Abstract
Purpose
To update the clinical practice guidelines for the use of natural and miscellaneous agents for the prevention and/or treatment of oral mucositis (OM).
Methods
A systematic review was conducted by the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer / International Society of Oral Oncology (MASCC/ISOO). The body of evidence for each intervention, in each cancer treatment setting, was assigned an evidence level. The findings were added to the database used to develop the 2014 MASCC/ISOO clinical practice guidelines. Based on the evidence level, the following guidelines were determined: Recommendation, Suggestion, and No Guideline Possible.
Results
A total of 78 papers were identified within the scope of this section, out of which 29 were included in this part, and were analyzed with 27 previously reviewed studies. A new Suggestion was made for oral glutamine for the prevention of OM in head and neck (H&N) cancer patients receiving radiotherapy with concomitant chemotherapy. The previous Recommendation against the use of parenteral glutamine for the prevention of OM in hematopoietic stem cell transplantation (HSCT) patients was re-established. A previous Suggestion for zinc to prevent OM in H&N cancer patients treated with radiotherapy or chemo-radiotherapy was reversed to No Guideline Possible. No guideline was possible for other interventions.
Conclusions
Of the vitamins, minerals, and nutritional supplements studied for the management of OM, the evidence supports a Recommendation against parenteral glutamine in HSCT patients and a Suggestion in favor of oral glutamine in H&N cancer patients for the management of OM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral mucositis (OM) is a debilitating complication of cancer therapy, that in addition to pain, nutritional and psychosocial consequences can lead to treatment delays, breaks, and dose reductions, potentially influencing treatment outcomes [1]. Therefore, extensive research has been conducted to find a remedy for OM.
Natural remedies, including herbal extracts (botanicals) and dietary supplements, have been a topic for prolific research in OM. Nutritional supplements are perceived as a needed element in patients with an unbalanced diet. Likewise, many herbal agents are considered to promote wound healing or to have analgesic effects.
The Mucositis Study Group (MSG) of the Multinational Association of Supportive Care in Cancer / International Society of Oral Oncology (MASCC/ISOO) has published clinical practice guidelines for OM [2,3,4]. In the 2014 guidelines update, the Natural and Miscellaneous section concluded the systematic review with 2 guidelines regarding vitamins, minerals, and nutritional supplements: (1) a Suggestion to use systemic zinc supplement to prevent OM in cancer patients receiving RT or RT-CT and (2) a Recommendation against the use of intravenous glutamine for the prevention of OM in patients receiving high-dose CT prior to hematopoietic stem cell transplantation (HSCT). No guideline was possible for any other agent [5, 6]. The aim of this project was to review newly acquired evidence and update the clinical practice guidelines for the use of natural and miscellaneous agents for the prevention and/or treatment of OM.
Methods
The methods are described in detail in Ranna et al. [7]. Briefly, a search for relevant papers indexed in the literature from January 1, 2011, to June 30, 2016, was conducted using Pubmed/Web of Science/EMBASE, with papers selected for review based on defined eligibility criteria.
Papers were reviewed by two independent reviewers and data were extracted using a standard electronic form. Studies were scored for their level of evidence (LoE) based on the Somerfield criteria, [8] and flaws were listed according to the Hadorn criteria [9]. A well-designed study was defined as a study with no major flaws per the Hadorn criteria.
Findings from the reviewed studies were merged with the evidence reviewed in the previous MASCC/ISOO guideline update. Then, findings from the reviewed studies were integrated into guidelines based on the overall LoE for each intervention. Guidelines were classified into 3 types: Recommendation, Suggestion, and No Guideline Possible.
Guidelines were specified based on the following variables: (1) the aim of the intervention (prevention or treatment of OM); (2) the treatment modality (RT, CT, RT-CT, or HSCT); and (3) the route of administration of the intervention.
The list of intervention keywords used for the literature search of the Natural and Miscellaneous section is presented in the Methods paper [7].
This report will cover the interventions categorized as vitamins, minerals, and nutritional supplements. The remaining agents will be described in the systematic review of Natural and Miscellaneous Agents for the Management of OM in Cancer Patients—Part 2.
Results
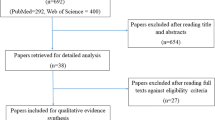
A total of 2653 papers were identified in the literature search: 1863 from PubMed and 790 from Web of Science. After careful assessment of the abstracts, 2563 articles were excluded due to repetition across databases, non-clinical studies, meta-analyses, and reviews. Three additional papers were transferred from other sections of the guidelines update. Ninety-three articles were retrieved for final review. After review of these full papers, 6 were moved to other sections, and 9 were excluded based on the eligibility criteria. A total of 78 papers are included in this section out of which 29 are included in this paper (part 1). These publications described 8 vitamins, minerals, and nutritional supplements and included in this part. These 29 new papers were merged with 27 publications that were reviewed in the previous guidelines update.
Zinc
Zinc is a vital electrolyte for homeostasis and is involved in biologic processes such as growth, wound healing, and immune reaction. Zinc is available in various forms, including zinc sulfate, zinc aspartate, and zinc l-carnosine. It is available commercially in combinations with other vitamins and elements. The specific compound affects the biologically available zinc dose. Table 1 summarizes the details of studies reviewed on zinc.
Zinc (systemic): H&N cancer, RT/RT-CT—prevention Guideline: No guideline possible (LoE I)
The efficacy of systemic zinc administration for the prevention of OM in H&N cancer patients receiving RT or RT-CT was studied in 6 randomized controlled trials (RCT). Of these, 4 reported that zinc was effective for the prevention of OM [10, 13, 15, 18] and 2 reported that zinc was ineffective [16, 17]. In these studies, the RT dose ranged between 50 and 70 Gy and exceeded 60 Gy in most patients. However, reports were not comparable in terms of type of zinc preparations and doses. Zinc sulfate was used in 4 RCTs of which zinc was found to be effective in 2 RCTs [10, 18] and ineffective in the other 2 [16, 17]. The doses administered in these RCTs ranged between 90 and 150 mg/day elemental zinc [10, 16,17,18]. Zinc magnesium aspartate (75 mg per day) was found to be effective in the prevention of OM in a well-designed RCT and its subsequent sub-analysis [13, 14]. Zinc l-carnosine, a zinc-containing molecule, was found effective in preventing OM compared with 4% sodium gualenate hydrate mouthwash [15].
