Abstract
Purpose
The aim of this study was to update the clinical practice guidelines for the use of agents for the prevention and/or treatment of gastrointestinal mucositis (GIM).
Methods
A systematic review was conducted by the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO). The body of evidence for each intervention, in each cancer treatment setting, was assigned an evidence level. Based on the evidence level, one of the following three guideline determinations was possible: Recommendation, Suggestion, and No Guideline Possible.
Results
A total of 78 papers across 13 interventions were examined of which 25 were included in the final review. No new guidelines were possible for any agent due to inadequate and/or conflicting evidence. Existing guidelines for probiotics and hyperbaric oxygen were unchanged.
Conclusions
Of the agents studied for the prevention and treatment of GIM, the evidence continues to support use of probiotics containing Lactobacillus spp. for prevention of chemoradiotherapy and radiotherapy-induced diarrhea in patients with pelvic malignancy, and hyperbaric oxygen therapy to treat radiation-induced proctitis. Additional well-designed research is encouraged to enable a decision regarding palifermin, glutamine, sodium butyrate, and dietary interventions, for the prevention or treatment of GIM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucositis is a common toxicity following chemotherapy and radiotherapy for cancer [1], often necessitating dose reductions and/or treatment breaks. These patients have twice the infection risk leading to a four-fold higher chance of death and three-fold longer hospital stays. Mucositis also adds substantial health care costs—US data from 2012 estimated a combined cost of $15,500 for each hospitalization due to severe mucositis, adding millions to annual health care expenditure [2]. Patients with mucositis may experience different patterns of toxicities and related consequences including, but not limited to, oral ulceration [3], increased infection rates [4], and diarrhea [5]. Gastrointestinal mucositis (GIM) refers specifically to mucosal injury, and related symptoms, distal to the oropharyngeal cavity. There is currently an unmet need for effective interventions for GIM in patients undergoing cancer therapy [1, 6]. Amifostine has been recommended for prevention of radiation-induced proctitis, and established antidiarrheal medications, loperamide and octreotide, continue to be recommended for treatment of chemotherapy-induced diarrhea [7,8,9]. However, there are limited options for most settings of GIM.
This paper reports the findings from a systematic review of the most recent literature assessing agents for prevention and treatment of GIM. This evaluation has been conducted on the background of previous reviews performed by The Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) published in 2004 [7], 2007 [8], and 2014 [9], with the first GIM-specific review published in 2013 [6]. Although much of the underlying pathobiology of mucositis is identical along the alimentary tract [10], beyond the oral cavity there are morphological and microbial differences related to regional specializations [10]. This makes diagnosis, inspection, and treatment of mucositis in these more distal regions all the more clinically challenging [11]. As such, our aim was to comprehensively review available literature and generate new evidence-based clinical practice guidelines for the prevention and/or treatment of GIM.
Methods
The methods related to this guidelines update are described in detail in Ranna et al. [12]. Briefly, a literature search for relevant papers published from 1 January 2011 to 30 June 2016 was conducted using PubMed and Web of Science, with papers selected for review based on defined inclusion and exclusion criteria. The list of keywords used for the literature search of this section is in Table 1.
Papers were reviewed by two independent reviewers, with data extracted using a standard electronic form. Studies were scored for their Level of evidence (LoE) based on Somerfield criteria [13] and flaws were listed according to Hadorn criteria [14]. A well-designed study was defined as a study with no major flaws as per the Hadorn criteria.
Findings from the reviewed studies were integrated into guidelines based on the overall LoE for each intervention. Guidelines were classified into Recommendation, Suggestion, and No Guideline Possible. Guidelines were separated based on the following: (1) the aim of the intervention (prevention or treatment); (2) the treatment modality (radiotherapy, chemotherapy, chemoradiotherapy, or hematopoietic stem cell transplantation); and (3) the route of administration of the intervention. Treatment modalities that included targeted therapies were excluded from this review.
Results
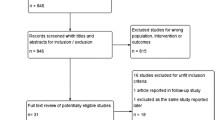
A total of 416 papers were identified in the literature search from PubMed and 535 from Web of Science. Four papers were transferred from other sections of the guidelines update. A further 15 were found by manual searches. After careful assessment of the abstracts, we excluded papers for reasons including: repetition across databases; pre-clinical nature of study; studies assessing mechanisms and not interventions; and systematic reviews. We retrieved 78 papers for final review. After review of these papers, we included 25 in this report, including 18 randomized controlled trials (Fig. 1). Studies were removed at the final review stage due to not investigating GIM directly; no clinical outcome measures presented; and repetitive studies (identical data presented more than once). The following 13 interventions were included in this review: antimicrobials (ciprofloxacine and metronidazole); famotidine; fat-modified diet; fiber; formalin; glutamine; hyperbaric oxygen; octreotide, palifermin; probiotics; sodium butyrate; steroids; and sucralfate (Table 2).
Antimicrobials (ciprofloxacine and metronidazole)
Guideline category: No Guideline Possible
Two papers investigating the combined use of antimicrobials (ciprofloxacin and metronidazole) for the treatment of chronic radiation-induced proctitis were reported since the previous guidelines update [15, 16]. One of these studies was a RCT for treatment of hemorrhagic radiation proctitis [15] that added to an earlier small cohort study by the same group [16]. It compared colonic irrigation and oral antibiotics with 4% formalin rectal administration. Both studies found a positive impact of antibiotics but due to the limited evidence available, no guideline was possible. No other antibiotic combinations or treatment settings have been examined since the previous GIM guidelines were published [6] (Box 1).
