Abstract
Purpose
Neutropenic complications remain the major dose-limiting toxicities of cancer chemotherapy. The aim of this study was to develop and internally validate a comprehensive and easily measurable scoring system for prediction of severe or febrile neutropenia in the first chemotherapy cycle of patients with solid tumors or lymphoma.
Methods
This prospective cohort study included consecutive patients at a tertiary referral hospital. Many clinical and laboratory-independent variables were measured at baseline. A multivariable logistic regression analysis was applied after unadjusted analysis, and the multivariable model was transformed into a simplified risk score based on 6 bootstrapped regression coefficients. The simplified scoring system was internally validated using cross-validation. All statistical tests were two-sided.
Results
A total of 305 patients were enrolled and followed during 1732 chemotherapy cycles. Of these, 259 were eligible for analysis. The multivariable model revealed 6 predictive factors for severe or febrile neutropenia (scores in parentheses): high-risk regimen without colony-stimulating factor (4 points), intermediate-risk regimen without colony-stimulating factor (3 points), age > 65 years and elevated ferritin (3 points), body mass index < 23 kg/m2 and body surface area < 2 m2 (2 points), estimated glomerular filtration rate < 60 mL/min/1.73m2 (2 points), and elevated C-reactive protein (1 point). The receiver operating characteristic curve was 0.832 (95% confidence interval [Cl], 0.767–0.897) for the simplified model and 0.816 (95% Cl, 0.771–0.860) for the cross-validation.
Conclusions
We developed and internally validated a user-friendly prediction model to guide personalized decision-making using available clinical data and few cost-effective laboratory tests. External validation in other centers with different patients is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced neutropenia continues to represent a serious and prevalent complication of many chemotherapy regimens, and it is often associated with hospitalization, treatment interruption, and dose reduction, which may compromise treatment outcomes [1,2,3,4].
Although granulocyte colony-stimulating factors (G-CSFs) have been shown to be prophylactically effective, they are expensive and associated with increased health-care costs [2]. Therefore, selective use of prophylactic G-CSFs in patients at high risk for neutropenic complications may optimize the health-care costs [5]. International clinical practice guidelines in oncology recommend prophylactic administration of a G-CSF if the risk of febrile neutropenia (FN) is 20% or higher [6,7,8,9]. Although these guidelines suggest FN risk calculation for each individual patient prior to chemotherapy and look forward to find a user-friendly risk prediction tool, no such comprehensive and clinically usable model has yet been widely accepted [6]. Thus, it seems essential to develop of a comprehensive and simplified model for prediction of neutropenic events.
Some previous studies have attempted to develop prediction models for neutropenia-related complications [10,11,12,13,14,15,16,17,18,19]. In a systematic review [20], Lyman et al. summarized several patient-, treatment-, and disease-related risk factors for FN and discussed the limitations of developed models. The retrospective design, small sample size, and lack of validation were mentioned the main limitations. In this systematic review, older age, poor performance status, low baseline white blood cell counts, low body mass index (BMI) or body surface area (BSA), advanced disease, use of myelosuppressive chemotherapy regimens, and the presence of comorbidities such as renal or hepatic disorders were considered as major significant predictors for the neutropenic events. Furthermore, the value of inflammatory markers in prediction of chemotherapy-induced myelotoxicity has been shown [19, 21]. Alexandre et al. hypothesized that the stimulation of acute phase protein responses (APPR) could be responsible for increased severe hematologic toxicity of chemotherapy [21]. Moreover, in a prospective study, patients with evidence of inflammation at baseline were more susceptible to FN [19]. Consequently, they recommended the baseline assessment of inflammatory status before initiation of cytotoxic chemotherapy. In the light of these studies, in addition to the majority of well-known and previously identified risk factors, we used a panel of cost-effective laboratory markers as the possible predictors in our study.
