disease with a favorable prognosis. However, some NAFLD patients show evidence of nonalcoholic steatohepatitis (NASH), which may progress to hepatic cirrhosis and hepatocellular carcinoma, with a resultant unfavorable prognosis [1, 2]. There are also racial differences in the prevalence of NAFLD. In Japan, the prevalence is reported to be 9–30% [3]. The prevalence of visceral fat-type obesity is high in Asian populations, and this may lead to insulin resistance and an increased incidence of NAFLD, even though the body mass index (BMI) of Asians is generally lower than that of African-Americans and Caucasians [4–6]. A high rate of NAFLD also occurs concomitantly with obesity, hyperglycemia, dyslipidemia, and hypertension (collectively referred to as metabolic syndrome) [3, 4, 7–9], but few large-scale long-term studies of the risk factors involved in the development of NAFLD have been reported.
Cigarettes contain more than 4,000 toxic chemicals, including tar, nicotine, and carbon monoxide. Cigarette smoking is a risk factor for the prevalence of and mortality from malignant cancers such as lung and esophageal cancers, lung diseases such as chronic obstructive pulmonary disease (COPD), and circulatory diseases [10–12]. An association of cigarette smoking with risk factors for NAFLD, such as insulin resistance, diabetes, and dyslipidemia, has also been reported [13–18]. However, a large-scale long-term study of the association between cigarette smoking and NAFLD has not been performed. Therefore, in this study, we investigated the factors involved in the development and cure of NAFLD in a follow-up study of a 10-year interval, and examined the association of smoking with the development of NAFLD.
Subjects and methods
Study design
We designed a retrospective follow-up study of a 10-year interval to investigate the effects of cigarette smoking on the development or cure of NAFLD. A total of 3,365 subjects underwent a complete medical health checkup including abdominal ultrasonography at the Kagoshima Kouseiren Medical Healthcare Center in both 1998 and 2008. Subjects positive for hepatitis B virus surface antigen (HBsAg) and hepatitis C virus antibody (HCV Ab) and those who did not undergo virus marker measurements were excluded. Alcohol intake was investigated by questionnaire, and the ethanol equivalent of alcohol consumption per day was calculated from the frequency of alcohol intake per month. Subjects who drank >20 g/day of ethanol were excluded from the study.
The diagnosis of fatty liver was based on the results of abdominal ultrasonography, which was performed by trained technicians. Fatty liver was diagnosed when hepatorenal echo contrast and liver brightness were observed [19, 20]. The diagnosis of fatty liver was subsequently confirmed by a specialist physician independently without reference to other data.
Although abdominal obesity (abdominal circumference >85 cm for men and >90 cm for women) is a necessary variable according to the Japanese criteria for diagnosing metabolic syndrome [21], waist measurements were not available for all the subjects in this study. In addition, a BMI of ≥25 has been proposed as a cutoff for the diagnosis of obesity in Asian people [19–22]. Therefore, we defined obesity as a BMI ≥25 and included it as one of the metabolic syndrome risk factors in this study. BMI was calculated by dividing body weight (kg) by the square of height (m2). Patients with hypertension were defined as those with a systolic blood pressure of ≥130 mmHg, those with a diastolic blood pressure of ≥85 mmHg, or those who were undergoing medical treatment for hypertension in 1998. Patients with dyslipidemia were defined as those with triglycerides of ≥150 mg/dL, those with HDL <40 IU/L, or those who were undergoing medical treatment of dyslipidemia in 1998. Patients with dysglycemia, including diabetes mellitus, were defined as those who had a fasting plasma glucose of ≥110 mg/dL or who were under medical treatment for diabetes in 1998. Thus, hypertension, dyslipidemia and dysglycemia were defined as risk factors for metabolic syndrome in this study according to the Japanese criteria for diagnosing this disorder [20, 21].