Box 1. Interventions for which no new evidence was added since the previous MASCC/ISOO systematic review [6, 9]
Prevention | ||
5-ASA* | PO | RT, pelvic ca |
Activated charcoal | PO | CT, solid ca |
Amifostine* | Enema, IV, SC | CT, CT-RT, RT, solid ca, pelvic ca |
Antidiarrheal program | PO | CT, lung ca |
Balsalazide | PO | RT, pelvic ca |
Budesonide | PO | CT, colorectal ca |
Celecoxib | PO | CT, solid ca |
Cholestyramine | PO | CT, RT, solid ca |
Chrysin | PO | CT, solid ca |
Circadian rhythm | n/a | RT, pelvic ca |
Glutamine | IV | CT, hematological |
Misoprostol* | Enema | RT, pelvic ca |
Neomycin | PO | CT, solid ca |
Physical activity | n/a | CT, RT, solid ca |
Sucralfate* | PO, enema | RT, pelvic ca |
Sulfasalazine* | PO | RT, pelvic ca |
Treatment | ||
Cefixime | PO | CT, solid ca |
Heater probes | Rectal | RT, pelvic ca |
Leucovorin | IV | RT, bone ca |
Metronidazole | Enema | RT, pelvic ca |
Octreotide* | IM, IV, SC | CT, CT-RT, solid ca, hematological |
Probiotics* | PO | RT, solid ca |
Sodium butyrate | Enema | RT, pelvic ca |
Sucralfate* | Enema | RT, pelvic ca |
Famotidine (oral)
Guideline category: No Guideline Possible
A single RCT investigating famotidine, a histamine H2 receptor antagonist, for the prevention of acute radiation-induced GIM in 36 patients with prostate cancer was successful [17]; however, due to a lack of supporting evidence, no guideline is possible.
Fat-modified diet
Guideline category: No Guideline Possible
The use of low fat or fat-modified diet to prevent radiotherapy-induced GIM was investigated in a multi-site RCT [18]. Patients with cancers of the pelvic region consumed the diets throughout radiotherapy; however, neither arm showed a significant improvement in GI symptoms. The compliance to the required fat intake for the control group was noted as a possible cause for a lack of effect seen in the study.
Fiber (with combinations of prebiotics, nutritional supplements, and probiotics)
Guideline category: No Guideline Possible
Studies have combined fiber (including using inulin and oligosaccharides often referred to as prebiotics) with additional interventions that we have grouped together due to this overarching dietary modification. Prebiotics are defined as “selectively fermented ingredients in the colon that produce specific changes in the composition and/or activity of gastrointestinal microbiota and have beneficial effects for host health” [40].
One paper examined fiber combined with a nutritional supplement, including vitamins, minerals, and probiotics, named Dixentil, for prevention of chemoradiotherapy-induced gastrointestinal toxicity [19]. This was a phase II study of 40 consecutive patients whom received a daily 10-ml vial of Dixentil (Gamfarma srl, Milano Italy) starting from 1st day of radiotherapy until the end of the scheduled treatment. Each vial contained 500 mg of galacto-oligosaccharides, 10 mg of L. casei, 10 mg of Lactobacillus acidophilus, 10 mg of zinc, 1 mg vitamin B1, 1 mg vitamin B2, 1 mg vitamin B6, and 10 mg nicotinamide. This study primarily focused on the safety and tolerance of Dixentil, being the first study to evaluate a nutritional supplement based on zinc, prebiotics, tyndalized probiotics, and vitamins for reducing the duration and frequency of severe and or persistent diarrhea. There was some indication of activity relative to historical control levels.
Hydrolyzed rice bran for prevention of GIM [20] was investigated in a small RCT (n = 20) of cervical cancer with limited evidence of effectiveness for diarrhea improvement. The main ingredient was water soluble fiber which is believed to carry anti-inflammatory properties, although the authors found no impact of fiber on immune cell activity in the study.
One RCT examined the capacity of prebiotics to prevent radiation-induced diarrhea [21]. Briefly, 38 women with gynecological cancer receiving radiotherapy were randomized to receive either prebiotics (6 g twice daily of an equal mix of inulin and fructo-oligosaccharide) or 6 g twice daily of matching placebo (maltodextrin). The main outcome measure was stool consistency as measured by the Bristol stool score, with no significant improvement in the prebiotic group for days with watery diarrhea. A previous paper in the same population confirmed that prebiotics modulated the levels of Lactobacillus and Bifidobacterium [41].
Finally, a small RCT investigated fiber (inulin plus partially hydrolyzed guar gum mixture) with Lactobacillus reuteri for prevention of radiotherapy-related proctitis in patients with prostate cancer [22]. Although effective, the small size of the study (n = 20) meant there was limited evidence to support a guideline. Overall, the use of fiber/prebiotics in studies for prevention of GIM had conflicting results regarding effectiveness and as such, no guideline is possible.
Formalin (rectal)
Guideline category: No Guideline Possible
One RCT investigated formalin rectal dab compared with sucralfate-prednisolone enema for treatment of hemorrhagic proctitis [23]. The study included 102 patients that had previously received radiotherapy for management of cervical carcinoma and found that formalin was superior to sucralfate. This supports the findings of Tsujinaka et al. 2005, which when reviewed in the previous update [6], showed that formalin was effective at treating hemorrhagic proctitis in patients treated with radiotherapy for pelvic cancers. However, due to the lack of studies in this area, no guideline is possible.