The efficacy of systemic zinc in preventing OM in H&N cancer patients was also evaluated in 2 case-control studies demonstrating conflicting results [11, 12].
Based on the evaluation of the quality of studies, the LoE is rated as I. Due to conflicting data about the efficacy, no guideline was possible.
Zinc (systemic): HSCT—prevention Guideline: No guideline possible (LoE II)
There was a single RCT assessing systemic zinc for the prevention of OM in patients undergoing HSCT [19] and a case-control study [20]. Both studies showed no efficacy. The LoE was II and no guideline was possible.
Zinc (systemic): Hematologic and solid cancers, CT—prevention Guideline: No guideline possible (LoE III)
There was a single RCT assessing systemic zinc administration for the prevention of OM in patients receiving CT for solid and hematologic malignancies [21]. This study concluded zinc was ineffective. The LoE was III and no guideline was possible.
Zinc (topical): Hematologic patients, CT—prevention Guideline: No guideline possible (LoE III)
There was a single RCT assessing topical zinc administration for the prevention of OM in patients receiving CT for leukemia [22]. Zinc sulfate was compounded as a 0.2% mouthwash and the patients were instructed to rinse twice a day for 2 weeks. The topical zinc was compared with 0.2% chlorhexidine mouthwash. The zinc mouthwash was not superior to chlorhexidine mouthwash. The LoE was III and no guideline was possible.
Supersaturated calcium phosphate rinse
Supersaturated calcium phosphate rinses (SCPRs) contain high concentration of calcium and phosphate ions, allowing them to diffuse into the intercellular spaces of the epithelium [23]. The calcium and phosphate ions are hypothesized to play a significant role in inflammatory processes and tissue repair. Table 2 summarizes the details of studies reviewed on SCPR.
Supersaturated calcium phosphate rinse: HSCT—prevention Guideline: No guideline possible (LoE III)
SCPR administered for the prevention of OM in patients undergoing HSCT was evaluated in 3 RCTs. Of these, 2 RCTs [24, 27] reported that SCPR was effective for the prevention of OM. A single RCT reported that it was ineffective [28]. This latter study compared a combination of SCPR and cryotherapy with cryotherapy alone. This study design may have reduced the risk of OM in both groups. Furthermore, in both study arms, 25% of the patients received reduced-intensity cytotoxic conditioning. These features in the study design might have hindered the SCPR’s effect (floor effect).
In addition, 1 comparative study and 1 cohort study have evaluated the efficacy of SCPR in preventing OM in patients undergoing HSCT [25, 26]. A comparison to historic control found that SCPR was effective [25] and the cohort study found it was effective only in a sub-group of patients receiving BEAM (carmustine, etoposide, cytarabine, and melphalan) as a conditioning regimen [26]. An additional study reported using SCPR as part of a multi-agent basic oral care protocol, which did not add information about the effect of SCPR alone [32]. Considering this literature, the LoE is III, and no guideline was possible.
Supersaturated calcium phosphate rinse: HSCT or CT—treatment Guideline: No guideline possible (LoE III)
One RCT evaluated the efficacy of SCPR in treating OM in a heterogenic group of patients including benign hematologic disorders, malignant hematologic diseases, and solid cancers. The patients were treated with CT as a definitive treatment or as a part of the conditioning regimen for HSCT [29]. No beneficial effect was found for SCPR compared with placebo. The LoE was III and no guideline was possible.
Supersaturated calcium phosphate rinse: RT-CT—prevention Guideline: No guideline possible (LoE III)
For SCPR administered for the treatment of OM in patients receiving RT (with or without CT) for H&N cancer, there was 1 RCT [30] showing no efficacy. There was also 1 comparative study showing ineffectiveness [31]. The LoE was III and no guideline was possible.
Glutamine
Glutamine is the most abundant amino acid in plasma and is a well-known nutrient used to increase cell proliferation as well as survival under metabolic stress conditions [33]. Glutamine is frequently used by rapidly dividing cells. In cancer patients, glutamine deficiency might develop, negatively affecting the function of host tissues [34]. Table 3 summarizes the details of studies reviewed on glutamine.
Glutamine (parenteral): HSCT—prevention Guideline: Recommendation against (LoE I)
The efficacy of parenteral glutamine in the prevention of OM in patients undergoing HSCT was studied in 6 RCTs [35, 37,38,39,40,41]. Of these, there was a single study, conducted in pediatrics, published since the publication of the previous guideline [41]. The glutamine regimens in all studies were comparable. Two RCTs [39, 40] found glutamine to be effective in preventing OM, while the 4 other RCTs [35, 37, 38, 41] did not demonstrate a beneficial effect. Moreover, in 1 RCT [38], a significant statistical correlation of glutamine treatment with relapse (p = 0.02) and mortality (p = 0.05) was documented [38]. Taking into account the lack of effectiveness in most studies as well as the potential risk, the panel decided to recommend against the use of parenteral glutamine for the prevention of OM in patients undergoing HSCT.
The LoE is I and the recommendation against is in line with the previous guideline about glutamine for this setting.
Glutamine (PO): HSCT—prevention Guideline: No guideline possible (LoE III)
Three RCTs have evaluated the effectiveness of oral glutamine for the prevention of OM in patients undergoing HSCT [42, 45, 46]. In a single RCT [46], oral glutamine was found to be effective, in another RCT [45], it was ineffective, while the third RCT [42] found it to be effective only in patients who underwent autologous HSCT and ineffective in patients who underwent allogeneic HSCT. In addition, 2 non-RCTs have also reported conflicting results for the efficacy of oral glutamine in preventing OM in this patient population [43, 44]. In light of the above conflicting results, the LoE is III and no guideline was possible.
