Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is a hepatic manifestation of the metabolic syndrome. The aim of this study was to evaluate a 6-month home-based lifestyle modification intervention delivered in collaboration with physicians, hygienists, registered dietitians, and nurses.
Methods
Outpatients with NAFLD diagnosed by abdominal ultrasonography were eligible for this study. Abdominal computed tomography (CT) scan evaluated liver fat deposition by the liver–spleen ratio (L/S ratio) and visceral fat accumulation as the visceral fat area (VFA; cm2). During the 6-month home-based lifestyle modification intervention, each patient was examined by physicians, nurses, hygienists, and registered dietitians, who provided individualized advice to the patients. Patients recorded their daily weight for self-control of weight with recommended diet and exercise regimens.
Results
Sixty-seven NAFLD patients were enrolled in this study and 22 patients (32.8%) completed the 6-month intervention. Nineteen of the 22 patients achieved significant improvements in body weight, body mass index (BMI), waist circumference, VFA, L/S ratio, and systolic blood pressure, with improved laboratory data. Overall, 39 patients withdrew from the intervention. The mean age of the patients who withdrew was 50.0 ± 11.0 years, which was significantly younger than that of the patients who were followed up (60.1 ± 10.1 years; P < 0.01).
Conclusions
The reduction in body weight achieved by NAFLD patients during the 6-month intervention was associated with improved fat deposition and liver function. This intervention offers a practical approach for treating a large number of NAFLD patients with lifestyle modification therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is commonly exhibited as a hepatic manifestation of the metabolic syndrome. NAFLD covers a spectrum of liver diseases ranging from benign simple steatosis to hepatic inflammation and fibrosis of nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma [1, 2]. There are currently no proven treatments for patients with NAFLD, although reduction in body weight improved the liver function in NAFLD [3–5]. It is apparent that changes in body weight and lifestyle should be the primary target for treating NAFLD. Previous studies have reported a beneficial effect of a recommended diet combined with an increase in physical activity on the progression of NAFLD [3–5], but these lifestyle modifications are rarely achieved and/or maintained in clinical practice. There are no established methods for intensive lifestyle modification in NAFLD, because of the difficulties in achieving and maintaining weight reduction [6, 7].
The aims of this study were: (1) to evaluate a 6-month home-based lifestyle modification intervention for Japanese NAFLD patients, which was supported by collaboration with physicians, hygienists, registered dietitians, and nurses; and (2) to clarify the ability of Japanese NAFLD patients to maintain weight loss over a 6-month period by lifestyle modification.
Patients and methods
Study population
This was a single-arm pilot trial of a home-based lifestyle modification intervention in outpatients with NAFLD who were attending Eguchi Hospital. All outpatients underwent abdominal ultrasonography, and NAFLD was diagnosed according to the following criteria: (1) slight diffuse increase in bright homogeneous echoes in the liver parenchyma with normal visualization of the diaphragm and portal and hepatic vein borders and normal hepatorenal echogenicity contrast; (2) diffuse increase in bright echoes in the liver parenchyma with slightly impaired visualization of the peripheral portal and hepatic vein borders; and (3) a marked increase in bright echoes at a shallow depth with deep attenuation, impaired visualization of the diaphragm, and marked vascular blurring [8]. The patients were included if they fulfilled the following criteria: (1) absence of markers of hepatitis B virus infection (HBV surface antigen and anti-HBc antibodies) or hepatitis C virus infection (anti-HCV antibodies); (2) no evidence of autoimmune liver disease or alcoholic liver disease (>20 g of alcohol per day); (3) no use of medications associated with steatosis or steatohepatitis, including steroids and tamoxifen; and (4) no use of insulin-sensitizing medications. All patients provided written informed consent, and the study protocol was approved by the Ethics Committee of Eguchi Hospital and was supervised by the Ethics Committee of Saga Medical School.
Procedures
The target of this home-based lifestyle modification intervention was a weight loss of 5% of the initial body weight within 6 months [9]. During the run-in period, at 3 months and at 6 months, meals were recorded for 3 days, using a self-administered questionnaire. Patients recorded their weight every day to determine self-control of weight and daily exercise, according to our previously applied method for moderately obese patients [10]. Each patient was examined by a physician every month to determine monthly improvements. Body weight and waist circumference were recorded and general compliance with the home-based lifestyle modification was assessed by interview by nurses at each monthly visit. All patients received lifestyle advice at each monthly visit in 30-min sessions from a single hygienist (H. H.). In addition, all patients received standardized nutritional counseling for adequate calorie intake, to reduce fat accumulation in the liver, in sessions delivered by two registered dietitians (M. U. and T. T.) every 3 months. The target dietary energy intake was defined as standard body weight × 25 − 30 kcal. All patients were free-living and consumed self-selected foods complying with standards developed by nutritional counseling. No vitamins or other nutritional supplements were prescribed. Exercise therapy was performed to achieve a target of 23 metabolic equivalent tasks (METs)·h/week (physical activity) + 4 METs·h/week (exercise) [11–14]. Reasons for dropping out were surveyed by nurses by telephone or mail. After patients had achieved compliance with the 6-month intervention, they were asked whether they wanted to receive periodic counseling to continue stable lifestyle modifications.