Glutamine (oral)
Guideline category: No Guideline Possible
There has been no previous guideline possible for oral glutamine to prevent or treat GIM in either hematological or solid cancers treated with chemotherapy despite 6 studies published over a decade ago (reviewed in [6]).
In the HSCT setting, this current guideline update found one new study [24], which was a historical case control study investigating oral glutamine for prevention of GIM in 22 patients receiving allogeneic HSCT. The oral supplement was referred to as GFO (15 g) and contained 3 g glutamine, 5 g dietary fiber, 1.5 g oligosaccharide, and 1.2 mg sodium. Two packages of GFO dissolved in 200 mL of water were administered to patients orally 3 times per day beginning 7 days prior to the start of conditioning and continued until 28 days after transplantation. This regimen was associated with decreased days of severe diarrhea compared with 22 matched historical controls; however, due to the limited evidence, there was no change to the former guideline.
With regard to lung cancer treated with chemoradiotherapy, a single historical case control study examining the role of oral glutamine in prevention of acute radiation-induced esophagitis was identified [25]. This study compared 56 patients that received 10 g of glutamine three times daily starting 1 week prior to first day of radiation and finishing 2 weeks after completion of radiotherapy to 48 patients that did not receive glutamine. Glutamine supplementation was associated with significantly decreased incidence of grade III esophagitis in patients receiving radiation with concurrent chemotherapy. Of note, another study that utilized the same database and patient files was subsequently published, albeit with fewer included patients, and found a consistent beneficial effect for glutamine [42]. This work adds to the studies previously included in the 2013 guidelines [43, 44]; however, due to the lack of randomized clinical trials for this indication and inconsistent results, no guideline is possible.
In regard to solid tumors in the pelvis (including genitourinary, gynecological and rectal) treated with concurrent chemotherapy and radiotherapy, there has been 2 RCTs [26, 27] and 1 non-RCT [28] since the last guidelines [6]. In patients with rectal cancer [27], oral glutamine was not effective at reducing severity of diarrhea, although the study was terminated prior to reaching the recruitment target. In patients with mixed pelvic cancers, oral glutamine caused significantly more acute radiation enteritis than placebo [26]. In contrast, in the non-RCT, patients with mixed pelvic tumors treated with glutamine had significant protection against severe diarrhea [28]. Due to the inconsistences seen in these studies, no guideline is possible.
No papers that have investigated IV glutamine have been published since the last update [6] (Box 1).
Hyperbaric oxygen therapy
Guideline category: Suggestion for treatment of RT-induced proctitis (LoE II)
There have been two recent studies investigating hyperbaric oxygen for treatment of pelvic radiotherapy-related proctitis [29, 30]. A cohort study found that daily hyperbaric oxygen was effective at reducing proctitis severity when started at the onset of chronic symptoms [30]. The majority of patients received radiotherapy in combination with hormone therapy for prostate cancer, and less than a quarter received chemotherapy. In contrast, a RCT of patients with over 12 months of gastrointestinal symptoms that had failed to respond to 3 months of optimal medical therapy, hyperbaric oxygen delivered 5 days a week was ineffective at resolving rectal bleeding or bowel symptoms at 12 months [29]. This RCT is the first to oppose the existing evidence supporting hyperbaric oxygen therapy (reviewed in [6]), and as such the panel continues to suggest hyperbaric oxygen therapy for treatment of radiation-induced proctitis in pelvic cancers. However, additional RCTs are urgently required given the now conflicting results.
Octreotide (intramuscular)
Guideline category: No Guideline Possible
A single RCT investigating the effectiveness of long-acting intramuscular octreotide for prevention of chemotherapy-induced-diarrhea in patients with colorectal cancer found no improvement compared with standard of care [31]. This is consistent with 2 previous RCTs; first, for prevention of chemoradiation-induced diarrhea in patients with anorectal cancer when compared with placebo [45], and second, in pelvic radiation-induced diarrhea when compared with placebo [46], which found no benefit for long-acting octreotide. Each study has been in a different population with different treatments and as such, no guideline was possible. The existing guideline that recommends the use of octreotide for treatment of chemotherapy-induced diarrhea is unchanged [6].
Palifermin
Guideline category: No Guideline Possible
As part of a larger RCT to investigate efficacy of different dose schedules of idarubicin for AML, Bradstock and colleagues recruited 155 patients from 23 centers to assess palifermin efficacy in preventing chemotherapy-related adverse events [32]. Incidence of severe GI toxicity was significantly different (p = 0.002) between placebo and palifermin, contributed mostly by decreased incidence of severe diarrhea in the palifermin group.
Two studies investigated the effectiveness of palifermin for prevention of GIM related to HSCT. A control-matched cohort study found that palifermin did not prevent clinically meaningful GIM in patients undergoing HSCT for non-Hodgkin and Hodgkin lymphoma [33]. In contrast, a retrospective case control study in the same setting found that palifermin was effective at reducing the number of days with moderate diarrhea [34]. Given the inconsistencies in findings across studies, no guideline is possible.
Probiotics
Guidelines category: No Guideline Possible for prevention of diarrhea due to chemotherapy alone
Probiotics are generally preparations that contain sufficient numbers of viable bacteria that are able to exert beneficial effects [6]. A RCT in colorectal cancer patients treated with irinotecan-based chemotherapy investigated prevention of diarrhea using a mixed probiotic formulation [35]. Patients were instructed to take three capsules per day for 12 weeks, each containing 10 billion CFU of bacteria (Bifidobacterium breve, Bifidobacterium bifidum, Bifidobacterium longum, Lactobacillus rhamnosus, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus plantarum, Streptococcus thermophilus, Lactobacillus brevis, Bifidobacterium infantis) and additives, inulin, maltodextrine, magnesium stearate, and ascorbic acid. Placebo capsules contained additives without probiotic bacteria. The study terminated due to slow accrual when 46 of 220 planned participants were recruited. Regardless, the authors reported that probiotics use was associated with significantly reduced incidence of severe and overall diarrhea.