Cigarette smoking was investigated by questionnaire, and the Brinkman index (BI) was calculated as the number of cigarettes smoked per day multiplied by the number of years that the subjects had smoked. Subjects who stopped smoking before 1998 (former smokers) or started smoking after 1998 (new smokers) were classified as nonsmokers in 1998, and those who stopped smoking after 1998 and before 2008 (new quitters) were classified as smokers in 1998. We performed further subanalysis using two groups (subjects who continued to smoke between 1998 and 2008, and those who did not smoke at all during this time) or three groups (the previous two groups in addition to new quitters). For alcohol consumption per day, the subjects were divided into 2 groups: those who did not drink alcohol (consumption 0 g/day) and light drinkers (mean consumption ≤20 g/day).
The study was approved by the ethics committees of the Kagoshima Prefectural Federation of Agricultural Cooperatives for Health and Welfare and the Kagoshima University Graduate School of Medical and Dental Sciences.
Statistical analysis
All analyses were performed using SPSS v.18 (SPSS, Inc., Chicago, IL, USA), with the significance level set at <5%. Continuous variables are shown as mean ± standard deviations (SD). Between-group comparison was performed by unpaired t test and Fisher’s exact test. Potential factors involved in the development or cure of NAFLD were analyzed by logistic regression analysis. Unadjusted and adjusted odds ratios (OR) and 95% confidence interval (95% CI) were calculated.
Results
Subjects’ baseline characteristics in 1998
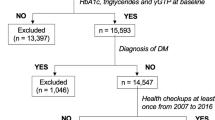
Of the initial 3,365 subjects, 76 were positive for HBsAg, 90 were positive for HCV Ab, and 2 were positive for both. Four hundred thirty-nine subjects were not tested for HBsAg or HCV Ab. In addition, 729 subjects drank >20 g/day of ethanol. On the basis of these data, 2,029 subjects were eligible for the study.
In 1998, 469 subjects (342 men and 127 women) and 1,560 subjects (772 men and 788 women) were included in the NAFLD and non-NAFLD groups, respectively. There was a significantly higher number of men in the NAFLD group, and the mean age in the NAFLD group was significantly lower than that in the non-NAFLD group (Table 1). The frequencies of obesity (BMI ≥25), hypertension, dyslipidemia, dysglycemia including diabetes mellitus, current cigarette smoking, and light alcohol drinkers were significantly higher in the NAFLD group compared to the non-NAFLD group (Table 1).
Comparison of subjects who developed NAFLD with non-NAFLD subjects
Two hundred sixty-six (17.1%) patients from the non-NAFLD group in 1998 were newly diagnosed with NAFLD in 2008 (164 men 21.2%, 102 women 12.9%) (Fig. 1). The baseline characteristics in 1998 were compared between the new-NAFLD and non-NAFLD groups. Age, frequency of male gender, obesity, dyslipidemia, and cigarette smoking differed significantly between the two groups (Table 2). These factors also had an independent association with NAFLD development (all subjects in Table 3), indicating that smokers were likely to develop NAFLD.
Furthermore, in a limited group of subjects that excluded former smokers (before 1998), new smokers after 1998 and those who quit between 1998 and 2008 (new quitters), cigarette smoking tended to be a risk factor for NAFLD development [adjusted odds ratio (AOR) 1.44, 95% CI 0.86–2.42 among the limited group of subjects in Table 3].
Association between cigarette smoking and the development of NAFLD
The association between smoking patterns and NAFLD was analyzed by classifying the subjects into 3 groups: BI = 0 (non-smokers), BI = 1–399, and BI ≥400. Of the 1,553 subjects in the non-NAFLD group in 1998 (excluding 7 subjects whose BI was not calculated because of lack of data), the risk of developing NAFLD correlated positively with BI in multivariate analysis adjusted for age, sex, obesity, hypertension, dyslipidemia, dysglycemia, and alcohol intake (Table 4).