Anthropometric assessment and blood chemistry examination were done every month. Body weight (kg) and height (m) were obtained. Body mass index (BMI) was calculated as the body weight in kilograms divided by the square of the height in meters (kg/m2). Waist circumference (cm) was measured at the umbilical level. Venous blood samples were taken from all patients at around 9:00 a.m. after a 12-h overnight fast, and the levels of aspartate aminotransferase (AST; IU/L), alanine aminotransferase (ALT; IU/L), total cholesterol (T-CHO; mg/dL), triglyceride (TG; mg/dL), fasting plasma glucose (FPG; mg/dL), and plasma insulin (µU/mL) were determined by enzyme immunoassays (Dainabot, Tokyo, Japan). As criteria for diabetes mellitus, the condition was defined as being present in patients with hypoglycemic therapy or those with an FPG of 126 or more at each visit. Insulin resistance was calculated by the homeostasis model of assessment-insulin resistance (HOMA-IR), using the following formula: HOMA-IR = fasting plasma insulin (µU/mL) × FPG (mg/dL)/405, which primarily reflects hepatic insulin resistance [15]. The metabolic syndrome was diagnosed according to the International Diabetes Federation (IDF) consensus worldwide definition of the metabolic syndrome [16]. The IDF definition requires central obesity (measured as ethnic group-specific thresholds for waist circumference; for people of Japanese origin: ≥80 cm for females and ≥90 cm for males) plus any two of the following four components: (1) serum triglycerides 150 mg/dL or more, or specific treatment for this lipid abnormality; (2) HDL-cholesterol 40 mg/dL or less in males and 50 mg/dL or less in females, or specific treatment for this lipid abnormality; (3) blood pressure 130/85 mmHg or more, or treatment for previously diagnosed hypertension; and (4) FPG 110 mg/dL or more, or previously diagnosed type 2 diabetes [17].
The abdominal computed tomography (CT) protocol and assessment was as follows. Before starting the intervention, the patient’s liver fat deposition was assessed by an abdominal CT scan to determine the liver–spleen ratio (L/S ratio) and visceral fat accumulation (visceral fat area; VFA, cm2). Unenhanced spiral acquisition through the liver was obtained during a breath-hold at 5.0-mm collimation, 15.0-mm/rotation table speed (HQ mode, pitch of 1: 3), 120 kV, and auto mA (Light Speed QXi; GE Yokogawa Medical Systems, Tokyo, Japan). Images were reconstructed at 10-mm increments. All patients underwent abdominal CT in the morning after a 12-h overnight fast. VFA was measured at the umbilical level and calculated using computer software (Fat Scan; N2 System, Osaka, Japan) [18]. Radiological assessments were made every 3 months after starting the treatment.
Continuous variables are summarized as mean ± SD. The Wilcoxon signed-ranks test was used to test continuous ordinal data for two qualitative variables. Proportions and categorical variables were tested by the χ 2 test. Differences were considered significant at P < 0.05. Univariate and multivariate logistic regression analyses were performed to determine factors associated with study withdrawal. All analyses were done using Statview for Windows (Version 5.0; SAS Institute, Cary, NC, USA).
Results
Characteristics of the patients and adherence to the intervention
From January 2007 to April 2008, a total of 75 outpatients were initially invited to participate in this 6-month home-based life style modification intervention. Eight patients did not provide written informed consent. After the screening period, 61 (91.0%; 30 males, 31 females) out of 67 patients were enrolled. The 61 patients included 23 patients with metabolic syndrome and 14 patients with diabetes mellitus. The clinical and biochemical characteristics of the 61 patients are summarized in Table 1. The mean ± SD age was 52.6 ± 12.1 years (range, 21–73) years, the mean BMI at baseline was 28.0 ± 3.8 kg/m2 (range, 21.4–37.5 kg/m2), the mean ALT was 45 ± 35 IU/L (range, 13–239 IU/L), and the mean L/S ratio was 1.04 ± 0.21 (range, 0.56–1.45). The baseline characteristics of the patients were not different between those who completed the intervention and those who withdrew, as shown in Table 1.