Guidelines category: Suggestion for prevention of chemoradiotherapy-induced diarrhea in pelvic malignancy (LoE III)
In regard to pelvic cancer patients treated with radiotherapy or chemoradiotherapy, we have assessed one additional RCT [36]. The study investigated a mixed probiotic formulation called Bifilact® which includes Lactobacillus acidophilus and Bifidobacterium longum in two different doses and schedules; either twice daily 1 billion or thrice daily 10 billion CFU. The placebo group received capsules containing the excipients. The lower dose was associated with significant decreases in grade 4 diarrhea in patients that had received prior pelvic surgery. There was no change in the study primary endpoint, incidence of grade 2–3 diarrhea. Given the consistent positive results seen in this trial and in the trials included in our previous guidelines [6], the panel continues to suggest that probiotics containing Lactobacillus spp., may be beneficial for prevention of chemoradiotherapy and radiotherapy-induced diarrhea in patients with pelvic malignancy.
Sodium butyrate (enema)
Guideline category: No Guideline Possible
One RCT for prevention of radiation-induced proctitis using sodium butyrate enemas was identified [37]. The study recruited 166 patients who were allocated to a variety of rectal doses of sodium butyrate (placebo, 1, 2, or 4 g). This study found no evidence of efficacy in reduction of incidence, severity, or duration of acute radiation-induced proctitis.
No trials were identified that examined treatment of radiation-induced proctitis as covered in the previous guidelines [6] (Box 1).
Steroid (beclomethasone)
Guideline category: No Guideline Possible
The steroid beclomethasone dipropionate was investigated in a RCT for prevention of radiotherapy-induced GIM in patients with prostate cancer [38]. Patients received beclomethasone suppositories throughout radiation and for 4 weeks following completion of treatment. There was no significant improvement seen in bowel symptoms at 3 or 12 months, although rectal bleeding was reduced.
Sucralfate (oral)
Guideline category: No Guideline Possible
A single RCT investigated oral sucralfate for treatment of hemorrhagic proctitis in patients that had received chemoradiation for pelvic malignancy [39]. All 122 patients received argon plasma coagulation prior to receiving sucralfate or placebo. In this study, there was no evidence for improved benefit by adding sucralfate. In previous studies investigating oral sucralfate for treatment of radiation-related proctitis, there has been some evidence of effectiveness [47, 48]; however, due to a lack of evidence, no guideline is possible. In contrast, for prevention of radiation-induced gastrointestinal side effects, the panel has an existing recommendation not to use sucralfate [6] and no recent studies have been published for this indication (Box 1).
Discussion
Following a systematic review of the most recent clinical literature on interventions for the prevention and treatment of GIM, the panel assessed the evidence for 13 interventions to support clinical practice guidelines. Due to a lack of new evidence or conflicting evidence in each setting, no new guidelines were formed. For two interventions, where new studies were found, an existing guideline was retained: (1) The panel continues to suggest that probiotics containing Lactobacillus spp. may be effective for prevention of diarrhea during chemoradiotherapy and radiotherapy for pelvic cancers; (2) The panel continues to suggest that hyperbaric oxygen therapy is an effective way to treat radiation-induced proctitis. Several clinical studies investigating new interventions, fiber, famotidine, fat-modified diet, and beclomethasone were reviewed, but due to limited evidence were unable to form guidelines.
The existing guideline for probiotics was supported by one new RCT [36] in patients with pelvic malignancy treated with radiotherapy (46%) and chemoradiotherapy (54%). Probiotics given at a standard dose of Bifilact® decreased the incidence of life-threatening diarrhea in patients that underwent pelvic surgery as part of the treatment plan. No benefit was seen in patients that did not receive pelvic surgery or received high-dose Bifilact®. Overall, the effect was marginal, and it was unclear if patients treated by single modality or combined therapy were more protected. One additional RCT in colorectal cancer patients, treated with irinotecan-based chemotherapy [35], showed significant reductions in severe diarrhea. Since this is the first study to examine probiotics in the chemotherapy-alone setting, it could not be incorporated with the existing evidence or guideline. Limiting the evidence is that each study to date has included different strains and doses of bacteria, and has been conducted in heterogenic cancer treatment settings. As such, the panel is still unable to make a strong recommendation regarding probiotics. Further work is required to find the optimal strains and dose for maximal protection, and to evaluate effectiveness across settings of radiotherapy and chemotherapy, with and without prior surgery. Future studies should track changes in fecal microbial composition throughout treatment as a biomarker of response to probiotics.
