Abstract
Purpose
This study aimed to prove the usefulness of the diagnostic plot, using the haemoglobin content of reticulocytes as a measure of functional iron deficiency (FID) and the ferritin index as a measure of iron availability, to customise anaemia treatment in cancer patients.
Methods
Based on results of this plot, cancer patients fulfilling practice guideline criteria to receive erythropoiesis-stimulating agents (ESAs) were allocated to treatment with ESAs alone, iron alone or the combination of both. Primary endpoint was the percentage of patients identified to require iron in addition or as an alternative to ESA therapy.
Results
Out of 303 patients screened, 286 were allocated to treatment: 204 patients were normochromic and iron replete and treated with ESAs alone, 22 had both FID and anaemia of chronic disease and were treated with ESAs and parenteral iron, and 60 were iron-depleted and treated with iron only. After 8 weeks, a haemoglobin increase >1 g/dL from baseline was shown by 56% of patients treated with ESAs alone, by 100% of patients receiving the combination, by 50% of normochromic and by 73% of hypochromic iron-depleted patients receiving iron only. Acute phase reaction did not diminish the response rate to ESAs.
Conclusions
The diagnostic plot was superior to transferrin saturation and ferritin in predicting iron availability in hypochromic patients treated with ESAs and proved useful to select treatment for anaemia in cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaemia in cancer patients is a significant factor of morbidity associated with the underlying disease and chemotherapy. It is the most important form of anaemia of chronic disease (ACD), which is characterised by a blunt erythropoietin response in addition to inadequate iron supply to the bone marrow [26, 40]. Among the common treatments are erythropoietin-stimulating agents (ESAs). However, two recent meta-analyses [9, 39] confirmed earlier safety concerns, which had resulted in restrictions on their use. Current anaemia treatment guidelines point at the need to rule out causes other than cancer or chemotherapy prior to ESA treatment. However, neither do they specify any diagnostic procedures [10, 11, 31–33], nor is any predictor of haematological response to ESAs available [10, 35]. Such a response is achieved in just about half of the patients, and adjunct iron treatment in anaemic patients with cancer is estimated to be widely underused as compared to dialysis patients [29].
Thus, to use ESAs most appropriately [9, 39], still biomarkers are needed (1) to identify responders, (2) to monitor response during treatment and (3) to estimate the iron balance and availability before and during ESA therapy.
The most likely reason for not responding to ESAs is functional iron deficiency (FID), a failure to provide stored iron to the erythroblasts. However, to date, parameters of iron status have not been predictive of a response to ESAs in anaemic iron-replete patients either [1, 13]. In 2002, the diagnostic plot was introduced [37] by combining the ferritin index (FI = soluble transferrin receptor (sTfR) in mg/L/log ferritin in µg/L), as marker of iron supply to the bone marrow [30], and the Hb content of reticulocytes, as indicator of FID [6], alternatively to ferritin and transferrin saturation (TSAT) [17]. The plot enables differentiation of four different categories (Fig. 1): In patients of quadrant 1, iron stores are replete and bone marrow releases red blood cells with normal Hb content (no iron deficiency = ‘NID patients’). In patients of quadrant 2, iron availability may be reduced, but without affecting erythropoiesis yet and Hb content of red blood cells remains normal (mild iron deficiency = ‘MID patients’). In patients of quadrant 3, iron availability runs short and results in storage and functional iron deficiency (iron deficiency anaemia = ‘IDA patients’). In quadrant 4, iron-replete patients present FID in combination with ACD (‘ACD/FID patients’).
Diagnostic plot for the differentiation of anaemia in cancer patients based on iron stores (sTfR/log ferritin) and iron demand for erythropoiesis (CHr) [37]. In patients with CRP values higher than 5 mg/L, the cutoff for the sTfR/log ferritin is 2.0 instead of 3.2
Infection and chronic inflammation may suppress erythropoietic activity and cause FID if stored iron is not mobilised and added to the labile iron pool. Consequently, the FI threshold of the diagnostic plot that indicates normal iron availability needs to be adjusted in patients with an acute phase reaction (C-reactive protein (CRP) >5 mg/L [17]) [37].
So far, three studies with limited data have demonstrated the diagnostic plot as a predictive model to adequately identify responders to ESAs, one in intensive care patients with ACD [36], one in anaemic low birth-weight infants [22] and one in anaemic patients with myeloma and lymphoma [23]. To further evaluate the practical benefit of the diagnostic plot, we conducted a first prospective trial in cancer patients with anaemia.