A simplified scoring system based on regression coefficients of a final multivariable model can help us to develop a clinically applicable model [22, 23]. Concerns about the overuse and underuse of G-CSFs, as well as their related complications, led us to develop a simplified scoring system for a better individualized decision-making. Since the first episode of severe neutropenia (SN) or FN occurs most frequently in the first cycle of cytotoxic chemotherapy [13], our primary outcome was the occurrence of SN or FN as a composite endpoint in the first cycle of a new chemotherapy regimen. This model could be externally validated later in other centers for all standard chemotherapy regimens.
Methods
Study design and participants
We reported this study based on the TRIPOD and STROBE statements [23, 24]. The study design was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (approval number 93.11.22.164). Written informed consent was obtained from the patients.
Between February 2015 and March 2016, a total of 305 consecutive adult patients with solid tumors or lymphoma who were beginning a new chemotherapy line were prospectively included in this cohort study and followed until June 2016. Patients for whom the nadir (days 10–14 after start of treatment) complete blood count (CBC) in the first cycle was not documented were considered as lost to follow-up and were excluded from the analysis. A total of 259 patients receiving at least one cycle of chemotherapy in a tertiary referral medical center (Shohada-e-Tajrish Medical Center, Tehran, Iran) were considered for the complete-case analysis. Data regarding types of cancers and treatment regimens of this study are summarized in Supplementary Table 1 (Online Resource).
Outcome and predictors
Our primary endpoint as a composite outcome was the occurrence of SN or FN in the first cycle of chemotherapy. Some previously identified predictors in addition to a panel of cost-effective inflammatory markers were considered as the primary possible predictors for the outcome of interest. Twenty-three readily accessible laboratory covariates were evaluated at baseline (Supplementary Table 2, Online Resource). A total of 14 laboratory and clinical candidate predictors were assessed in unadjusted analysis (Table 1). Final selected predictors were assessed in adjusted analysis (Table 2). Further details regarding the predictors and outcome assessment are explained in the Supplementary Methods (Online Resource).
Statistical methods
A detailed report regarding the statistical methods used, predictor handling, selection procedure, collinearity and interaction terms, model development, validation, sample size, missing data, and risk groups is provided in the Supplementary Methods (Online Resource). Briefly, the univariable and multivariable logistic regression analyses were used. The model discrimination and calibration were assessed. To improve the model applicability, we transformed the multivariable model into a simplified scoring system. The simplified model was internally validated using fivefold cross-validation. The statistical analyses were performed using the IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, N.Y., USA). All statistical tests were two-sided.
Results
Participants
A total of 305 patients were prospectively included in this study and followed through 1732 cycles. Of these, 46 were considered lost to follow-up and were excluded from the regression analyses. The median number of chemotherapy cycles was 6 (range, 1–16). The patient flow diagram is shown in Supplementary Fig. 1 (Online Resource). Thirty-two patients developed at least one episode of FN during all cycles of chemotherapy. Results showed that 51% of the first FN events occurred in the first cycle. Of 259 eligible patients, 56 (21.6%) experienced severe or febrile neutropenia in the first chemotherapy cycle. Patients’ characteristics are summarized in Table 3.
Model development and specification
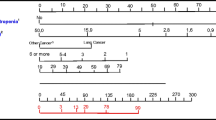
The predictor selection procedure is shown in Supplementary Fig. 1 (Online Resource). Unadjusted associations between 14 candidate predictors and the outcome are indicated in Table 1. The components and characteristics of the adjusted model (e.g., the regression coefficients, bootstrap P values, risk ratios, and scores of the predictors) are summarized in Table 2. After bootstrapping, the model was transformed into a clinically usable scoring system. Based on the point score of each predictor (Table 2), a total risk score was calculated for each patient. Although the total score of the patients in this study ranged between 0 and 8, the maximum possible score in the scoring system was 12. Patients with a total risk score of ≥ 4 were expected to experience the outcome. By using this cutoff score, we predicted a group of patients with a high probability of SN or FN occurrence, for whom the prophylactic G-CSF could be considered. Figure 1 demonstrates a relation between the risk score and percent of the event in each score. Three clinical examples indicating how to use the simplified model are presented in Supplementary Table 3 (Online Resource). In total, 181 patients had a risk score of less than 4 points. Of these, only 10 patients (5.5%) experienced the outcome. In contrast, among 78 patients identified as being at high risk for the neutropenic events (using a cutoff score of ≥ 4 points), a total of 46 (58.9%) experienced the outcome.