For palifermin, one RCT provided evidence that 60 μg/kg/day palifermin for 3 days before and after induction chemotherapy for AML was effective in decreasing incidence of severe GIM following chemotherapy [32]. In contrast, a case-matched cohort study found that palifermin was ineffective in reducing GIM related to BEAM chemotherapy and HSCT in patients with lymphomas [33]. Finally, a study that retrospectively assessed GIM in two groups of patients (9 that received palifermin and pegfilgrastim, and 10 that received neither) found palifermin treatment was associated with significantly fewer days with grade 2 or worse diarrhea [34]. A previous study by Johannson et al. reported protective effects on mucosal barrier, but not clinical symptoms, of palifermin in the setting of autologous HSCT after conditioning chemotherapy for lymphomas using the same dose and schedule of palifermin [49]. However, since the three studies are not in agreement regarding the effectiveness of palifermin in high-dose chemotherapy for hematological cancers, no guideline is possible. Furthermore, the previous guidelines reviewed the paper by Rosen et al. that found palifermin to be ineffective against GIM following 5-FU-based chemotherapy for metastatic colorectal cancer [50]. This differs from the extensive preclinical evidence that reported reduced GIM with palifermin following chemotherapies used for colorectal cancer [51, 52]. As such, the effectiveness of palifermin appears to be related to the type of chemotherapy and the addition of total body irradiation.
Hyperbaric oxygen therapy continues to be investigated as an intervention for radiation-induced proctitis, especially in the context of chronic hemorrhagic disease. While there is a relative lack of RCTs in this setting, the cumulative evidence does support its use in over 10 studies spanning 20 years. The one exception noted is the recent RCT by Glover et al. which included patients with long-standing symptoms resistant to optimal therapy. They found hyperbaric oxygen therapy was ineffective at resolving rectal bleeding or bowel symptoms at 12 months [29]. This RCT is the first to oppose the existing evidence and indicates that radiation morbidity refractory to standard management may not be effectively treated with this approach. However, additional RCTs are urgently required given the now conflicting results.
The current review evaluated sodium butyrate enemas for the prevention of radiation-induced proctitis. The included trial was a relatively large, well-designed study [37] that examined increasing doses of sodium butyrate throughout radiation treatment. However, there was no evidence of effectiveness in reducing the incidence, severity, or duration of proctitis. In contrast, the previous guidelines included a number of studies that investigated the use of sodium butyrate for treatment of radiation-induced proctitis [6], and topical sodium butyrate was considered effective for treatment of existing acute radiation proctitis. However, the panel noted the studies were relatively small with a combined evidence level of III [6]. As such, there is conflicting evidence for effectiveness in the prevention and treatment setting, and no guideline is possible for either.
In January 2019, the literature search was repeated in an effort to capture late-breaking evidence published July 2016 to December 2018. This identified a further 4 relevant studies investigating prevention and treatment of GIM but did not impact on existing guidelines or lead to any new guidelines. A double-blind RCT investigated 3% Aloe vera ointment for treatment of acute radiation-induced proctitis [53]. Nine patients received 1 g Aloe vera twice daily for 4 weeks, while 11 patients received placebo ointment. Aloe vera was effective at reducing diarrhea and fecal urgency, but was not able to reduce hemorrhage or rectal pain. A randomized non-blinded and non-controlled study investigated high protein (36 g daily) oral nutritional supplement in pre-cachectic patients treated with chemotherapy for colorectal cancer [54]. The study recruited 47 to the supplement group and 48 to the control group, and found no significant differences in toxicity between groups. An elemental diet, consisting predominantly of amino acids, for prevention of gastrointestinal toxicity in patients receiving stem cell transplant for hematological cancers was evaluated in a prospective cohort study [55]. Elemental diet was received by 52 consecutive patients and results were compared with previous 21 consecutive patients. Severe diarrhea incidence was not significantly different between the diet (50%) and non-diet (26%) groups (p = 0.08). Finally, a randomized study compared enteral nutrition with high levels of omega-3 fatty acid (900 mg/day) with lower levels of omega-3 fatty acid (250 mg/day) supplementation for prevention of toxicities during neoadjuvant chemotherapy for esophageal cancer [56]. A total of 31 patients were randomized to the high omega-3 group and 30 to the lower omega-3 group. The incidence of severe diarrhea was 16% vs 37% (p = 0.068) in favor of the high-dose group.
A limitation of this review was the exclusion of papers that included targeted therapies; a decision made a priori to allow direct comparison with previous recommendations. In future reviews, inclusion of emerging multimodal regimens will be critical. Further, the classical definition of GIM will need to move with the field, to broaden its definition to include biological therapy-specific toxicities [57, 58]. Finally, as the combination use of chemotherapy and radiotherapy with targeted agents or immunotherapy will increase the risk of GIM, so will the need for tailored preventative and therapeutic interventions grow.