Glutamine (PO): H&N cancer, RT-CT—prevention Guideline: Suggestion (LoE II)
Oral glutamine was found to be effective in preventing OM due to RT-CT in H&N cancer patients in 2 RCTs [47, 48]. In one RCT, 10 g of oral glutamine consumed 3 times a day throughout the concomitant RT-CT course, along the 6 weeks of treatment, significantly reduced the severity of OM and its associated pain [48]. In the other RCT, 10 g of oral glutamine given 2 h before RT, beginning at the first RT session and continued all along the RT course, significantly reduced the severity and duration of OM [47]. This study had a heterogeneous patient population, with 65% of the patients to receive RT with concurrent CT, and 35% of the patients receiving RT alone. Therefore, the LoE is II and the panel suggested the use of oral glutamine in H&N cancer patients undergoing RT-CT. The suggestion is with caution due to the higher mortality rate seen in HSCT patients treated with parenteral glutamine [38].
Glutamine (topical): H&N cancer, RT—prevention Guideline: No guideline possible (LoE III)
A single RCT [49] demonstrated the effectiveness of topical glutamine in preventing OM in H&N cancer patients undergoing RT. LoE was classified as III. Therefore, it was not possible to provide any guideline.
Glutamine (parenteral): Hematologic malignancies, CT—prevention Guideline: No guideline possible (LoE III)
A single RCT [50] has evaluated the benefit of parenteral glutamine in preventing OM in 16 AML patients undergoing chemotherapy (8 vs. 8 patients). This study, as well as 2 non-RCTs [34, 51], failed to demonstrate any beneficial effect of glutamine. Accordingly, LoE is III, and no guideline was possible.
Glutamine (PO): Solid cancers, CT—prevention Guideline: No guideline possible (LoE II)
A RCT in breast cancer patients evaluating a rapid-uptake formulation of glutamine for the prevention of OM reported positive results [52]. Another small RCT in various solid tumors in pediatric and adult patients reported oral glutamine to be effective in the prevention of OM [55]. Two additional cohort studies involving small patient populations (n = 9 and n = 14) also suggested oral glutamine to be effective [53, 54]. Due to the variability in the glutamine formulation and the patient population, no guideline is possible.
Elemental diet
Elemental diet (ED) formulations have been widely used in Japan for improving nutritional status in patients [61]. These formulas contain amino acids, carbohydrates, vitamins, minerals, and minimal fat and are considered to be a good source of l-glutamine.
Elemental diet (PO): HSCT—prevention Guideline: No guideline possible (LoE IV)
The use of an ED for the prevention of OM in patients undergoing HSCT was assessed in a single cohort study [58] and found to be ineffective. Therefore, no guideline was possible.
Elemental diet (PO): Solid cancers, CT—prevention Guideline: No guideline possible (LoE III)
A small RCT [59] tested the efficacy of ED for the prevention of OM in a heterogeneous group of cancer patients treated with chemotherapy. Ten patients were treated with glutamine, 10 patients were treated with both glutamine and ED, and an additional 10 patients received neither glutamine nor ED and served as the control group. The incidence of grade ≥ 2 OM was the highest in the glutamine group, followed by the control group, and the least in the glutamine plus ED group (p = 0.04). ED was found to be effective also in a cohort study of 22 colorectal cancer patients treated with 5-fluorouracil (FU)-based chemotherapy [60].
Elemental diet (PO, swish and swallow): H&N cancer, RT/RT-CT—prevention Guideline: No guideline possible (LoE IV)
A single retrospective cohort study evaluating the use of ED for the prevention of OM associated with RT (with or without CT) demonstrated that the degree of OM was much lower in patients using ED [61]. Since it was applied as swish and swallow, it is assumed to have both topical and systemic effect.
Vitamin E
Vitamin E refers to eight fat-soluble compounds (α-, β-, γ-, δ-tocopherol, and α-, β-, γ-, δ-tocotrienol) that act as antioxidants. α-Tocopherol, the most common form of vitamin E in human tissues, is considered to have cytoprotective and anti-inflammatory properties [62]. Table 4 summarizes the details of studies reviewed on vitamin E.
Vitamin E (PO, swish and swallow): Hematologic patients, CT—treatment Guideline: No guideline possible (LoE III)
A single RCT [63] compared the efficacy of swish and swallow vitamin E, pycnogenol, and glycerin (vehicle-control) for the treatment of OM in 72 pediatric patients receiving CT for hematologic malignancies. Both vitamin E and pycnogenol were found to be effective compared with the vehicle (p < 0.001 each). However, there was no significant difference between the efficacy observed in the active arms (p = 0.89).
Another RCT compared vitamin E tablets to vitamin E oil swish and swallow [67]. There was no control arm in this study. The study recruited 80 children who were undergoing CT for hematological malignancies, 40 in each arm. Vitamin E swish and swallow was superior to vitamin E pills in the treatment of OM (p < 0.001).
Vitamin E (topical): Solid cancer, CT—treatment Guideline: No guideline possible (LoE III)
A single RCT evaluated vitamin E for the treatment of OM in 17 solid cancer patients receiving CT [64]. Vitamin E mouthwash shortened the duration of OM (p = 0.025). No other studies in this category were identified. Therefore, no guideline is possible.
Vitamin E (topical): Solid cancers, CT—prevention Guideline: No guideline possible (LoE III)
Topical vitamin E was tested for the prevention of OM in a RCT enrolling 16 pediatric patients treated with CT [65]. It is unclear how many patients were enrolled in each arm as the study reported the sample size in terms of cycles of treatment. Most of these patients were diagnosed with solid cancer. There was no difference between the groups in regard to OM severity.
Vitamin E (swish and swallow): H&N cancer, RT—prevention Guideline: No guideline possible (LoE II)
The efficacy of vitamin E for the prevention of OM in H&N cancer patients treated with RT was tested in a RCT [66]. Vitamin E was applied as a swish and swallow oil. This RCT concluded that vitamin E is effective (p = 0.038). No other studies were available in this category. Therefore, no guideline is possible.
Selenium
Selenium is an essential trace element with anti-oxidative and anti-inflammatory properties. It is an important cofactor for glutathione peroxidase, which scavenges free radicals [68]. Table 5 summarizes the details of studies reviewed on selenium.
Selenium (PO): HSCT—prevention Guideline: No guideline possible (LoE III)
The efficacy of oral selenium for the prevention of OM in patients treated with high-dose CT as a part of the conditioning regimen prior to HSCT was evaluated in a single RCT [69]. Selenium was found to be effective in reducing the severity and duration of OM. No other studies on the efficacy of selenium in preventing OM were identified; therefore, no guideline was possible.