Methods
Patients
Eligible for this study were adult patients with a malignant tumour and an anaemia fulfilling the European practice guideline criteria for ESA treatment of 2004 [10]. The anaemia had to be developed at least three cycles and at least 6 weeks prior to the end of chemotherapy as ESAs should be given concomitantly. Excluded were patients with an anaemia attributable to factors other than cancer or chemotherapy, an overt iron deficiency as suggested by ferritin concentrations <20 µg/L, a contraindication for ESA or iron preparations or a life expectancy <3 months, and furthermore, pregnant or breast-feeding women. All enrolled patients gave written informed consent.
Study design and protocol
This was an open, controlled, parallel-group, multicenter, prospective trial enrolling patients from October 2004 to October 2006. Within 3 weeks of enrolment, patients underwent an initial clinical and laboratory screen. Baseline laboratory tests included a complete blood and reticulocyte count, erythropoietin, vitamin B12 and folate levels and a biochemistry profile including markers of iron metabolism. The latter comprised ferritin, sTfR, FI, TSAT, CRP and CHr.
Based on these markers and the resulting quadrant of the diagnostic plot, patients were allocated to treatment: NID patients received erythropoietin ß (NeoRecormon®, 30,000 U subcutaneously once per week), which based on routine diagnostic procedures would have been the standard treatment for all study patients. Therefore, these patients were taken as a reference for the evaluation of secondary endpoints. Due to the iron impairment indicated by the plot results, MID patients were allocated to treatment with oral iron (ferrous sulphate 300 mg daily), IDA patients to treatment with parenteral iron (Fe saccharate; Venofer® 200 mg once per week) and ACD/FID patients to treatment with the combination of erythropoietin ß and parenteral iron once per week (Fig. 1).
Hb and the parameters of the diagnostic plot were re-measured in weeks 2, 4, 8 and 12. Based on the results, treatment was either stopped (if Hb was >12 g/dL), modified (if quadrant had changed) or continued for a maximum of 12 weeks. Patients who had to be transfused during the study were considered non-responders; their laboratory data were excluded from the present analysis. The study protocol was approved by the competent regional Ethics Committee (Medical Association North Rhine) and conducted in accordance with the principles of GCP and the Declaration of Helsinki.
Clinical endpoints
As the primary objective was to validate the plot as a diagnostic tool for the differentiation of iron states, the primary endpoint was the percentage of patients identified by the diagnostic plot to require iron either in addition or as alternative to ESA therapy. Iron requirements were verified based on the haemoglobin response to the allocated treatment. Accordingly, response rates and time to response were secondary endpoints as well as the tolerability and safety of iron therapy, the percentage of patients with an APR, its impact on treatment response and the rate of transfusions and infections.
Response to the different treatment regimens was analysed by changes of Hb and sTfR concentrations on week 4, 8 and 12 in comparison to baseline. At each of these time points, patients with an Hb increase >1 g/dL from baseline were considered responders [32]. The increase in sTfR concentration was measured as putative indicator of proliferative bone marrow response to ESA treatment [15].
Diagnostic testing
All laboratory measurements were performed centrally. Blood counts and CHr were measured using the Advia 120 haematology analyser (Siemens Diagnostics, Frankfurt, Germany), CRP and TSAT using the COBAS 6000 chemistry analyser (Roche Diagnostics, Mannheim, Germany), ferritin and sTfR concentration using an Elecsys 2010 analyser (Roche Diagnostics, Mannheim, Germany) and erythropoietin using the Immulite analyser (Siemens diagnostics, Frankfurt, Germany).
The threshold used in the diagnostic plot for CHr should be the same for measurements with analysers from Sysmex, whereas those used for the FI (3.2 and 2.0) are specific to the sTfR assay from Roche.
Statistical analysis
The statistical analysis was performed by an external organisation (WISP, Langenfeld, Germany). Groups of patients were compared based on their original quadrant and assignment to treatment even though these may have changed during the course of the study. Between-group and pair-wise comparisons of metric variables were performed with the respective Wilcoxon test and those of frequencies using Fisher’s exact test (two-sided).