References
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB, Mucositis Study Section of the MASCC and the ISOO (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025
Carlotto A, Hogsett VL, Mairorini EM, Razulis JG, Sonis ST (2013) The economic burden of toxicities associated with cancer treatment: review of the literature and analysis of nausea and vomiting, diarrhoea, oral mucositis and fatigue. Pharmacoeconomics 31:753–766
Gibson RJ, Cummins AG, Bowen JM, Logan RM, Healey T, Keefe DMK (2006) Apoptosis occurs early in the basal layer of the oral mucosa following cancer chemotherapy. Asia Pac J Clin Oncol 2:39–49
Blijlevens NM, Donnelly JP, De Pauw BE (2000) Mucosal barrier injury: biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: an overview. Bone Marrow Transplant 25:1269–1278
Stringer AM, Al-Dasooqi N, Bowen JM, Tan TH, Radazum M, Logan RM, Mayo B, Keefe DMK, Gibson RJ (2013) Biomarkers of chemotherapy-induced diarrhea: a clinical study of intestinal microbiome alterations, intestinal inflammation and circulating matrix metalloproteinases. Support Care Cancer 21:1843–1852
Gibson RJ, Keefe DM, Lalla RV, Bateman E, Blijlevens N, Fijlstra M, King EE, Stringer AM, van der Velden WJ, Yazbeck R, Elad S, Bowen JM, For The Mucositis Study Group of the Multinational Association of Supportive Care in Cancer International Society of Oral Oncology (MASCC/ISOO) (2013) Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer 21:313–326
Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis ST (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100:2026–2046
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, DB MG, Hutchins RD, Peterson DE, Mucositis Study Section of the MASCC and the ISOO (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Lalla R, Bowen J, Barasch A, Elting L, Epstein J, Keefe D, McGuire D, Migliorati C, Nicolatou-Galitis O, Peterson D, Raber-Durlacher J, Sonis S, Elad S, Al-Dasooqi N, Brennan M, Gibson R, Fulton J, Hewson I, Jensen SB, Logan R, Ohrn KEO, Sarri T, Saunders D, von Bultzingslowen I, Yaron N (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120:1453–1461
Keefe DM (2004) Gastrointestinal mucositis: a new biological model. Support Care Cancer 12:6–9
Wardill HR, Bowen JM, Gibson RJ (2012) Biomarkers of small intestinal mucosal damage induced by chemotherapy: an emerging role for the (13)C sucrose breath test. J Support Oncol 11:61–67
Ranna V, Cheng K, Castillo D, Porcello L, Vaddi A, Lalla R, Bossi P, Elad S (2019) Development of the MASCC/ISOO clinical practice guidelines for mucositis: an overview of the methods support care. Support Care Cancer. https://doi.org/10.1007/s00520-019-04891-1
Somerfield MR, Padberg JJ, Pfisher DG, Bennett CL, Recht A, Smith TJ, Weeks JC, Winn RJ, Durant JR (2000) ASCO clinical practice guidelines: process, progress, pitfalls, and prospects. Class Pap Curr Comm 4:881–886
Hadorn DC, Baker D, Hodges JS, Hicks N (1996) Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol 49:749–754
Sahakitrungruang C, Patiwongpaisarn A, Kanjanasilp P, Malakorn S, Atittharnsakul P (2012) A randomized controlled trial comparing colonic irrigation and oral antibiotics administration versus 4% formalin application for treatment of hemorrhagic radiation proctitis. Dis Colon Rectum 55:1053–1058
Sahakitrungruang C, Thum-Umnuaysuk S, Patiwongpaisarn A, Atittharnsakul P, Rojanasakul A (2011) A novel treatment for haemorrhagic radiation proctitis using colonic irrigation and oral antibiotic administration. Color Dis 13:e79–e82
Razzaghdoust A, Mozdarani H, Mofid B, Aghamiri SM, Heidari AH (2014) Reduction in radiation-induced lymphocytopenia by famotidine in patients undergoing radiotherapy for prostate cancer. Prostate 74:41–47
Wedlake LJ, McGough C, Shaw C, Klopper T, Thomas K, Lalji A, Dearnaley DP, Blake P, Tait D, Khoo VS, Andreyev HJ (2012) Clinical trial: efficacy of a low or modified fat diet for the prevention of gastrointestinal toxicity in patients receiving radiotherapy treatment for pelvic malignancies. J Hum Nutr Diet 25:247–259
Scartoni D, Desideri I, Giacomelli I, Di Cataldo V, Di Brina L, Mancuso A, Furfaro I, Bonomo P, Simontacchi G, Livi L (2015) Nutritional supplement based on zinc, prebiotics, probiotics and vitamins to prevent radiation-related gastrointestinal disorders. Anticancer Res 35:5687–5692
Itoh Y, Mizuno M, Ikeda M, Nakahara R, Kubota S, Ito J, Okada T, Kawamura M, Kikkawa F, Naganawa S (2015) A randomized, double-blind pilot trial of hydrolyzed rice bran versus placebo for radioprotective effect on acute gastroenteritis secondary to chemoradiotherapy in patients with cervical cancer. Evid Based Complement Alternat Med 2015:974390
Garcia-Peris P, Velasco C, Hernandez M, Lozano MA, Paron L, de la Cuerda C, Breton I, Camblor M, Guarner F (2016) Effect of inulin and fructo-oligosaccharide on the prevention of acute radiation enteritis in patients with gynecological cancer and impact on quality-of-life: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr 70:170–174
Nascimento M, Aguilar-Nascimento JE, Caporossi C, Castro-Barcellos HM, Motta RT (2014) Efficacy of synbiotics to reduce acute radiation proctitis symptoms and improve quality of life: a randomized, double-blind, placebo-controlled pilot trial. Int J Radiat Oncol Biol Phys 90:289–295