Folinic acid
Methotrexate (MTX) is one of the most widely used anti-cancer agents. Folinic acid, also known as leucovorin, is considered to reverse the action of MTX and therefore is usually used in cases of MTX toxicity [72]. Table 5 summarizes the details of studies reviewed on folinic acid.
Folinic acid (PO/topical): HSCT—prevention Guideline: No guideline possible (LoE IV)
Our literature search failed to identify any RCT assessing the efficacy of folinic acid for the prevention of OM in patients undergoing HSCT. A single cohort study [70] found systemic folinic acid to be effective in preventing MTX-induced OM in patients undergoing HSCT (p = 0.014). A trend toward efficacy in preventing OM was found for folinic acid mouthwash (p = 0.051). Considering the LoE, no guideline was possible.
Calcitriol
Calcitriol, also called 1,25-dihydroxycholecalciferol, is the active metabolite of vitamin D. Vitamin D is a group of fat-soluble hormones responsible for increasing intestinal absorption of calcium, magnesium, and phosphate [73]. Vitamin D is speculated to have anti-inflammatory properties related to its ability to diminish the release of TNF-α and to increase the synthesis of interleukin10 [71]. Table 5 summarizes the details of studies reviewed on calcitriol.
GCalcitriol (PO): HSCT—prevention Guideline: No guideline possible (LoE III)
The use of calcitriol for prevention of OM in patients undergoing HSCT was evaluated in a single RCT [71]. Twenty-eight patients with Fanconi anemia undergoing HSCT were treated with either oral calcitriol or placebo. There was no difference in OM incidence or severity between the two groups.
Discussion
This systematic review is the first part of the update about natural and miscellaneous agents for the management of OM. This part included literature about minerals, vitamins, and supplemental diet. Based on the literature review, several changes have been made to the MASCC/ISOO clinical practice guidelines for the management of OM.
In regard to glutamine, parenteral administration in HSCT patients in order to prevent OM yielded a Recommendation against its use. This guideline appeared in the previous guidelines [6]; however, the LoE was elevated from II to I due to a new well-designed RCT [41]. A new Suggestion was made for oral glutamine in H&N cancer patients treated with RT-CT for the prevention of OM. This guideline is based on 2 new RCTs [47, 48]. These studies demonstrated that glutamine at a dose range of 10–30 mg/day, delivered throughout the RT-CT, may be effective to prevent OM. This positive guideline for PO glutamine takes into account the negative guideline for parenteral glutamine by attaching a note to this guideline and advising caution due to the higher relapse and mortality rate in parenteral glutamine administration in HSCT patients [38]. It is unclear if the discrepancy between the outcome of parenteral glutamine in HSCT and PO glutamine for RT-CT relies on the mode of administration or on the different underlying diseases and treatment modalities. Of note, in one of the RCTs used to develop this Suggestion, oral glutamine was delivered as a swish-and-swallow [47], and there may be a combined topical and systemic effect in this study.
In regard to zinc, this guideline update reverses the Suggestion made for zinc in the 2014 MASCC/ISOO guidelines for H&N cancer patients treated with RT or RT-CT [4]. This guideline change is based on 2 new RCTs that reported a lack of effectiveness [16, 17]. It is noted that there is an additional new RCT that confirmed the previous RCTs showing positive results with zinc [18]. However, due to the conflicting evidence, it is impossible to conclude a positive effect for zinc.
The publications about zinc used various compounds. The guideline generalized the conclusion because the number of studies was too low to break down the evidence by specific zinc compound. Based on the current evidence, it is impossible to tell if a certain type of zinc is superior compared with other types of zinc in respect of OM prevention. Since the absorption of the zinc is depended on the zinc compound (for example, out of 220-mg zinc sulfate, 50-mg elemental zinc is bioavailable), it is advised to specify in future research the type of zinc compound.
In regard to SCPR, 2 RCTs in patients undergoing HSCT showed some level of effectiveness, while another RCT found that SCPR did not confer any additional benefit in HSCT patients receiving cryotherapy. Evidence from other types of studies was also conflicting. Therefore, the panel concluded that there was conflicting evidence that warrants additional studies prior to instituting a guideline [24, 27, 28].
Other vitamins and minerals for which the literature search identified new studies include vitamin E, selenium, folinic acid, and calcitriol. Due to limited evidence, no guideline was possible for any of these vitamins and minerals. Furthermore, when a combination protocol was studied, the nature of the mixed intervention did not allow a conclusion about any particular component of the combination [74]. While the previous guideline update identified several studies about vitamin A effects on OM [6], no new studies were found on this topic in this review. Therefore, the status of the previous guideline is unchanged—no guideline possible.
While the timeframe of this literature review ended in mid-2016, several RCTs on the management of OM were published since then. Although not influencing the present guidelines, they are worth mentioning: In a single RCT, oral glutamine has significantly delayed the onset as well as the severity of OM in H&N cancer patients receiving RT-CT [75]. However, glutamine was found to be ineffective in another RCT [76], although it slightly reduced OM compared with placebo. Additionally, a large prospective cohort study has found oral glutamine to be effective in preventing OM [77]. Likewise, a non-blinded RCT in solid cancer patients treated with CT reported that glutamine formulated as sodium azulene sulfonate l-glutamine was effective in preventing OM [78].
Publications post-mid-2016 were also found for ED. A small RCT has evaluated the efficacy of ED in preventing CT-induced OM in esophageal cancer patients [79]. The severity of OM was significantly lower in the ED arm as reported by the patients, but not as measured by the providers. Another RCT failed to demonstrate a beneficial effect of ED in preventing OM in esophageal cancer patient treated with RT with or without concomitant CT [80].
In the search for recently published articles about zinc, a RCT reported that zinc sulfate reduced the incidence and severity of OM in leukemia patients undergoing CT [81]. Additionally, zinc was reported to be effective in 2 comparative studies; one conducted in HSCT and the second conducted in RT for H&N cancer [12, 82].
A search for recently published articles about SCPR identified a large RCT, which concluded that SCPR was ineffective in the management of OM in H&N cancer patients treated with RT/RT-CT [83]. A recent RCT in pediatric patients [84] and comparative studies about SCPR indicated conflicting results about the effectiveness in preventing OM [23, 85].