Results
Eleven cancer outpatient clinics in Germany screened 342 patients of whom 303 were eligible. There were no significant differences among patients with different status of iron stores and haematopoiesis at baseline regarding age, gender, body mass index, type or stage of tumour or performance status (Table 1).
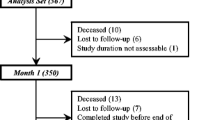
In 286 patients who were assigned to treatment due to the results of the diagnostic plot, treatment response was evaluable; 265 patients were treated and analysed per protocol. Of these, 227, 217 and 151 completed weeks 4, 8 and 12, respectively (Fig. 2). No serious adverse event attributable to ESA or iron treatment was reported.
Baseline iron and inflammation status
Assessment of the diagnostic plot
At baseline, about four in five patients had a normal FI (NID and ACD/FID patients), indicating normal iron availability for erythropoiesis; 60 patients (21%) had increased values (MID and IDA patients), indicating reduced iron supply to the bone marrow (Table 1). Forty-two patients (15%) fell into Q3 and Q4 (CHr ≤ 28 pg) indicating FID; of these, 20 patients (7%) had classical IDA, and 22 patients (8%) had a combination of FID and ACD. The ferritin concentration was significantly higher in patients with NID and ACD/FID compared to those with MID and IDA (p < 0.0001). Average sTfR was normal in the former and elevated in the latter groups. Endogenous erythropoietin concentrations did not vary significantly among quadrants (Table 1).
Assessment of transferrin saturation
TSAT levels were highest in NID patients with 145 of them (71%) having values ≥20% (Table 1). Corresponding numbers (frequencies) in MID, IDA and ACD/FID patients were 18 (45%), seven (35%) and 11 (50%), respectively. TSAT was significantly lower in patients with CHr ≤ 28 pg (IDA and ACD/FID) compared to patients with CHr > 28 pg (NID and MID) (means ± SD, 18.1 ± 13.3% vs. 30.8 ± 21.2%, p < 0.0001).
Assessment of inflammation
Elevated CRP values (>5 mg/L) were measured in 65%, 84%, 79% and 86% of NID , MID, IDA and ACD/FID patients, respectively. CRP levels were significantly higher in patients with FI ≤ 3.2 (NID and ACD/FID) than in patients with FI > 3.2 (MID and IDA; means ± SD, 35.8 ± 49.9 vs. 14.1 ± 19.1, p < 0.0001; Table 1). CRP levels were highest in patients with ACD/FID or with ferritin >800 µg/L (means ± SD, 60 ± 73 mg/L), TSAT ≤ 20% (41 ± 49 mg/L), sTfR ≥5 mg/L (41 ± 63 mg/L) and CHr ≤ 28 pg (59 ± 77 mg/L).
Response to treatment
Hb response
In all groups, Hb increased significantly and continuously during treatment (baseline vs. 12 weeks: all p < 0.001; Fig. 3). Mean Hb increases and the proportion of responders among patients with hypochromic red cells (IDA and ACD/FID) were greater than among those with normochromic red cells (NID and MID; Fig. 4). The average Hb increase of IDA and ACD/FID patients was about 2 g/dL after 8 weeks; however, 18% of the ACD/FID patients had a Hb increase of 1–2 g/dL. Of the 60 patients treated with iron alone, 39 (65%) responded. These patients, who without the diagnostic plot would have been treated with ESAs, showed an Hb increase comparable to both ESA-treated groups. In patients with normochromic anaemia and ESA-only therapy, the response rate after 8 weeks was not diminished through infection or chronic inflammation (71.3%, 84.0%, 76.9% and 88.5% in patients without, with developing, resolving, or persistent APR, respectively, based on CRP levels below or above 5 mg/L at baseline and week 8).
Transfusion rate
Over the whole 12-week study period, 95 of the 265 per-protocol patients (36%) received at least one blood transfusion: 79 out of 194 NID patients (41%), six out of 31 MID patients (19%), three out of 19 IDA patients (16%) and seven out of 21 ACD/FID patients (33%). From weeks 5 to 12, overall 49 patients (18%) still required at least one transfusion, 43 patients with NID (22%), one with MID (3%), one with IDA (5%) and four with ACD/FID (19%), respectively.
sTfR response
ESA-treated NID and ACD/FID patients demonstrated a significant sTfR increase of more than 60% from baseline regardless of Hb response (p ≤ 0.001). In the iron-only-treated IDA patients, sTfR concentration decreased in responders (baseline, 7.3 ± 4.0 vs. week 8, 5.3 ± 3.0 mg/L), whereas in non-responders, it remained stable over 8 weeks of therapy (baseline, 7.4 ± 4.0 vs. week 8, 7.2 ± 2.0 mg/L, p > 0.1). In MID patients, no significant sTfR change during treatment was measured, regardless of Hb response.