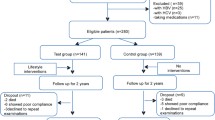
The treatment time course of the patients during the 6-month intervention is shown in Fig. 1. Six patients did not attend the first counseling session. Within the first 2 months, 21 patients (31.3%) had withdrawn from the intervention. At month 3, 31 patients (46.3%) had adhered to the visiting schedule and received the prescribed program. Overall, 22 patients (32.8%) completed the 6-month intervention.
Clinical course of all 75 patients through 6 months. Six patients did not attend the first counseling session. Within the first 2 months, 21 patients (31.3%) had withdrawn from the intervention. At month 3, 31 patients (46.3%) had attended the counseling sessions and received the prescribed intervention. A total of 22 patients (32.8%) completed the 6-month intervention. NAFLD nonalcoholic fatty liver disease
Clinical response to the 6-month home-based lifestyle modification intervention
A total of 22 out of the 67 NAFLD patients were followed up for 6 months. At 6 months, there were significant improvements in 19 patients (86.4%) in terms of body weight, BMI, waist circumference, L/S ratio, and systolic blood pressure, as shown in Table 2. In addition, VFA, as determined by CT scan, decreased significantly. In terms of laboratory findings, liver function and triglyceride and glucose metabolism, including insulin resistance, were significantly improved at the end of the intervention (P < 0.05; Table 2). These improvements were evident at 3 months because most parameters were improved at this time, as shown in Table 2. There were no significant differences in total cholesterol or HDL-cholesterol levels at 6 months. All of the 22 patients who completed the intervention were followed up as outpatients of Eguchi Hospital and showed alleviation of NAFLD, based on improvements in the L/S ratio. The number of patients meeting the criteria for metabolic syndrome decreased from 23 to 2 during the 6-month intervention. All 14 patients with diabetes mellitus had an FPG of 126 mg/dL or more before starting the intervention and only 1 patient had an FPG of 126 mg/dL or more after 6 months.
Reasons for withdrawing from the 6-month home-based lifestyle modification intervention
Figure 2 shows the age distribution of the patients who completed and those who withdrew from the intervention. The mean age of the patients who withdrew was 50.0 ± 11.0 years, which was significantly younger than that of the patients who were followed up (60.1 ± 10.1 years; P < 0.01). There was no significant difference in the number of males and females between the two groups. The reasons for withdrawing from the intervention were surveyed by nurses. The main reasons cited by the patients who withdrew were as follows: 18 patients reported difficulty in participating in the planned monthly visits due to work commitments; 9 patients did not fully understand their diseases; 3 patients lost interest in the regimen; 2 patients reported difficulty in adhering to the visits due to family nursing care commitments; and 7 patients reported other personal problems.
Age distribution of the patients who were followed up and those who withdrew from the home-based lifestyle modification intervention. The mean age of the patients who withdrew was 50.0 ± 11.0 years, which was significantly younger than that of the patients who were followed up (60.1 ± 10.1 years; P < 0.01) yo years old
Discussion
The 6-month home-based lifestyle modification intervention in the present study resulted in clinically relevant improvements in body weight, visceral fat accumulation, liver fat deposition, liver function (ALT levels), and insulin resistance in Japanese patients with NAFLD, when the patients continued the regimen in collaboration with physicians, hygienists, registered dietitians, and nurses. In an earlier study [19], the severity of liver steatosis was correlated with the amount of visceral fat accumulation. In the present study, we found that the reduction in visceral fat accumulation by appropriate therapy might ameliorate liver steatosis and improve liver function in patients with NAFLD. Although there is currently little evidence to support the effectiveness of body weight reduction, some studies have indicated that weight reduction through lifestyle modification is effective for the management of NAFLD [20–23]. The observation period in the present pilot study was only 6 months, and longer-term follow up is required to confirm the clinical benefit of the intervention.
Body weight reduction, as found in this and other studies, should be considered as part of the treatment regimen fopr NAFLD. It is, however, well known that patient withdrawal from an intervention is a significant barrier to weight reduction therapy in obese patients [10]. In the present study, because we included a relatively large number of NAFLD patients as outpatients for therapy, the home-based lifestyle modification intervention was done in collaboration with interdisciplinary medical staff, in addition to physicians, to maintain the motivation of the patients. The withdrawal rate in this study during the 6-month period was relatively high (67.2%), compared with 35.3% in our previous study [10], in which moderately obese patients were followed up more intensively every 1–2 weeks by physicians only. The total number of patients included in that study was limited by the intensive physician involvement. These two factors; namely, the intensive patient management and the large number of treated patients, are controversial, but this problem must be overcome to enable such a therapeutic approach to be taken for weight reduction in patients with NAFLD. The present study revealed several patient-related factors and/or reasons for withdrawal, including the patients’ ages, lack of understanding about NAFLD, and lack of interest about their treatment, in addition to other reasons. These factors should be considered when designing a regimen to be delivered in collaboration with interdisciplinary medical staff for body weight reduction therapy in NAFLD patients.