In summary, for the interventions reviewed in this paper, the available evidence supported guidelines for glutamine. Likewise, based on additional evidence, we reversed a previous guideline for zinc. Considering the growing body of evidence, the guidelines about the agents covered in this section will require updating in the future.
Change history
24 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00520-021-06141-9
References
Elad S, Zadik Y, Yarom N (2017) Oral complications of nonsurgical cancer therapies. Atlas Oral Maxillofac Surg Clin North Am 25:133–147
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE, Mucositis study section of the Multinational Association of Supportive Care in C, the International Society for Oral O (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis ST, Mucositis study section of the Multinational Association for Supportive Care in C, International Society for Oral O (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100:2026–2046
Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S, Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in C, International Society of Oral O (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120:1453–1461
Jensen SB, Jarvis V, Zadik Y, Barasch A, Ariyawardana A, Hovan A, Yarom N, Lalla RV, Bowen J, Elad S, Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral O (2013) Systematic review of miscellaneous agents for the management of oral mucositis in cancer patients. Support Care Cancer 21:3223–3232
Yarom N, Ariyawardana A, Hovan A, Barasch A, Jarvis V, Jensen SB, Zadik Y, Elad S, Bowen J, Lalla RV, Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral O (2013) Systematic review of natural agents for the management of oral mucositis in cancer patients. Support Care Cancer 21:3209–3221
Ranna V, Cheng K, Castillo D, Porcello L, Vaddi A, Lalla R, Bossi P, Elad S (2019) Development of the MASCC/ISOO Clinical Practice Guidelines for Mucositis: an overview of the methods. Support Care Cancer
Somerfield M, Padberg J, Pfister D, Bennett C, Recht A, Smith T, Weeks J, Winn R, Durant J (2000) ASCO clinical practice guidelines: process, progress, pitfalls, and prospects. Class Pap Curr Comments 4:881–886
Hadorn DC, Baker D, Hodges JS, Hicks N (1996) Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol 49:749–754
Ertekin MV, Koc M, Karslioglu I, Sezen O (2004) Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys 58(1):167–174
Doi H, Fujiwara M, Suzuki H, Niwa Y, Nakayama M, Shikata T, Odawara S, Takada Y, Kimura T, Kamikonya N, Hirota S (2015) Polaprezinc reduces the severity of radiation-induced mucositis in head and neck cancer patients. Mol Clin Oncol 3:381–386
Suzuki A, Kobayashi R, Shakui T, Kubota Y, Fukita M, Kuze B, Aoki M, Sugiyama T, Mizuta K, Itoh Y (2016) Effect of polaprezinc on oral mucositis, irradiation period, and time to discharge in patients with head and neck cancer. Head Neck 38:1387–1392
Lin LC, Que J, Lin LK, Lin FC (2006) Zinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head-and-neck cancers: a double-blind, randomized study. Int J Radiat Oncol Biol Phys 65:745–750
Lin YS, Lin LC, Lin SW, Chang CP (2010) Discrepancy of the effects of zinc supplementation on the prevention of radiotherapy-induced mucositis between patients with nasopharyngeal carcinoma and those with oral cancers: subgroup analysis of a double-blind, randomized study. Nutr Cancer 62:682–691
Watanabe T, Ishihara M, Matsuura K, Mizuta K, Itoh Y (2010) Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int J Cancer 127:1984–1990
Sangthawan D, Phungrassami T, Sinkitjarurnchai W (2013) A randomized double-blind, placebo-controlled trial of zinc sulfate supplementation for alleviation of radiation-induced oral mucositis and pharyngitis in head and neck cancer patients. J Med Assoc 96:69–76
Gorgu SZ, Ilknur AF, Sercan O, Rahsan H, Nalan A (2013) The effect of zinc sulphate in the prevention of radiation induced oral mucositis in patents with head and neck cancer. Int J Radiat Res 11:111–116
Moslemi D, Babaee N, Damavandi M, Pourghasem M, Moghadamnia AA (2014) Oral zinc sulphate and prevention of radiation-induced oropharyngeal mucositis in patients with head and neck cancers: a double blind, randomized controlled clinical trial. Int J Radiat Res 12:235–241
Mansouri A, Hadjibabaie M, Iravani M, Shamshiri AR, Hayatshahi A, Javadi MR, Khoee SH, Alimoghaddam K, Ghavamzadeh A (2012) The effect of zinc sulfate in the prevention of high-dose chemotherapy-induced mucositis: a double-blind, randomized, placebo-controlled study. Hematol Oncol 30:22–26
Hayashi H, Kobayashi R, Suzuki A, Ishihara M, Nakamura N, Kitagawa J, Kanemura N, Kasahara S, Kitaichi K, Hara T, Tsurumi H, Moriwaki H, Itoh Y (2014) Polaprezinc prevents oral mucositis in patients treated with high-dose chemotherapy followed by hematopoietic stem cell transplantation. Anticancer Res 34:7271–7277
Arbabi-kalati F, Arbabi-kalati F, Deghatipour M, Ansari Moghadam A (2012) Evaluation of the efficacy of zinc sulfate in the prevention of chemotherapy-induced mucositis: a double-blind randomized clinical trial. Arch Iran Med 15:413–417
Mehdipour M, Taghavi Zenoz A, Asvadi Kermani I, Hosseinpour A (2011) A comparison between zinc sulfate and chlorhexidine gluconate mouthwashes in the prevention of chemotherapy-induced oral mucositis. Daru 19:71–73
Bhatt N, Naithani R, Gupta SK (2017) Supersaturated calcium phosphate rinse in prevention and treatment of mucositis in patients undergoing hematopoietic stem. Cell Transplant Exp Clin Transplant 15:567–570