Nelamangala Ramakrishnaiah VP, Javali TD, Dharanipragada K, Reddy KS, Krishnamachari S (2012) Formalin dab, the effective way of treating haemorrhagic radiation proctitis: a randomized trial from a tertiary care hospital in South India. Color Dis 14:876–882
Iyama S, Sato T, Tatsumi H, Hashimoto A, Tatekoshi A, Kamihara Y, Horiguchi H, Ibata S, Ono K, Murase K, Takada K, Sato Y, Hayashi T, Miyanishi K, Akizuki E, Nobuoka T, Mizugichi T, Takimoto R, Kobune M, Hirata K, Kato J (2014) Efficacy of enteral supplementation enriched with glutamine, fiber, and oligosaccharide on mucosal injury following hematopoietic stem cell transplantation. Case Rep Oncol 7:692–699
Topkan E, Parlaks C, Topuk S, Pehlivan B (2012) Influence of oral glutamine supplementation on survival outcomes of patients treated with concurrent chemoradiotherapy for locally advanced non-small cell lung cancer. BMC Cancer 12:502
Vidal-Casariego A, Calleja-Fernández A, de Urbina-González JJ, Cano-Rodríguez I, Cordido F, Ballesteros-Pomar MD (2014) Efficacy of glutamine in the prevention of acute radiation enteritis: a randomized controlled trial. JPEN J Parenter Enteral Nutr 38:205–213
Rotovnik Kozjek N, Kompan L, Soeters P, Oblak I, Mlakar Mastnak D, Možina B, Zadnik V, Anderluh F, Velenik V (2011) Oral glutamine supplementation during preoperative radiochemotherapy in patients with rectal cancer: a randomised double blinded, placebo controlled pilot study. Clin Nutr 30:567–570
Kucuktulu E, Guner A, Kahraman I, Topbas M, Kucuktulu U (2013) The protective effects of glutamine on radiation-induced diarrhea. Support Care Cancer 21:1071–1075
Glover M, Smerdon GR, Andreyev HJ, Benton BE, Bothma P, Firth O, Gothard L, Harrison J, Ignatescu M, Laden G, Martin S, Maynard L, McCann D, Penny CEL, Phillips S, Sharp G, Yarnold J (2016) Hyperbaric oxygen for patients with chronic bowel dysfunction after pelvic radiotherapy (HOT2): a randomised, double-blind, sham-controlled phase 3 trial. Lancet Oncol 17:224–233
Tahir AR, Westhuyzen J, Dass J, Collins MK, Webb R, Hewitt S, Fon P, McKay M (2015) Hyperbaric oxygen therapy for chronic radiation-induced tissue injuries: Australasia’s largest study. Asia Pac J Clin Oncol 11:68–77
Hoff PM, Saragiotto DF, Barrios CH, del Giglio A, Coutinho AK, Andrade AC, Dutra C, Forones NM, Correa M, Portella Mdo S, Passos VQ, Chinen RN, van Eyll B (2014) Randomized phase III trial exploring the use of long-acting release octreotide in the prevention of chemotherapy-induced diarrhea in patients with colorectal cancer: the LARCID trial. J Clin Oncol 32:1006–1011
Bradstock KF, Link E, Collins M, Di Iulio J, Lewis ID, Schwarer A, Enno A, Marlton P, Hahn U, Szer J, Cull G, Seymour JF, Australasian Leukaemia and Lymphoma Group (2014) A randomized trial of prophylactic palifermin on gastrointestinal toxicity after intensive induction therapy for acute myeloid leukaemia. Br J Haematol 167:618–625
Herbers AH, van der Velden WJ, de Haan AF, Donnelly JP, Blijlevens NM (2014) Impact of palifermin on intestinal mucositis of HSCT recipients after BEAM. Bone Marrow Transplant 49:8–10
Campbell P, Friebe A, Foulstone P, Grigg A, Hempton J, Bajel A (2012) Impact of palifermin on mucosal toxicity in autologous stem cell transplants using busulfan-melphalan conditioning chemotherapy for Hodgkin and non-Hodgkin lymphoma. Leuk Lymphoma 53:1415–1416
Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, Miskovska V, Bystricky B, Beniak J, Medvecova L, Lagin A, Svetlovska D, Spanik S, Zajac V, Mardiak J, Drgona L (2015) Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther Med 23:356–362
Demers M, Dagnault A, Desjardins J (2014) A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr 33:761–767
Maggio A, Magli A, Rancati T, Fiorino C, Valvo F, Fellin G, Ricardi U, Munoz F, Cosentino D, Cazzaniga LF, Valdagni R, Vavassori V (2014) Daily sodium butyrate enema for the prevention of radiation proctitis in prostate cancer patients undergoing radical radiation therapy: results of a multicenter randomized placebo-controlled dose-finding phase 2 study. Int J Radiat Oncol Biol Phys 89:518–524
Fuccio L, Guido A (2012) Topical rectal beclomethasone dipropionate treatment for the prevention of radiation-induced bleeding. Gut 61:1369
Chruscielewska-Kiliszek MR, Regula J, Polkowski M, Rupinski M, Kraszewska E, Pachlewski J, Czaczkowska-Kurek E, Butruk E (2013) Sucralfate or placebo following argon plasma coagulation for chronic radiation proctitis: a randomized double blind trial. Color Dis 15:e48–e55
Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid M (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17:259–275
García-Peris P, Velasco C, Lozano MA, Moreno Y, Paron L, de la Cuerda C, Bretón I, Camblor M, García-Hernández J, Guarner F, Hernández M (2012) Effect of a mixture of inulin and fructo-oligosaccharide on Lactobacillus and Bifidobacterium intestinal microbiota of patients receiving radiotherapy: a randomised, double-blind, placebo-controlled trial. Nutr Hosp 27:1908–1915
Tutanc OD, Aydogan A, Akkucuk S, Sunbul AT, Zincircioglu SB, Alpagat G, Erden ES (2013) The efficacy of oral glutamine in prevention of acute radiotherapy-induced esophagitis in patients with lung cancer. Contemp Oncol (Pozn) 17:520–524