Association of metabolic syndrome risk factors and cigarette smoking with NAFLD development
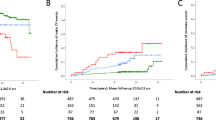
The association of obesity, hypertension, dyslipidemia, and dysglycemia (four metabolic syndrome risk factors) with the incidence of NAFLD was analyzed. As shown in Fig. 2, the incidence of NAFLD was 13.2% in subjects with no metabolic syndrome risk factors, and 19.0, 22.5, and 24.7% in those with 1, 2 and ≥3 factors, respectively. The risk of NAFLD was significantly correlated with number of metabolic syndrome risk factors (Table 5). Cigarette smoking at baseline was also found to be an independent risk factor for NAFLD development in this model (all subjects in Table 5).
Frequency of the development of nonalcoholic fatty liver disease (NAFLD) for subjects with different numbers of metabolic syndrome risk factors in smokers and nonsmokers. The incidence of NAFLD was higher in smokers than in nonsmokers. The incidence of NAFLD in smokers with one or more metabolic syndrome risk factor was higher than in those with none, but did not differ among those with 1, 2, or ≥3 factors. * Metabolic syndrome risk factors are obesity, hypertension, dyslipidemia and dysglycemia, as defined in “Subjects and methods”
The incidence of NAFLD increased as the number of metabolic syndrome risk factors increased in nonsmokers (Fig. 2). In contrast, the incidence in smokers with one or more metabolic syndrome risk factors (≥35%) was higher than in those with none, but did not differ among those with 1, 2, or ≥3. The incidence of NAFLD was significantly higher in smokers than in nonsmokers, regardless of the number of metabolic syndrome risk factors.
Furthermore, in the limited group of subjects including continuing smokers and nonsmokers only, cigarette smoking at baseline was a risk factor for NAFLD development, although it did not achieve statistical significance (<0.05) in multivariate analysis (AOR 1.64, 95% CI 0.99–2.72 among the limited group of subjects in Table 5).
Comparison of subjects in whom NAFLD was cured with those with persistent NAFLD
Of the 469 subjects in the NAFLD group in 1998, NAFLD was cured in 170 (36.2%) in 2008 (116 men 33.9%, 54 women 42.5%) (Fig. 1). A comparison of baseline characteristics in 1998 between the NAFLD-cured and NAFLD-persistent groups showed a significant difference in age, but not in sex, obesity, hypertension, dyslipidemia, dysglycemia, cigarette smoking, or light alcohol intake (Table 2). In multivariate analysis using these factors, only age had an independent association with cure of NAFLD. The frequency of NAFLD cure was 31.7% in subjects with no metabolic syndrome risk factors, compared with 36.1, 42.7, and 31.4% in those with 1, 2 and ≥3 factors, respectively, showing no association between NAFLD cure and the number of metabolic syndrome risk factors (P = 0.21).
Influence of smoking cessation on NAFLD status or NAFLD development
The association between smoking cessation and risk of NAFLD was analyzed using subjects who never smoked, those who smoked consistently from 1998 to 2008, and new quitters who were smokers in 1998 but had stopped by 2008. The frequency of NAFLD cure in new quitters was similar (10.1%) to those in continuing smokers (8.0%) and nonsmokers (8.2%, Table 6). In contrast, the frequency of the development of NAFLD in new quitters (22.7%) was higher than that in nonsmokers (10.5%) and was similar to that in continuing smokers (17.9%). Furthermore, after adjusting for age, obesity, dyslipidemia, sex, hypertension, dysglycemia and alcohol intake, compared with nonsmokers, the odds ratios of the development of NAFLD among new quitters and continuing smokers were 2.73 (95% CI 1.71–4.36) and 1.47 (95% CI 0.90–2.42), respectively (Table 7). In addition, after adjusting for an increase in BMI from 1998 to 2008 (Table 7, model 4), the odds ratio in new quitters decreased more compared to that in continuing smokers (2.91–1.94 vs. 2.29–1.91).
Discussion
During the 10-year period of the study, 17.1% of the subjects developed NAFLD. Cigarette smoking was an independent risk factor for NAFLD, in addition to age, obesity, dyslipidemia, and the total number of metabolic syndrome risk factors. The Brinkman index (a smoking index) was also associated with NAFLD development. Metabolic syndrome risk factors are known to be related to NAFLD, but this is the first follow-up study over a 10-year period to show that cigarette smoking is an independent risk factor for NAFLD development, as well as for metabolic syndrome risk factors. However, in multivariate analysis, the association between cigarette smoking and NAFLD development did not reach statistical significance in the limited group of subjects, which may be due in part to the modest sample size. In addition, subanalysis using subjects who quit smoking demonstrated that smoking cessation seems to be a risk for NAFLD development, a result which can be partially explained by an increase in BMI.