Papas AS, Clark RE, Martuscelli G, O'Loughlin KT, Johansen E, Miller KB (2003) A prospective, randomized trial for the prevention of mucositis in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 31:705–712
Wasko-Grabowska A, Rzepecki P, Oborska S, Barzal J, Mlot B, Gawronski K, Wasko M, Szczylik C (2012) A supersaturated calcium phosphate solution seems to effectively prevent and treat oral mucositis in haematopoietic stem cell transplanted cancer patients - single centre experience. J BUON 17:363–368
Wasko-Grabowska A, Rzepecki P, Oborska S, Barzal J, Gawronski K, Mlot B, Szczylik C (2011) Efficiency of supersaturated calcium phosphate mouth rinse treatment in patients receiving high-dose melphalan or BEAM prior to autologous blood stem cell transplantation: a single-center experience. Transplant Proc 43:3111–3113
Markiewicz M, Dzierzak-Mietla M, Frankiewicz A, Zielinska P, Koclega A, Kruszelnicka M, Kyrcz-Krzemien S (2012) Treating oral mucositis with a supersaturated calcium phosphate rinse: comparison with control in patients undergoing allogeneic hematopoietic stem cell transplantation. Support Care Cancer 20:2223–2229
Svanberg A, Ohrn K, Birgegard G (2015) Caphosol((R)) mouthwash gives no additional protection against oral mucositis compared to cryotherapy alone in stem cell transplantation. A pilot study. Eur J Oncol Nurs 19:50–53
Raphael MF, den Boer AM, Kollen WJ, Mekelenkamp H, Abbink FC, Kaspers GJ, Zomer-Kooijker K, Molmans BH, Tissing WJ (2014) Caphosol, a therapeutic option in case of cancer therapy-induced oral mucositis in children?: results from a prospective multicenter double blind randomized controlled trial. Support Care Cancer 22:3–6
Lambrecht M, Mercier C, Geussens Y, Nuyts S (2013) The effect of a supersaturated calcium phosphate mouth rinse on the development of oral mucositis in head and neck cancer patients treated with (chemo)radiation: a single-center, randomized, prospective study of a calcium phosphate mouth rinse + standard of care versus standard of care. Support Care Cancer 21:2663–2670
Stokman MA, Burlage FR, Spijkervet FK (2012) The effect of a calcium phosphate mouth rinse on (chemo) radiation induced oral mucositis in head and neck cancer patients: a prospective study. Int J Dent Hyg 10:175–180
Bhatt V, Vendrell N, Nau K, Crumb D, Roy V (2010) Implementation of a standardized protocol for prevention and management of oral mucositis in patients undergoing hematopoietic cell transplantation. J Oncol Pharm Pract 16:195–204
Cluntun AA, Lukey MJ, Cerione RA, Locasale JW (2017) Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3:169–180
Yildirim ZK, Bidev D, Buyukavci M (2013) Parenteral glutamine supplementation has no effect on chemotherapy-induced toxicity in children with non-Hodgkin lymphoma. J Pediatr Hematol Oncol 35:371–376
van Zaanen HC, van der Lelie H, Timmer JG, Furst P, Sauerwein HP (1994) Parenteral glutamine dipeptide supplementation does not ameliorate chemotherapy-induced toxicity. Cancer 74(10):2879–2884 1994 Nov 15 74: 2879-2884
Kuskonmaz B, Yalcin S, Kucukbayrak O, Cetin N, Cetin M, Tezcan I, Uckan D (2008) The effect of glutamine supplementation on hematopoietic stem cell transplant outcome in children: a case-control study. Pediatr Transplant 12(1):47–51
Schloerb PR, Skikne BS (1999) Oral and parenteral glutamine in bone marrow transplantation: a randomized, double-blind study. JPEN J Parenter Enteral Nutr 23:117–122
Pytlik R, Benes P, Patorkova M, Chocenska E, Gregora E, Prochazka B, Kozak T (2002) Standardized parenteral alanyl-glutamine dipeptide supplementation is not beneficial in autologous transplant patients: a randomized, double-blind, placebo controlled study. Bone Marrow Transplant 30:953–961
Piccirillo N, De Matteis S, Laurenti L, Chiusolo P, Sora F, Pittiruti M, Rutella S, Cicconi S, Fiorini A, D'Onofrio G, Leone G, Sica S (2003) Glutamine-enriched parenteral nutrition after autologous peripheral blood stem cell transplantation: effects on immune reconstitution and mucositis. Haematologica 88:192–200
Blijlevens NM, Donnelly JP, Naber AH, Schattenberg AV, DePauw BE (2005) A randomised, double-blinded, placebo-controlled, pilot study of parenteral glutamine for allogeneic stem cell transplant patients. Support Care Cancer 13:790–796
Uderzo C, Rebora P, Marrocco E, Varotto S, Cichello F, Bonetti M, Maximova N, Zanon D, Fagioli F, Nesi F, Masetti R, Rovelli A, Rondelli R, Valsecchi MG, Cesaro S (2011) Glutamine-enriched nutrition does not reduce mucosal morbidity or complications after stem-cell transplantation for childhood malignancies: a prospective randomized study. Transplantation 91:1321–1325
Anderson PM, Ramsay NK, Shu XO, Rydholm N, Rogosheske J, Nicklow R, Weisdorf DJ, Skubitz KM (1998) Effect of low-dose oral glutamine on painful stomatitis during bone marrow transplantation. Bone Marrow Transplant 22(4):339–344
Cockerham MB, Weinberger BB, Lerchie SB (2000) Oral glutamine for the prevention of oral mucositis associated with high-dose paclitaxel and melphalan for autologous bone marrow transplantation. Ann Pharmacother 34(3):300–303
Iyama S, Sato T, Tatsumi H, Hashimoto A, Tatekoshi A, Kamihara Y, Horiguchi H, Ibata S, Ono K, Murase K, Takada K, Sato Y, Hayashi T, Miyanishi K, Akizuki E, Nobuoka T, Mizugichi T, Takimoto R, Kobune M, Hirata K, Kato J (2014) Efficacy of enteral supplementation enriched with glutamine, fiber, and oligosaccharide on mucosal injury following hematopoietic stem cell transplantation. Case Rep Oncol 7:692–699