Algara M, Rodríguez N, Viñals P, Lacruz M, Foro P, Reig A, Quera J, Lozano J, Fernández-Velilla E, Membrive I, Dengra J, Sanz X (2007) Prevention of radiochemotherapy-induced esophagitis with glutamine: results of a pilot study. Int J Radiat Oncol Biol Phys 69:342–349
Jazieh AR, Younas A, Safa M, Redmond K, Buncher R, Howington J (2007) Phase I clinical trial of concurrent paclitaxel, carboplatin, and external beam chest irradiation with glutamine in patients with locally advanced non-small cell lung cancer. Cancer Investig 25:294–298
Zachariah B, Gwede CK, James J, Ajani J, Chin LJ, Donath D, Rosenthal SA, Kane BL, Rotman M, Berk L, Kachnic LA (2010) Octreotide acetate in prevention of chemoradiation-induced diarrhea in anorectal cancer: randomized RTOG trial 0315. J Natl Cancer Inst 102:547–556
Martenson JA, Halyard MY, Sloan JA, Proulx GM, Miller RC, Deming RL, Dick SJ, Johnson HA, Tai TH, Zhu AW, Keit J, Stien KJ, Atherton PJ (2008) Phase III, double-blind study of depot octreotide versus placebo in the prevention of acute diarrhea in patients receiving pelvic radiation therapy: results of North Central Cancer Treatment Group N00CA. J Clin Oncol 26:5248–5253
Franzén L, Hellsing U, Henriksson R, Littbrand B (1998) Managing side-effects in radiotherapy with regard to the gastrointestinal tract. Recent Results Cancer Res 108:127–133
Henriksson R, Franzén L, Littbrand B (1991) Prevention of irradiation-induced bowel discomfort by sucralfate: a double-blind, placebo-controlled study when treating localized pelvic cancer. Am J Med 91:151S–157S
Johansson JE, Hasseus B, Johansson P, Eklof C, Ohman D, Stockelberg D (2009) Gut protection by palifermin during autologous haematopoietic SCT. Bone Marrow Transplant 43:807–811
Rosen LS, Abdi E, Davis ID, Gutheil J, Schnell FM, Zalcberg J, Cesano A, Gayko U, Chen MG, Clarke S (2006) Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy. J Clin Oncol 24:5194–5200
Farrell CL, Rex KL, Chen JN, Bready JV, DiPalma CR, Kaufman SA, Rattan A, Scully S, Lacey DL (2002) The effects of keratinocyte growth factor in preclinical models of mucositis. Cell Prolif 35:78–85
Gibson RJ, Bowen JM, Keefe DM (2005) Palifermin reduces diarrhea and increases survival following irinotecan treatment in tumor-bearing DA rats. Int J Cancer 116:464–470
Sahebnasagh A, Ghasemi A, Akbari J, Alipour A, Lashkardoost H, Ala S, Salehifar E (2017) Successful treatment of acute radiation proctitis with aloe vera: a preliminary randomized controlled clinical trial. J Altern Complement Med 23:858–865
Ziętarska M, Krawczyk-Lipiec J, Kraj L, Zaucha R, Małgorzewicz S (2017) Chemotherapy-related toxicity, nutritional status and quality of life in precachectic oncologic patients with, or without, high protein nutritional support. A prospective, randomized study. Nutrients 9:E1108
Morishita T, Tsushita N, Imai K, Sakai T, Miyao K, Sakemura R, Kato T, Niimi K, Ono Y, Sawa M (2016) The efficacy of an oral elemental diet in patients undergoing hematopoietic stem cell transplantation. Intern Med 55:3561–3569
Miyata H, Yano M, Yasuda T, Yamasaki M, Murakami K, Makino T, Nishiki K, Sugimura K, Motoori M, Shiraishi O, Mori M, Doki Y (2017) Randomized study of the clinical effects of ω-3 fatty acid-containing enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Nutrition 33:204–210
van Sebille YZA, Gibson RJ, Wardill HR, Bowen JM (2016) Gastrointestinal toxicities of first and second generation small molecule HER TKIs in advanced non small cell lung cancer. Curr Opin Palliat Support Care 10:152–156
Loriot Y, Perlemuter G, Malka D, Penault-Llorca F, Boige V, Deutsch E, Massard C, Armand JP, Soria JC (2008) Drug insight: gastrointestinal and hepatic adverse effects of molecular-targeted agents in cancer therapy. Nat Clin Pract Oncol 5:268–278
Acknowledgments
We would like to acknowledge the expert assistance of our research librarians during the development of the database search terms and paper retrieval; Lorraine Porcello (Bibby Dental Library, Eastman Institute for Oral Health, University of Rochester Medical Center, Rochester, NY, USA) and Daniel A. Castillo (Edward G. Miner Library, University of Rochester Medical Center, Rochester, NY, USA). On behalf of the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
These authors disclose no relevant conflict of interest: JC, NB, NA, EB, KC, CDM, BM, AS, WT, HW, YVS, VR, AV, KKFC, SE. JB and RG are MASCC board members.
PB has served an advisory role for AstraZeneca, Helsinn, and Kyowa Kyrin and received grants from Merck, Kyowa Kyrin, and Roche. DK is on the advisor board for Zealand Pharma and Helsinn, the speakers bureau for Merck and Mundipharma, and a consultant for Entrinsic Health Solutions.
RVL has served as a consultant for Colgate Oral Pharmaceuticals, Galera Therapeutics, Ingalfarma SA, Monopar Therapeutics, Mundipharma, and Sucampo Pharma; has received research support to his institution from Galera Therapeutics, Novartis, Oragenics, and Sucampo Pharma, and has received stock in Logic Biosciences.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bowen, J.M., Gibson, R.J., Coller, J.K. et al. Systematic review of agents for the management of cancer treatment-related gastrointestinal mucositis and clinical practice guidelines. Support Care Cancer 27, 4011–4022 (2019). https://doi.org/10.1007/s00520-019-04892-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04892-0