Optimal iron supply
CHr response
ACD/FID patients who were all Hb responders to the combined ESA/iron treatment showed a continuous increase in CHr. In contrast, among ESA-only treated NID patients, responders, but not the non-responders, showed a continuous decline of CHr during treatment (day 1 vs. week 12, p < 0.0001), indicating FID (Table 2). Of the iron-only-treated IDA patients, responders showed an increase in CHr, whereas in the non-responders and MID patients, CHr remained stable (data not shown).
FI response
In all patients treated with ESAs, FI increased significantly (day 1 vs. week 12, p < 0.0001) regardless of Hb response (Table 2). Within 12 weeks of corresponding treatment, the FI of NID patients moved 49.2% of responders to Q2 or Q3, indicating a reduced iron supply for erythropoiesis, whereas this happened to only two ACD/FID patients (19%). Of the patients treated with iron alone (MID and IDA), only Hb responders showed a decrease of FI during treatment (data not shown).
TSAT assessment
Of the NID patients receiving ESAs only, 75.6% had a TSAT value ≥20% and a ferritin level ≥100 µg/L at baseline; 71% showed a mean Hb increase of 2 g/dL after 12 weeks (Fig. 4). However, values of TSAT ≥ 20% and ferritin ≥ 100 µg/L were also measured in 47.8% of ACD/FID patients although they had FID (CHr ≤ 28 pg).
Discussion
Evidence from clinical trials supports the use of ESAs below critical Hb concentrations. However, in the absence of reliable diagnostic procedures to figure out the cause of anaemia and to predict ESA response, practice guidelines recommend to initiate conventional therapy and observe haematopoietic response first [10, 31]. Guidelines further recommend to consider relevant diagnostic testing, in particular of FID. This was the first study using a diagnostic rules-based approach to determine the most appropriate anaemia therapy. It also allowed a comparative evaluation of potential diagnostic markers.
STfR has been recommended as marker of erythropoietic activity [7, 15]. Our data confirm that an increase of sTfR may indicate not only stimulation of erythropoiesis during ESA therapy but also that it has no predictive value of the Hb response [35] as sTfR increased in both responders and non-responders.
Practice guidelines for chronic kidney disease recommend to initiate ESA therapy in haemodialysis patients, if TSAT is ≥20% and ferritin ≥ 100 µg/L [27]. Based on these guidelines, iron supplementation would have been withheld in all patients falling into Q4 of the diagnostic plot. However, these patients actually showed a higher response rate (p = 0.0035 in week 12, Fig. 4) and a greater Hb increase (Fig. 3) than those treated with ESAs alone confirming results of earlier studies [2, 5, 20, 28]. Thus, our data indicate superiority of the diagnostic plot based on FI and CHr to serum ferritin and TSAT in recognising FID.
FID may result either from an ESA overstimulation of erythropoiesis and accordingly an iron need exceeding the capacity of delivery, or from an impaired iron delivery from reticulo-endothelial to erythropoietic cells [12]. The ratio of FID to the delivery of iron as represented by CHr and FI in the diagnostic plot provides a new approach to detect iron-deficient states when monitoring ESA therapy [14]. In ESA-only treated patients, the shift of nearly half of the responders from Q1 to Q2 or Q3 indicated that markedly stimulated erythropoiesis depleted afore normal iron stores. That this happened to only 19% of patients under the combination treatment confirms the benefit of intravenous iron supplementation and the relevance of the FI to indicate reduced iron availability.
CHr enables an assessment of the incorporation of iron into Hb of red cells. Thus, it is a direct indicator of functional availability of iron to the erythron [38]. In recent years, CHr has been established as an indicator of FID and iron management in ESA-treated dialysis patients [19]. Our study confirmed CHr as indicator of functional iron availability in cancer patients. All ESA-only treated patients showed a comparable increase of FI, but like in the DRIVE study [16], only responders exhibited a decline in CHr, whereas it increased in iron-supplemented ACD/FID responders (Table 2). These results suggest that non-responders to ESA therapy can mobilise stored iron but are unable to incorporate it into the erythron.