Coghlin Dickson TM, Wong RM, Offrin RS, Shizuru JA, Johnston LJ, Hu WW, Blume KG, Stockerl-Goldstein KE (2000) Effect of oral glutamine supplementation during bone marrow transplantation. JPEN J Parenter Enteral Nutr 24(2):61–66
Aquino VM, Harvey AR, Garvin JH, Godder KT, Nieder ML, Adams RH, Jackson GB, Sandler ES (2005) A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: a pediatric blood and marrow transplant consortium study. Bone Marrow Transplant 36(7):611–616
Chattopadhyay S, Saha A, Azam M, Mukherjee A, Sur PK (2014) Role of oral glutamine in alleviation and prevention of radiation-induced oral mucositis: a prospective randomized study. South Asian J Cancer 3:8–12
Tsujimoto T, Yamamoto Y, Wasa M, Takenaka Y, Nakahara S, Takagi T, Tsugane M, Hayashi N, Maeda K, Inohara H, Uejima E, Ito T (2015) L-glutamine decreases the severity of mucositis induced by chemoradiotherapy in patients with locally advanced head and neck cancer: a double-blind, randomized, placebo-controlled trial. Oncol Rep 33:33–39
Huang EY, Leung SW, Wang CJ, Chen HC, Sun LM, Fang FM, Yeh SA, Hsu HC, Hsiung CY (2000) Oral glutamine to alleviate radiation-induced oral mucositis: a pilot randomized trial. Int J Radiat Oncol Biol Phys 46(3):535–539
Sornsuvit C, Komindr S, Chuncharunee S, Wanikiat P, Archararit N, Santanirand P (2008) Pilot study: effects of parenteral glutamine dipeptide supplementation on neutrophil functions and prevention of chemotherapy-induced side-effects in acute myeloid leukaemia patients. J Int Med Res 36(6):1383–1391
Ward E, Smith M, Henderson M, Reid U, Lewis I, Kinsey S, Allgar V, Bowers D, Picton SV (2009) The effect of high-dose enteral glutamine on the incidence and severity of mucositis in paediatric oncology patients. Eur J Clin Nutr 63(1):134–140
Peterson DE, Jones JB, Petit RG (2007) 2nd Randomized, placebo-controlled trial of Saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based chemotherapy. Cancer 109(2):322–331
Skubitz KM, Anderson PM (1996) Oral glutamine to prevent chemotherapy induced stomatitis: a pilot study. J Lab Clin Med 127(2):223–228
Rubio IT, Cao Y, Hutchins LF, Westbrook KC, Klimberg VS (1998) Effect of glutamine on methotrexate efficacy and toxicity. Ann Surg 227(5):772–778; discussion 778-80
Anderson PM, Schroeder G, Skubitz KM (1998) Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer 83:1433–1439
Jebb SA, Osborne RJ, Maughan TS, Mohideen N, Mack P, Mort D, Shelley MD, Elia M (1994) 5-fluorouracil and folinic acid-induced mucositis: no effect of oral glutamine supplementation. Br J Cancer 70:732–735
Choi K, Lee SS, Oh SJ, Lim SY, Jeon WK, Oh TY, Kim JW (2007) The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin Nutr 26:57–62
Morishita T, Tsushita N, Imai K, Sakai T, Miyao K, Sakemura R, Kato T, Niimi K, Ono Y, Sawa M (2016) The efficacy of an Oral elemental diet in patients undergoing hematopoietic stem cell transplantation. Intern Med 55:3561–3569
Tanaka Y, Takahashi T, Yamaguchi K, Osada S, Shimokawa T, Yoshida K (2016) Elemental diet plus glutamine for the prevention of mucositis in esophageal cancer patients receiving chemotherapy: a feasibility study. Support Care Cancer 24:933–941
Ogata Y, Ishibashi N, Yamaguchi K, Uchida S, Kamei H, Nakayama G, Hirakawa H, Tanigawa M, Akagi Y (2016) Preventive effects of amino-acid-rich elemental diet Elental(R) on chemotherapy-induced oral mucositis in patients with colorectal cancer: a prospective pilot study. Support Care Cancer 24:783–789
Harada K, Ferdous T, Horinaga D, Uchida K, Mano T, Mishima K, Park S, Hanazawa H, Takahashi S, Okita A, Fukunaga M, Maruta J, Kami N, Shibuya K, Ueyama Y (2016) Efficacy of elemental diet on prevention for chemoradiotherapy-induced oral mucositis in patients with oral squamous cell carcinoma. Support Care Cancer 24:953–959
Galli F, Azzi A, Birringer M, Cook-Mills JM, Eggersdorfer M, Frank J, Cruciani G, Lorkowski S, Ozer NK (2017) Vitamin E: emerging aspects and new directions. Free Radic Biol Med 102:16–36
Khurana H, Pandey RK, Saksena AK, Kumar A (2013) An evaluation of vitamin E and pycnogenol in children suffering from oral mucositis during cancer chemotherapy. Oral Dis 19:456–464
Wadleigh RG, Redman RS, Graham ML, Krasnow SH, Anderson A (1992) Cohen MH vitamin E in the treatment of chemotherapy-induced mucositis. Am J Med 92(5):481–484
Sung L, Tomlinson GA, Greenberg ML, Koren G, Judd P, Ota S, Feldman BM (2007) Serial controlled N-of-1 trials of topical vitamin E as prophylaxis for chemotherapy-induced oral mucositis in paediatric patients. Eur J Cancer 43(8):1269–1275
Ferreira PR, Fleck JF, Diehl A, Barletta D, Braga-Filho A, Barletta A, Ilha L (2004) Protective effect of alpha-tocopherol in head and neck cancer radiation-induced mucositis: a double-blind randomized trial. Head Neck 26(4):313–321
El-Housseiny AA, Saleh SM, El-Masry AA, Allam AA (2007) The effectiveness of vitamin “E” in the treatment of oral mucositis in children receiving chemotherapy. J Clin Pediatr Dent 31:167–170
Ryan-Harshman M, Aldoori W (2005) The relevance of selenium to immunity, cancer, and infectious/inflammatory diseases. Can J Diet Pract Res 66:98–102
Jahangard-Rafsanjani Z, Gholami K, Hadjibabaie M, Shamshiri AR, Alimoghadam K, Sarayani A, Mojtahedzadeh M, Ostadali-Dehaghi M, Ghavamzadeh A (2013) The efficacy of selenium in prevention of oral mucositis in patients undergoing hematopoietic SCT: a randomized clinical trial. Bone Marrow Transplant 48:832–836