Our study had several important limitations. First, outpatients were included if they had both biochemical and ultrasonographic findings indicating NAFLD, but it was not possible to distinguish between simple fatty liver and NASH using these methods. Further studies focusing on histological changes in addition to anthropometric and laboratory findings are required to distinguish between simple fatty liver and NASH. Second, this study was a pilot trial and was not a controlled trial, and the patients who withdrew were not followed up.
In conclusion, this 6-month home-based lifestyle modification intervention was effective for outpatients with NAFLD in terms of weight reduction, with a decrease in visceral fat accumulation. It is important to determine approaches to improve adherence when treating larger numbers of patients with continuous counseling.
References
Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of non-alcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–40.
Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375–8.
Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with nonalcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–81.
Suzuki A, Lindor K, St Saver J, Lymp J, Mendes F, Muto A, et al. Effects of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060–6.
Riley P, Sudarshi D, Johal M, Benedict A, Panteli J, Crook M, et al. Weight loss, dietary advice and statin therapy in non-alcoholic fatty liver disease: a retrospective study. Int J Clin Pract. 2008;62:374–81.
Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–6.
Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;22:2082–90.
Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in fatty liver disease. Gastroenterology. 2002;123:745–50.
de Luis DA, Aller R, Izaola O, Sagrado MG, Conde R, Gonzalez JM. Effect of a hypocaloric diet on transaminases in nonalcoholic fatty liver disease and obese patients, relation with insulin resistance. Diabetes Res Clin Pract. 2008;79:74–8.
Fujimoto K, Sakata T, Etou H, Fukagawa K, Ookuma K, Terada K, et al. Charting of daily weight pattern reinforces maintenance of weight reduction in moderately obese patients. Am J Med Sci. 1992;303:145–50.
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl):S498–516.
Nakagaichi M, Tanaka K. Development of a 12-min treadmill walk test at a self-selected pace for the evaluation of cardiorespiratory fitness in adult men. Appl Human Sci. 1998;17:281–8.
Astrand PO, Rodahl K. Applied sports physiology. In: Astrand PO, Rodahl K, Dahl HA, editors. Textbook of work physiology. Physiological bases of exercise. 3rd ed. New York: McGraw-Hill; 1986. p. 646–82.
Ministry of Health, Labour and Welfare of Japan. Exercise and physical activity reference for health promotion 2006. http://www.nih.go.jp/eiken/programs/pdf/epar2006.pdf. Jul 2006.
Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care. 1996;19:1138–41.
International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. http://www.idf.org/webdata/docs/MetS_def_update2006.pdf. Cited 21 Jan 2009.
Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group. The metabolic syndrome––a new worldwide definition. Lancet. 2005;366:1059–61.
Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–6.
Ishibashi E, Eguchi Y, Eguchi T, Matsunobu A, Oza N, Nakashita S, et al. Waist circumference correlates with hepatic fat accumulation in male Japanese patients with non-alcoholic fatty liver disease, but not in females. J Gastroenterol Hepatol. 2008;23:908–13.
Ballentani S, Dalle Grave R, Suppini A, Marchesini G, Fatty Liver Italian Network. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology. 2008;47:746–54.
Chan HL, de Silva HJ, Leung NW, Lim SG, Farrell GC, Asia-Pacific Working Party on NAFLD. How should we manage patients with non-alcoholic fatty liver disease in 2007? J Gastroenterol Hepatol. 2007;22:801–8.
de Piano A, Prado WL, Caranti DA, Siqueira KO, Stella SG, Lofrano M, et al. Metabolic and nutritional profile of obese adolescents with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2007;44:446–52.
Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. Br Med J. 2007;334:299.
Acknowledgments
The authors would like to thank the medical staff at Eguchi Hospital; Ms. Yukie Watanabe, Chieko Ogawa, and Reiko Sonoda for their assistance, and Professor Kyuichi Tanikawa (International Institute for Liver Research) for his excellent advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oza, N., Eguchi, Y., Mizuta, T. et al. A pilot trial of body weight reduction for nonalcoholic fatty liver disease with a home-based lifestyle modification intervention delivered in collaboration with interdisciplinary medical staff. J Gastroenterol 44, 1203–1208 (2009). https://doi.org/10.1007/s00535-009-0115-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-009-0115-x