Model performance
The final bootstrapped model was well calibrated (Hosmer-Lemeshow p = .708, Supplementary Table 4, Online Resource). A cutoff probability of 0.18 for the full model and a cutoff score of 4 points for the simplified model provided the optimal sensitivity and specificity on the basis of the receiver operating characteristic (ROC) curve. As indicated in Fig. 2, the full model and simplified model had the area under the ROC curve of 0.845 (95% confidence interval [CI], 0.788–0.903) and 0.832 (95% Cl, 0.767–0.897), respectively. The simplified model retained the ability to predict the outcome as excellently as the full model (the ROCs between 0.8 and 0.9 are considered excellent discrimination) [25]. The scoring system had the sensitivity of 82.1%, specificity of 84.2%, positive predictive value (PPV) of 58.9%, and negative predictive value (NPV) of 94.5%. The simplified model was internally validated by cross-validation. The area under the ROC curve for the cross-validation was 0.816 (95% Cl, 0.771–0.860).
Receiver operating characteristic (ROC) curve of the outcome predicted group for the full model and simplified model. The straight line shows the relation between sensitivity and 1-specificity under the null hypothesis of no discrimination. The area under the ROC curve was 0.845 (95% confidence interval [CI], 0.788–0.903) and 0.832 (95% CI, 0.767–0.897) for the full model and simplified model, respectively
Discussion
Interpretation
Multivariable model
Development of a unique, comprehensive, and clinically usable model to predict the chemotherapy-induced neutropenic complications in patients with different tumor types is highly recommended [6]. Lyman et al. have developed an adjusted model based on some laboratory and clinical predictors [13]. However, this model has not been simplified for clinical decision-making. By “simplified model or scoring system,”, we mean a simplified format of the underlying regression model to ease its use in practice [22]. As stated in the Supplementary Methods (Online Resource), this simplification could be done, for example, by transforming (such as rounding) the regression coefficients of predictors included in the final model to easy-to-sum integers which are then associated with outcome. It should be noted that a simple unadjusted analysis based on the baseline values and significant differences indicated in Table 3 is not adequate to predict the risk in place of the model. All final predictors in the model have been adjusted for the other independent variables. In fact, adjusted regression coefficients of a multivariable model would provide an improved estimation of the risk. In a prospective study, López-Pousa et al. presented a prediction model for the first cycle chemotherapy-induced neutropenia in patients with different solid tumors [15]. In another study of patients with non-Hodgkin lymphoma, a multivariable model was also developed by Pettengell et al. for the first cycle FN occurrence [17]. The PPVs of these two models were markedly lower (17% for López-Pousa et al.’s model and 28% for Pettengell et al.’s model) compared with that of ours (i.e., 58.9%) in patients either with solid tumors or lymphoma. The low PPV of these models denotes that they classified many patients as high risk, while they did not actually develop the event. Furthermore, using genetic factors in addition to patient-related and chemotherapy-related risk factors, Pfeil et al. developed a multivariable prediction model for FN occurrence in early breast cancer patients receiving chemotherapy [11]. Nevertheless, overall predictive ability of the model remained poor even after including some genetic factors in the model.
There are only few studies which have developed a simplified risk score for prediction of neutropenic complications in patients with different tumor types. In a SEER-based study, Hosmer et al. developed a simplified risk score to predict the first cycle occurrence of FN in elderly patients with breast, lung, colorectal, and prostate cancer [14]. Of note, the model sensitivity and PPV were considerably low (24 and 12%, respectively). In another study of 266 patients with hematologic malignancies, Moreau et al. developed a general myelotoxicity score to predict the occurrence of FN [16]. A score (0–4) was assigned to each drug according to its expected rate of SN. The major characteristics of the mentioned models are summarized in Table 4.