Sugita J, Matsushita T, Kashiwazaki H, Kosugi M, Takahashi S, Wakasa K, Shiratori S, Ibata M, Shono Y, Shigematsu A, Obara M, Fujimoto K, Endo T, Nishio M, Kondo T, Hashino S, Tanaka J, Asaka M, Imamura M (2012) Efficacy of folinic acid in preventing oral mucositis in allogeneic hematopoietic stem cell transplant patients receiving MTX as prophylaxis for GVHD. Bone Marrow Transplant 47:258–264
Hamidieh AA, Sherafatmand M, Mansouri A, Hadjibabaie M, Ashouri A, Jahangard-Rafsanjani Z, Gholami K, Javadi MR, Ghavamzadeh A, Radfar M (2016) Calcitriol for oral mucositis prevention in patients with Fanconi anemia undergoing hematopoietic SCT: a double-blind, randomized, placebo-controlled trial. Am J Ther 23:e1700–e1708
Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncologist 11:694–703
Holick MF (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80:1678S–1688S
Chitapanarux I, Pisprasert V, Tharavichitkul E, Jakrabhandu S, Klunklin P, Onchan W, Supawongwattana B, Traisathit P, Rattanachaiwong S, Sattasiri WM (2016) Randomized study of nutritional status and treatment toxicities of oral arginine, glutamine, and omega-3 fatty acids during concurrent chemoradiotherapy for head and neck cancer patients. Funct Foods Health Dis 6:121–132
Pattanayak L, Panda N, Dash MK, Mohanty S, Samantaray S (2016) Management of chemoradiation-induced mucositis in head and neck cancers with oral glutamine. J Glob Oncol 2:200–206
Lopez-Vaquero D, Gutierrez-Bayard L, Rodriguez-Ruiz JA, Saldana-Valderas M, Infante-Cossio P (2017) Double-blind randomized study of oral glutamine on the management of radio/chemotherapy-induced mucositis and dermatitis in head and neck cancer. Mol Clin Oncol 6:931–936
Pachon Ibanez J, Pereira Cunill JL, Osorio Gomez GF, Irles Rocamora JA, Serrano Aguayo P, Quintana Angel B, Fuentes Pradera J, Chaves Conde M, Ortiz Gordillo MJ, Garcia Luna PP (2018) Prevention of oral mucositis secondary to antineoplastic treatments in head and neck cancer by supplementation with oral glutamine. Nutr Hosp 35:428–433
Nihei S, Sato J, Komatsu H, Ishida K, Kimura T, Tomita T, Kudo K (2018) The efficacy of sodium azulene sulfonate L-glutamine for managing chemotherapy-induced oral mucositis in cancer patients: a prospective comparative study. J Pharm Health Care Sci 4:20
Okada T, Nakajima Y, Nishikage T, Ryotokuji T, Miyawaki Y, Hoshino A, Tokairin Y, Kawada K, Nagai K, Kawano T (2017) A prospective study of nutritional supplementation for preventing oral mucositis in cancer patients receiving chemotherapy. Asia Pac J Clin Nutr 26:42–48
Ishikawa T, Yasuda T, Doi T, Okayama T, Sakamoto N, Gen Y, Dohi O, Yoshida N, Kamada K, Uchiyama K, Handa O, Takagi T, Konishi H, Yagi N, Kokura S, Naito Y, Itoh Y (2016) The amino acid-rich elemental diet Elental(R) preserves lean body mass during chemo- or chemoradiotherapy for esophageal cancer. Oncol Rep 36:1093–1100
Rambod M, Pasyar N, Ramzi M (2018) The effect of zinc sulfate on prevention, incidence, and severity of mucositis in leukemia patients undergoing chemotherapy. Eur J Oncol Nurs 33:14–21
Hayashi H, Kobayashi R, Suzuki A, Yamada Y, Ishida M, Shakui T, Kitagawa J, Hayashi H, Sugiyama T, Takeuchi H, Tsurumi H, Itoh Y (2016) Preparation and clinical evaluation of a novel lozenge containing polaprezinc, a zinc-L-carnosine, for prevention of oral mucositis in patients with hematological cancer who received high-dose chemotherapy. Med Oncol 33:7
Wong KH, Kuciejewska A, Sharabiani MTA, Ng-Cheng-Hin B, Hoy S, Hurley T, Rydon J, Grove L, Santos A, Ryugenji M, Bhide SA, Nutting CM, Harrington KJ, Newbold KL (2017) A randomised controlled trial of Caphosol mouthwash in management of radiation-induced mucositis in head and neck cancer. Radiother Oncol 122:207–211
Treister N, Nieder M, Baggott C, Olson E, Chen L, Dang H, Krailo M, August A, Sung L (2017) Caphosol for prevention of oral mucositis in pediatric myeloablative haematopoietic cell transplantation. Br J Cancer 116:21–27
Kiprian D, Jarzabski A, Kawecki A (2016) Evaluation of efficacy of Caphosol in prevention and alleviation of acute side effects in patients treated with radiotherapy for head and neck cancers. Contemp Oncol (Pozn) 20:389–393
Acknowledgments
The authors are thankful for the medical librarians for their valuable contribution to this project:
Lorraine Porcello, MSLIS, MSIM – Bibby Dental Library, Eastman Institute for Oral Health, University of Rochester Medical Center, Rochester, NY, USA; Daniel A. Castillo, MLIS – Edward G. Miner Library, University of Rochester Medical Center, Rochester, NY, USA.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Per the MASCC Guidelines Policy, employees of commercial entities were not eligible to serve on this MASCC Guidelines Panel.
The authors disclose no conflict of interest (NY, AH, AA, SBJ, MG, HIH, AK, AM, GO, MP, NMN, TR, ASL, NST, EZ, VR, AV, KF, AB, SE). PB has served an advisory role for AstraZeneca, Helsinn, and Kyowa Kyrin and received grants from Merck, Kyowa Kyrin, and Roche.
RVL has served as a consultant for Colgate Oral Pharmaceuticals, Galera Therapeutics, Ingalfarma SA, Monopar Therapeutics, Mundipharma, and Sucampo Pharma; has received research support to his institution from Galera Therapeutics, Novartis, Oragenics, and Sucampo Pharma; and has received stock in Logic Biosciences.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yarom, N., Hovan, A., Bossi, P. et al. Systematic review of natural and miscellaneous agents for the management of oral mucositis in cancer patients and clinical practice guidelines—part 1: vitamins, minerals, and nutritional supplements. Support Care Cancer 27, 3997–4010 (2019). https://doi.org/10.1007/s00520-019-04887-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04887-x