Cigarette smoking had been previously associated with chronic liver diseases such as chronic hepatitis C and B, primary biliary cirrhosis, and alcoholic liver diseases [23–25], but the association between NAFLD and cigarette smoking had not been fully elucidated. Suzuki et al. [26] reported that initiation of cigarette smoking in patients with NAFLD was associated with ALT elevation in a 1-year follow-up survey, but the association between the development of NAFLD and cigarette smoking was not fully investigated. Chavez-Tapia et al. [27] showed that smoking was not associated with NAFLD in univariate regression analysis, but found that the risk of NAFLD tended to increase in subjects who smoked ≥10 (OR = 1.16 [95% CI 0.76–1.64]) and ≥20 (OR = 1.54 [95% CI 0.94–2.52]) packs per year compared to nonsmokers. These results may depend on the number of subjects and the duration of the study, and require confirmation in larger long-term longitudinal studies.
Increases in BMI partially explained the excess risk of NAFLD development in smokers in 1998, especially in new quitters (Table 7). Other mechanisms are also speculated, for instance smoking-induced fatty changes and fibrosis in the liver [28–40]. H2O2 and nicotine produced by smoking reduce adiponectin expression [30, 31]. Smoking also promotes the production of activated NADPH oxidase-induced reactive oxygen species, which enhances oxidative stress and lipid peroxidation due to impaired antioxidative action [32–34]. Yuan et al. [35] reported that cigarette smoking inactivated 5′-adenosine monophosphate-activated protein kinase (AMPK) by dephosphorylation and promoted triglyceride accumulation in hepatocytes via activation of sterol regulatory element-binding protein-1 (SREBP-1), inducing fatty liver in mice fed a high-fat diet. In heavy smokers, tissue becomes hypoxic due to elevation of carbon monoxide and hemoglobin levels and impairment of oxygen transport by red blood cells, which induces erythropoietin production and promotes iron absorption in the intestines [36]. Excess iron is thought to be deposited in the liver and to eventually induce hepatocellular damage and fibrosis [36]. In addition, oxidative stress produces necrotizing inflammation in fatty liver [37]. In obese rats, cigarette smoking elevated ALT and caused hepatocellular ballooning and lobular inflammation [38]. Smoking also promotes the production of inflammatory cytokines and hepatic fibrosis-associated molecules [38–40]. Further investigation of the mechanism whereby smoking influences development or progression of NAFLD in humans is required.
There is a sex difference in the incidence of NAFLD, with men being more likely to develop fatty liver compared to women [7, 41, 42]. A similar result was obtained in this study. The prevalence of fatty liver has been shown to be about 25% in men in their 30s–60s, while it gradually increases with age in women and reaches a similar prevalence after 60 years of age [7]. Sex hormones are involved in this change, and postmenopausal reduction of estrogen levels is thought to promote visceral fat accumulation and induce insulin resistance [43]. The involvement of smoking in increasing testosterone levels has been proposed [44], suggesting that sex hormones are involved in cigarette smoking-induced NAFLD [45]. Therefore, smoking and changes in sex hormones may both be related to the development or progression of NAFLD.