Predictors
The use of some certain myelosuppressive agents or regimens has been known as a key independent predictor for the neutropenic events in prior studies [13, 16, 26]. However, it is not possible to include all myelosuppressive regimens as separate covariates in a single model. One of the ideal predictors, which could be included in a multivariable prediction model, is the FN risk category of chemotherapy regimens. We demonstrated that the interaction between the risk group of regimens and prophylactic G-CSF use could be considered as a highly potent and significant independent predictor for the occurrence of FN or SN. This sensible interaction was surprisingly ignored in the previous prediction models. Thus, the inclusion of this novel potent predictor into the prediction model could result in a reduced omitted-variable bias in our study compared to prior studies. Moreover, since the FN risk category is not specific to particular chemotherapy regimens, it can be simply used in the future even with the emergence of new chemotherapy regimens.
Previous studies have indicated that low BMI or low BSA is associated with an increased risk of neutropenic events [12, 16, 27, 28]. In the study performed by Pettengell et al., a higher weight was also found to have a protective role against developing FN in the first cycle of the treatment [17]. Capping the dose in obese patients and using the ideal body weight in dose calculation may explain these associations [29, 30]. In fact, reducing the total dose or using idealized body weight (i.e., expected weight of a healthy normal person calculated based on age, sex, and height) rather than actual body weight in chemotherapy dose calculation could lead to reduced toxicity. In addition, the altered pharmacokinetics of some cytotoxic drugs in overweight patients may be another explanation [30, 31]. Thus, further pharmacokinetics research is recommended [30]. When the rational interactions were assessed in this study, the interaction between BMI (< 23 kg/m2) and BSA (< 2 m2) was found to be a stronger and more significant predictor for the outcome than any individual variable.
The association between renal disease as a chronic comorbidity and neutropenic complications is widely reported [13, 32,33,34]. In an adjusted analysis by Hurria et al., a creatinine clearance < 50 mL/min estimated by the Cockcroft-Gault equation was associated with an increased risk of FN in older breast cancer patients receiving adjuvant chemotherapy [34]. Moreover, in agreement with our study, Lyman et al. also found a significant association between estimated glomerular filtration rate (eGFR) as a continuous variable and first cycle SN or FN in an adjusted analysis [13]. To generate an easily measureable risk score, we used the eGFR as a dichotomous variable (i.e., the eGFR < 60 mL/min/1.73 m2). In patients with renal disorders, metabolism and excretion of a drug might be altered even if the drug is eliminated by non-renal transport and metabolism [35]. Additionally, increased toxicity as a result of drug accumulation may be related to altered plasma protein binding and distribution volume of the drug [36].
Consistent with this study, some previous reports have indicated that higher levels of serum ferritin and C-reactive protein (CRP) were associated with an increased risk of infection or FN after either chemotherapy or allogeneic hematopoietic cell transplantation [37,38,39]. Moreover, in a non-homogenous population of patients with advanced cancers, Alexandre et al. indicated that an elevated inflammatory status is correlated with increased risk of chemotherapy-induced severe hematologic toxicity [21]. Possible explanations for this association are reduced cytochrome P450 3A (CYP 3A) activity and consequently overexposure to any substrate of CYP 3A, bone marrow sensitivity to a given exposure, and also increased cytotoxicity of chemotherapy-induced DNA damage in the case of APPR stimulation [21]. In a pharmacokinetic study by Alexandre et al., ferritin plasma concentration was correlated with increased concentration of midazolam, representing a reduced CYP 3A activity [19]. Although elevated ferritin was found to be an independent prediction factor in our study, a stronger relationship was identified for its interaction with age > 65 years. Of note, in spite of highly significant p value, the fairly wide range of CI for the relative risk of this interaction should be considered. This uncertainty may be related to the low exposure rate of this combined variable (approximately 10 and 1% in patients with and without the outcome, respectively). However, further investigation is warranted to validate this potent interaction and also to clarify the rationale behind it.
Our reason for not considering the performance status as a candidate predictor of the event in spite of prior promising evidences was its subjective nature and bias associated with inter-observer variability [40].
Limitations
The lack of external validation is the main limitation. Furthermore, although we included patients with different geographic characteristics and a wide range of tumor types referred from across the country, we declare that no single population is completely representative of the entire population. Thus, external validation in other centers with different patients is necessary before implementing this prediction rule in clinical practice. Moreover, we emphasize that the number of events in our study is fairly small. Of note, the reported CIs are relatively wide, and thus, results should be interpreted with caution. In addition, the systematic error related to the estimation of eGFR should be mentioned as another limitation.
Implications
All clinical and laboratory parameters included in this model are readily available and easily measurable in clinical setting. After external validation in a large population of patients across different centers and geographical locations, this scoring system might be easily used in clinical practice for better stratifying patients at higher risk of neutropenic complications and those who might benefit from prophylactic G-CSF administration. We declare that this fairly small study should be considered as a pilot study and further research with a sufficiently large sample size is required to be sure for clinical applicability of the model as a risk score.
Conclusions
We developed an internally validated scoring system based on few readily accessible and easily assessable predictors to define a group of patients who are at high risk of neutropenic complications. This clinical prediction rule may rationalize the use of myeloid growth factors, minimize the health-care costs, and maximize the patients’ quality of life. However, before using this prediction rule in clinical practice, it should be prospectively tested and/or updated in other centers with different patients to assess its generalizability and external validity.
References
Weycker D, Li X, Tzivelekis S, Atwood M, Garcia J, Li Y, Reiner M, Lyman GH (2017) Burden of chemotherapy-induced febrile neutropenia hospitalizations in US clinical practice, by use and patterns of prophylaxis with colony-stimulating factor. Support Care Cancer 25(2):439–447
Dinan MA, Hirsch BR, Lyman GH (2015) Management of chemotherapy-induced neutropenia: measuring quality, cost, and value. J Natl Compr Cancer Netw 13(1):e1–e7
Weycker D, Li X, Edelsberg J, Barron R, Kartashov A, Xu H, Lyman GH (2015) Risk and consequences of chemotherapy-induced febrile neutropenia in patients with metastatic solid tumors. J Oncol Pract 11(1):47–54
Culakova E, Thota R, Poniewierski MS, Kuderer NM, Wogu AF, Dale DC, Crawford J, Lyman GH (2014) Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med 3(2):434–444
Kuderer NM, Lyman GH (2011) Personalized medicine and cancer supportive care: appropriate use of colony-stimulating factor support of chemotherapy. J Natl Cancer Inst 103(12):910–913
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade J, Wozniak A, Armitage J, American Society of Clinical Oncology (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33(28):3199–3212
Aapro MS, Bohlius J, Cameron D, Dal Lago L, Donnelly JP, Kearney N, Lyman G, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber D, Zielinski C, European Organisation for Research and Treatment of Cancer (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32
Crawford J, Caserta C, Roila F, ESMO Guidelines Working Group (2010) Hematopoietic growth factors: ESMO clinical practice guidelines for the applications. Ann Oncol 21(suppl 5):v248–v251
Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I, Griffiths EA, Hough S, Kloth DD, Kuter DJ, Lyman GH, Mably M, Mukherjee S, Patel S, Perez LE, Poust A, Rampal R, Roy V, Rugo HS, Saad AA, Schwartzberg LS, Shayani S, Talbott M, Vadhan-Raj S, Vasu S, Wadleigh M, Westervelt P, Burns JL, Pluchino L (2017) Myeloid growth factors, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15(12):1520–1541 doi:1540–1405
Aapro M, Ludwig H, Bokemeyer C, Gascón P, Boccadoro M, Denhaerynck K, Krendyukov A, Gorray M, MacDonald K, Abraham I (2016) Predictive modeling of the outcomes of chemotherapy-induced (febrile) neutropenia prophylaxis with biosimilar filgrastim (MONITOR-GCSF study). Ann Oncol 27(11):2039–2045
Pfeil AM, Vulsteke C, Paridaens R, Dieudonné A-S, Pettengell R, Hatse S, Neven P, Lambrechts D, Szucs TD, Schwenkglenks M, Wildiers H (2014) Multivariable regression analysis of febrile neutropenia occurrence in early breast cancer patients receiving chemotherapy assessing patient-related, chemotherapy-related and genetic risk factors. BMC Cancer 14(1):201
Chan A, Chen C, Chiang J, Tan SH, Ng R (2012) Incidence of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer 20(7):1525–1532
Lyman GH, Kuderer NM, Crawford J, Wolff DA, Culakova E, Poniewierski MS, Dale DC (2011) Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 117(9):1917–1927
Hosmer W, Malin J, Wong M (2011) Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer. Support Care Cancer 19(3):333–341
Lopez-Pousa A, Rifà J, Casas de Tejerina A, Gonzalez-Larriba J, Iglesias C, Gasquet J, Carrato A, DELFOS Study Group (2010) Risk assessment model for first-cycle chemotherapy-induced neutropenia in patients with solid tumours. Eur J Cancer Care 19(5):648–655
Moreau M, Klastersky J, Schwarzbold A, Muanza F, Georgala A, Aoun M, Loizidou A, Barette M, Costantini S, Delmelle M, Dubreucq L, Vekemans M, Ferrant A, Bron D, Paesmans M (2009) A general chemotherapy myelotoxicity score to predict febrile neutropenia in hematological malignancies. Ann Oncol 20(3):513–519
Pettengell R, Bosly A, Szucs TD, Jackisch C, Leonard R, Paridaens R, Constenla M, Schwenkglenks M, Impact of Neutropenia in Chemotherapy-European Study Group (INC-EU) (2009) Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: data from the INC-EU prospective observational European neutropenia study. Br J Haematol 144(5):677–685
Lyman GH, Crawford J, Kuderer NM, Wolff D, Culakova E, Poniewierski MS, Dale DC (2008) Final risk prediction model for neutropenic complications in patients receiving cancer chemotherapy. Blood 112(11):1312–1312
Alexandre J, Rey E, Girre V, Grabar S, Tran A, Montheil V, Rabillon F, Dieras V, Jullien V, Hérait P, Pons G, Treluyer J, Goldwasser F (2007) Relationship between cytochrome 3A activity, inflammatory status and the risk of docetaxel-induced febrile neutropenia: a prospective study. Ann Oncol 18(1):168–172
Lyman GH, Abella E, Pettengell R (2014) Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol 90(3):190–199
Alexandre J, Gross-Goupil M, Falissard B, Nguyen M-L, Gornet J-M, Misset J-L, Goldwasser F (2003) Evaluation of the nutritional and inflammatory status in cancer patients for the risk assessment of severe haematological toxicity following chemotherapy. Ann Oncol 14(1):36–41
Mehta HB, Mehta V, Girman CJ, Adhikari D, Johnson ML (2016) Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol 79:22–28
Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 162 (1):W1–73
Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, Initiative STROBE (2007) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med 4(10):1628–1654 (e1297)
Hosmer DW, Lemeshow S (2000) Assessing the fit of the model. In: Applied logistic regression, 2nd edn. Wiley, New York, pp 143–202
Hershman D, Hurley D, Wong M, Morrison VA, Malin JL (2009) Impact of primary prophylaxis on febrile neutropenia within community practices in the US. J Med Econ 12(3):203–210
Lyman GH, Delgado DJ (2003) Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer 98(11):2402–2409