Many cross-sectional studies have shown that metabolic syndrome risk factors are associated with NAFLD [3, 4, 7–9], but causal relationships cannot be proven by cross-sectional studies alone. Associations of changes in body weight and metabolic syndrome with the development and cure of NAFLD have been demonstrated in longitudinal studies [19, 26, 46, 47]. Hamaguchi et al. [19] followed 4,401 healthy subjects for an average period of 414 days and found that new NAFLD developed in 308 subjects (10% of the non-NAFLD subjects). The presence of metabolic syndrome was most strongly associated with newly developed NAFLD, and body weight gain was also an independent risk factor. NAFLD was cured in 113 subjects (16% of the NAFLD patients) during the observation period, with body weight loss being the most important factor, indicating that weight loss is more important than the absence of metabolic syndrome. In our study, the incidence of NAFLD development after 10 years was investigated based on the number of metabolic syndrome risk factors in 1998, and was found to increase as the number of risk factors increased (Fig. 2; Table 5). In addition, the number of metabolic syndrome risk factors in 1998 was not associated with cure of NAFLD after 10 years. Body weight loss during the 10-year period was an independent factor contributing to NAFLD cure (data not shown), similar to the findings of Hamaguchi et al. [19]. In contrast, in Hamaguchi et al., the Brinkman index did not differ between the NAFLD and non-NAFLD groups at baseline, which is inconsistent with our results. This may be because the mean period of 414 days in Hamaguchi et al. was not long enough to investigate the influence of smoking. In addition, the results of our analysis are very important because a significant association between cigarette smoking and the development of NAFLD was present in a population in which a strong association between the development of NAFLD and metabolic syndrome risk factors was evident.
Risk factor modification such as weight loss and medication for insulin resistance and dyslipidemia should cure or prevent NAFLD. In this study, we found that cigarette smoking is a risk factor for NAFLD. Therefore, smoking cessation is likely to decrease the risk of NAFLD among current smokers. However, in our subanalysis of three groups that included new quitters, and continuing smokers and nonsmokers, smoking cessation seemed to convey a higher risk for developing NAFLD than continuing to smoke (Table 7). It has been reported that smoking is a risk factor for diabetes mellitus, but smoking cessation is also associated with substantial weight gain and may lead to a higher short-term risk of type 2 diabetes [17, 29]. Despite the fact that we were unable to clarify the effect of smoking cessation on the treatment or development of NAFLD because (1) our study population was too small, (2) the date when smoking was stopped was not considered, and (3) metabolic syndrome risk factors that arose or were cured during the 10-year period and the effects of treatment on these diseases were not fully considered, we speculate that smoking cessation without weight gain is likely to be beneficial for patients with NAFLD.
Subjects who drank ≤20 g/day of alcohol were included in our study. In a cross-sectional study in Japanese men, low alcohol consumption (40–140 g/week) significantly reduced the incidence of fatty liver (AOR = 0.824 [95% CI 0.683–0.994]) [20]. We have also found that alcohol intake may inhibit the development of fatty liver through an association of alcohol drinking pattern with obesity [48]. In the current study, intake of a small amount of alcohol had an inhibitory effect on NAFLD development in multivariate analysis that included the number of metabolic syndrome risk factors as a variable (Table 5). In animal models, cigarette smoking and alcohol intake have been shown to contribute to the development and exacerbation of fatty liver [49]. Thus, further studies of the apparent synergistic effect of alcohol intake and cigarette smoking on NAFLD are required in humans.
There are several limitations in this study. Firstly, ultrasonography is effective for diagnosing fatty liver, but detecting fatty liver in patients with ≤30% liver fat or in obese patients is relatively difficult [4]. Moreover, as simple fatty liver and NASH cannot be distinguished by ultrasonography, an association of cigarette smoking with fatty liver severity could not be shown. Secondly, although the frequency of NAFLD development in continuing smokers was higher than that in continuing nonsmokers in the limited group of subjects, the difference was not statistically significant in multivariate analysis (Table 5). This may be due to the modest sample size. Finally, our study was a follow-up study of a 10-year interval in which the data was obtained at only two points, in 1998 and in 2008. Longitudinal studies such as those involving the evaluation of NAFLD incidence and smoking patterns over time using several points during a 10-year period may provide more convincing evidence of the contribution of cigarette smoking to NAFLD development.