Scott S, Chrischilles E, Link B, Delgado D, Fridman M, Stolshek B (2003) Days of prophylactic filgrastim use to reduce febrile neutropenia in patients with non-Hodgkin's lymphoma treated with chemotherapy. J Manag Care Pharm 9(2 Supp A):15–21
Lyman GH, Sparreboom A (2013) Chemotherapy dosing in overweight and obese patients with cancer. Nat Rev Clin Oncol 10(8):451–459
Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, Morrison VA, Pini TM, Runowicz CD, Rosner GL, Shayne M, Sparreboom A, Sucheston LE, Lyman GH, American Society of Clinical Oncology (2012) Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 30(13):1553–1561
Barpe DR, Rosa DD, Froehlich PE (2010) Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur J Pharm Sci 41(3):458–463
Li X, Luthra R, Morrow PK, Fisher MD, Reiner M, Barron RL, Langeberg WJ (2016) Comorbidities among patients with cancer who do and do not develop febrile neutropenia during the first chemotherapy cycle. J Oncol Pharm Pract 22(5):679–689
Chao C, Page J, Yang S-J, Rodriguez R, Huynh J, Chia V (2014) History of chronic comorbidity and risk of chemotherapy-induced febrile neutropenia in cancer patients not receiving G-CSF prophylaxis. Ann Oncol 25(9):1821–1829
Hurria A, Hurria A, Brogan K, Panageas KS, Pearce C, Norton L, Jakubowski A, Howard J, Hudis C (2005) Effect of creatinine clearance on patterns of toxicity in older patients receiving adjuvant chemotherapy for breast cancer. Drugs Aging 22(9):785–791
Nolin T, Naud J, Leblond F, Pichette V (2008) Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther 83(6):898–903
Olyaei AJ, Foster TA, Lerma E (2015) Drug dosing in chronic kidney disease. In: Hornblower SRFS, Turner PNN, Goldsmith CND et al (eds) Oxford textbook of clinical nephrology, 4th edn. Oxford University Press, USA, pp 2911–2918
Hong J, Woo HS, Ahn HK, Sym SJ, Park J, Cho EK, Shin DB, Lee JH (2016) Pre-treatment blood inflammatory markers as predictors of systemic infection during induction chemotherapy: results of an exploratory study in patients with acute myeloid leukemia. Support Care Cancer 24(1):187–194
Kanda J, Mizumoto C, Ichinohe T, Kawabata H, Saito T, Yamashita K, Kondo T, Takakura S, Ichiyama S, Uchiyama T, Ishikawa T (2011) Pretransplant serum ferritin and C-reactive protein as predictive factors for early bacterial infection after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 46(2):208–216
Sato M, Kako S, Oshima K, Sato K, Terasako K, Kimura S-I, Nakasone H, Okuda S, Yamazaki R, Higuchi T, Nishida J, Kanda Y (2010) Prediction of infectious events by high-sensitivity C-reactive protein level before undergoing chemotherapy for acute myeloid leukaemia. Scand J Infect Dis 42(2):97–101
Sørensen J, Klee M, Palshof T, Hansen H (1993) Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer 67(4):773–775
Acknowledgements
This study was supported by Shahid Beheshti University of Medical Sciences [grant number 6620] and Behnam Daheshpour Charity Organization. The funders of this study were not involved in performing the study (e.g., data collection, analysis, and interpretation) as well as preparation of this report. We would like to thank Dr. A. Rezazadeh Mafi for critically reading the manuscript and Drs. H.R. Mirzaei, A. Rakhsha, A. Mousavizadeh, M. Malekzadeh, A.S. Yousefi Kashi, M. Houshyari, P. Hajian, and S. Azghandi for providing the patient data. We also thank the nursing staff of the oncology ward, especially Mrs. P. Peyghambarlou, for the administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 87.5 kb)
Rights and permissions
About this article
Cite this article
Razzaghdoust, A., Mofid, B. & Moghadam, M. Development of a simplified multivariable model to predict neutropenic complications in cancer patients undergoing chemotherapy. Support Care Cancer 26, 3691–3699 (2018). https://doi.org/10.1007/s00520-018-4224-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4224-z