In conclusion, metabolic syndrome risk factors increase the risk of NAFLD. In this retrospective study, we found that cigarette smoking, a risk factor for metabolic syndrome, was also a risk factor for NAFLD development independent of metabolic syndrome risk factors. In addition, although NAFLD development became more likely as the Brinkman index increased, smoking cessation was also a likely risk factor for NAFLD development, which is partially explainable by an increase in BMI. Therefore, having never smoked is important for the prevention of NAFLD in nonsmokers, and additional treatment or prevention of metabolic syndrome risk factors may be necessary to encourage the cessation of cigarette smoking to treat or prevent NAFLD in smokers.
References
Michitaka K, Nishiguchi S, Aoyagi Y, Hiasa Y, Tokumoto Y, Onji M, et al. Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol. 2010;45:86–94.
Kawada N, Imanaka K, Kawaguchi T, Tamai C, Ishihara R, Matsunaga T, et al. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44:1190–4.
Amarapurkar DN, Hashimoto E, Laurentius LA, Sollano JD, Chen PJ, Goh KL, Asia-Pacific Working Party on NAFLD. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788–93.
Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560–78.
Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes Rev. 2002;3:141–6.
Hsieh SD, Yoshinaga H, Muto T, Sakurai Y, Kosaka K. Health risks among Japanese men with moderate body mass index. Int J Obes Relat Metab Disord. 2000;24:358–62.
Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954–61.
Radu C, Grigorescu M, Crisan D, Lupsor M, Constantin D, Dina L. Prevalence and associated risk factors of non-alcoholic fatty liver disease in hospitalized patients. J Gastrointestin Liver Dis. 2008;17:255–60.
Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabetic Med. 2005;22:1141–5.
Ezzati M, Lopez AD. Regional, disease specific patterns of smoking-attributable mortality in 2000. Tob Control. 2004;13:388–95.
Inoue M, Hanaoka T, Sasazuki S, Sobue T, Tsugane S. JPHC Study Group Impact of tobacco smoking on subsequent cancer risk among middle-aged Japanese men and women: data from a large-scale population-based cohort study in Japan—the JPHC study. Prev Med. 2004;38:516–22.
Hudson NL, Mannino DM. Tobacco use: a chronic illness? J Community Health. 2010;35:549–53.
Wada T, Urashima M, Fukumoto T. Risk of metabolic syndrome persists twenty years after the cessation of smoking. Intern Med. 2007;46:1079–82.
Fagard RH, Nilsson PM. Smoking and diabetes—the double health hazard! Prim Care Diabetes. 2009;3:205–9.
Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance—a potential link with the insulin resistance syndrome. J Intern Med. 1993;233:327–32.
Will JC, Galuska DA, Ford ES, Mokdad A, Calle EE. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int J Epidemiol. 2001;30:540–6.
Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152:10–7.
Campbell Chelland S, Moffatt RJ, Stamford BA. Smoking and smoking cessation—the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis. 2008;201:225–35.
Hamaguchi M, Kojima T, Takebe N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–8.
Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, et al. Light and moderate alcohol consumption significantly reduces the prevalence of fatty liver in the Japanese male population. Am J Gastroenterol. 2009;104:2189–95.
Japanese Society of Internal Medicine. Definition criteria of the metabolic syndrome for Japanese population. J Jpn Soc Int Med. 2005;94:188–203.
World Health Organization Western Pacific Region, International Association for the Study of Obesity/International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. Melbourne: Health Communications Australia; 2000.
Bataller R. Time to ban smoking in patients with chronic liver disease. Hepatology. 2006;44:1394–6.
El-Zayadi AR. Heavy smoking and the liver. World J Gastroenterol. 2006;12:6098–101.
Zein CO. Clearing the smoke in chronic liver diseases. Hepatology. 2010;51:1487–90.
Suzuki A, Lindor K, Saver J, Lymp J, Mendes F, Muto A, et al. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatology. 2005;43:1060–6.
Chavez-Tapia NC, Lizardi-Cervera J, Perez-Bautista O, Ramos-Ostos MH, Uribe M. Smoking is not associated with nonalcoholic fatty liver disease. World J Gastroenterol. 2006;12:5196–200.
Husain K, Scott BR, Reddy SK, Somani SM. Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol. 2001;25:89–97.
Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–9.
Iwashima Y, Katsuya T, Ishikawa K, Kida I, Ohishi M, Horio T, et al. Association of hypoadiponectinemia with smoking habit in men. Hypertension. 2005;45:1094–100.
Kawamoto R, Tabara Y, Kohara K, Miki T, Ohtsuka N, Kusunoki T, et al. Smoking status is associated with serum high molecular adiponectin levels in community-dwelling Japanese men. J Atheroscler Thromb. 2010;17:423–30.
Agarwal R. Smoking, oxidative stress and inflammation: impact on resting energy expenditure in diabetic nephropathy. BCM Nephrol. 2005;6:13.
Avti PK, Kumar S, Pathak CM, Vaiphei K, Khanduja KL. Smokeless tobacco impairs the antioxidant defense in liver, lung, and kidney of rats. Toxicol Sci. 2006;89:547–53.
Muriel P. Role of free radicals in liver diseases. Hepatol Int. 2009;3:526–36.
Yuan H, Shyy JYJ, Mokdad A. Second hand smoke stiulates lipid accumulation in the liver by modulating AMPK and SREBP-1. J Hepatol. 2009;51:535–47.
El-Zayadi AR, Selim O, Hamdy H, El-Tawil A, Moustafa H. Heavy cigarette smoking induces hypoxic polycythemia (erythrocytosis) and hyperuricemia in chronic hepatitis C patients with reversal of clinical symptoms and laboratory parameters with therapeutic phlebotomy. Am J Gastroenterol. 2002;97:1264–5.
Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92.
Azzalini L, Ferrer E, Ramalho LN, Moreno M, Domínguez M, Colmenero J, et al. Cigarette smoking exacerbates nonalcoholic fatty liver disease in obese rats. Hepatology. 2010;51:1567–76.
Zeidel A, Beilin B, Yardeni I, Mayburd E, Smirnov G, Bessler H. Immune response in asymptomatic smokers. Acta Anaesthesiol Scand. 2002;46:959–64.
Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:258–65.
Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Tani S, Goto M. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27:142–9.
Anezaki Y, Ohshima S, Ishii H, Kinoshita N, Dohmen T, Kataoka E, et al. Sex difference in the liver of hepatocyte-specific Pten-deficient mice: a model of nonalcoholic steatohepatitis. Hepatol Res. 2009;39:609–18.
Suzuki A, Manal FA. Nonalcoholic fatty liver disease in women. Women’s Health. 2009;5:191–203.
Manjer J, Johansson R, Lenner P. Smoking as a determinant for plasma levels of testosterone, androstenedione, and DHEAs in postmenopausal women. Eur J Epidemiol. 2005;20:331–7.
Economou F, Xyrafis X, Livadas S, Androulakis II, Argyrakopoulou G, Christakou CD, et al. In overweight/obese but not in normal-weight women, polycystic ovary syndrome is associated with elevated liver enzymes compared to controls. Hormones (Athens). 2009;8:199–206.
John BD, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–54.
Oza N, Eguchi Y, Mizuta T, Ishibashi E, Kitajima Y, Horie H, et al. A pilot trial of body weight reduction for nonalcoholic fatty liver disease with a home-based lifestyle modification intervention delivered in collaboration with interdisciplinary medical staff. J Gastroenterol. 2009;44:1203–8.
Hiramine Y, Imamura Y, Uto H, Koriyama C, Horiuchi M, Oketani M, et al. Alcohol drinking patterns and the risk of fatty liver in Japanese men. J Gastroenterol. doi:10.1007/s00535-010-0336-z.
Bailey SM, Mantena SK, Millender-Swain T, Cakir Y, Jhala NC, Chhieng D, et al. Ethanol and tobacco smoke increase hepatic steatosis and hypoxia in the hypercholesterolemic apoE(−/−) mouse: implications for a “multihit” hypothesis of fatty liver disease. Free Radic Biol Med. 2009;46:928–38.
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamabe, A., Uto, H., Imamura, Y. et al. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol 46, 769–778 (2011). https://doi.org/10.1007/s00535-011-0376-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0376-z