Apart from iron deficiency, infection and inflammation were shown to play a major role in the poor response of patients with chronic kidney disease to ESAs [25]. In these patients, high CRP in association with low CHr and TSAT levels are supposed to explain the lack of response to further iron therapy [18]. In addition, inflammation may induce erythropoietin resistance [4, 25]. That during chronic inflammation higher ESA doses are required is supported by data from the European Survey of Anaemia Management (ESAM) [21]: Despite high doses of ESAs, patients with CRP above 50 mg/L showed lower Hb values than those with CRP below 50 mg/L. In our study, ACD/FID patients consistently showed decreased CHr and mean TSAT and CRP levels of 18.7 ± 7.7% and 83 ± 87 mg/L, respectively. However, still 82% responded to ESAs and intravenous iron treatment with an Hb increase of ≥2 g/dL within 8 weeks. In the ESA-only treated group, the response rate was independent of CRP as well (Table 2). By now, it remains unclear whether this is due to differences in patient populations or ESA doses.
Surprisingly, the diagnostic plot identified in the current study about one in five anaemic patients of an average cancer population qualifying for ESA therapy to be just iron deficient. In contrast, the number of ACD/FID patients, who, as expected, responded well to the combination therapy, was rather small. Overall, based on the diagnostic plot, ESAs alone appeared not to be the most appropriate treatment option for about one in four patients fulfilling current guideline criteria for ESA treatment. Moreover, in view of the relatively moderate response of NID patients to ESAs alone, it is likely that they would have benefited from supplemental iron as well. This would be in line with results from previous studies (reviewed in [1]), and the continuous decrease of CHr in NID responders may suggest the development of FID under ESA therapy without concomitant iron support.
Thus, the original question raised in this study of which patients need iron in addition to ESAs might rather read which patients need ESAs in addition to iron. Whether iron generally should be given along with ESA therapy in cancer patients is still under debate [3, 8, 24, 34]. However, none of the published trials, including ours, indicate an emerging safety risk and to our knowledge, there has been no dedicated safety study of iron therapy in anaemic cancer patients yet. At least our data provide good reason to extensively use CHr and FI as markers for erythropoiesis and iron metabolism before initiating any unneeded or potentially harmful therapy with ESAs or transfusions [29].
In conclusion, the plot reflects the relationship between FID and iron availability and during ESA therapy the combined assessment of CHr and FI is predictive of a treatment response. The study confirmed the value of intravenous iron supplementation to improve response to ESAs. For the first time, the diagnostic plot enabled to identify anaemic cancer patients with iron deficiency who otherwise would be treated with ESAs. The plot was confirmed as an indicator of response to ESAs and of FID and appears a promising tool to decide upon treatment in cancer patients with anaemia. However, this needs to be substantiated by further studies.
References
Auerbach M (2008) Should intravenous iron be the standard of care in oncology? J Clin Oncol 26:1579–1581
Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, Balan S, Barker L, Rana J (2004) Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol 22:1301–1307
Auerbach M, Coyne D, Ballard H (2008) Intravenous iron: from anathema to standard of care. Am J Hematol 83:580–588
Barany P (2001) Inflammation, serum C-reactive protein, and erythropoietin resistance. Nephrol Dial Transplant 16:224–227
Bastit L, Vandebroek A, Altintas S, Gaede B, Pinter T, Suto TS, Mossman TW, Smith KE, Vansteenkiste JF (2008) Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol 26:1611–1618
Baynes RD (1996) Assessment of iron status. Clin Biochem 29:209–215
Beguin Y, Loo M, R’Zik S, Sautois B, Lejeune F, Rorive G, Fillet G (1995) Quantitative assessment of erythropoiesis in haemodialysis patients demonstrates gradual expansion of erythroblasts during constant treatment with recombinant human erythropoietin. Br J Haematol 89:17–23
Bohlius J (2008) Is intravenous iron supplementation with erythropoiesis-stimulating agents beneficial in cancer patients with anemia? Nat Clin Pract Oncol 5:688–689
Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A (2009) Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 373:1532–1542
Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Osterborg A, Repetto L, Soubeyran P (2004) EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer. Eur J Cancer 40:2201–2216
Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Osterborg A, Repetto L, Soubeyran P (2007) EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 43:258–270
Bovy C, Tsobo C, Crapanzano L, Rorive G, Beguin Y, Albert A, Paulus JM (1999) Factors determining the percentage of hypochromic red blood cells in hemodialysis patients. Kidney Int 56:1113–1119
Brugnara C (2000) Reticulocyte cellular indices: a new approach in the diagnosis of anemias and monitoring of erythropoietic function. Crit Rev Clin Lab Sci 37:93–130
Brugnara C (2003) Iron deficiency and erythropoiesis: new diagnostic approaches. Clin Chem 49:1573–1578
Cazzola M, Beguin Y, Kloczko J, Spicka I, Coiffier B (2003) Once-weekly epoetin beta is highly effective in treating anaemic patients with lymphoproliferative malignancy and defective endogenous erythropoietin production. Br J Haematol 122:386–393
Coyne D, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala AR (2007) Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol 18:975–984
Dati F, Schumann G, Thomas L, Aguzzi F, Baudner S, Bienvenu J, Blaabjerg O, Blirup-Jensen S, Carlstrom A, Petersen PH, Johnson AM, Milford-Ward A, Ritchie RF, Svendsen PJ, Whicher J (1996) Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP Reference Material (CRM 470). International Federation of Clinical Chemistry. Community Bureau of Reference of the Commission of the European Communities. College of American Pathologists. Eur J Clin Chem Clin Biochem 34:517–520
El-Khatib M, Duncan HJ, Kant KS (2006) Role of C-reactive protein, reticulocyte haemoglobin content and inflammatory markers in iron and erythropoietin administration in dialysis patients. Nephrology (Carlton) 11:400–404
Fishbane S, Shapiro W, Dutka P, Valenzuela OF, Faubert J (2001) A randomized trial of iron deficiency testing strategies in hemodialysis patients. Kidney Int 60:2406–2411
Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR (2007) Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 12:231–242
Horl WH, Jacobs C, Macdougall IC, Valderrabano F, Parrondo I, Thompson K, Carveth BG (2000) European best practice guidelines 14–16: inadequate response to epoetin. Nephrol Dial Transplant 15(Suppl 4):43–50
Kasper DC, Widness JA, Haiden N, Berger A, Hayde M, Pollak A, Herkner KR (2008) Characterization and differentiation of iron status in anemic very low birth weight infants using a diagnostic nomogram. Neonatology 95:164–171
Katodritou E, Terpos E, Zervas K, Speletas M, Kapetanos D, Kartsios C, Verrou E, Banti A, Effraimidou S, Christakis J (2007) Hypochromic erythrocytes (%): a reliable marker for recognizing iron-restricted erythropoiesis and predicting response to erythropoietin in anemic patients with myeloma and lymphoma. Ann Hematol 86:369–376
Katodritou E, Verrou E, Zervas K (2008) Intravenous iron: a useful therapeutic tool but not a panacea. Am J Hematol 83:521–523
McDougall IC, Cooper AC (2002) Erythropoietin resistance: the role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transplant 17:39–43
Means RT Jr, Krantz SB (1992) Progress in understanding the pathogenesis of the anemia of chronic disease. Blood 80:1639–1647
National Kidney Foundation (2001) IV. NKF-K/DOQI clinical practice guidelines for anemia of chronic kidney disease: update 2000. Am J Kidney Dis 37:S182–S238
Pedrazzoli P, Farris A, Del Prete S, Del Gaizo F, Ferrari D, Bianchessi C, Colucci G, Desogus A, Gamucci T, Pappalardo A, Fornarini G, Pozzi P, Fabi A, Labianca R, Di Costanzo F, Secondino S, Crucitta E, Apolloni F, Del Santo A, Siena S (2008) Randomized trial of intravenous iron supplementation in patients with chemotherapy-related anemia without iron deficiency treated with darbepoetin alpha. J Clin Oncol 26:1619–1625
Pedrazzoli P, Rosti G, Secondino S, Siena S (2009) Iron supplementation and erythropoiesis-stimulatory agents in the treatment of cancer anemia. Cancer 115:1169–1173
Punnonen K, Irjala K, Rajamaki A (1997) Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 89:1052–1057
Rizzo JD, Lichtin AE, Woolf SH, Seidenfeld J, Bennett CL, Cella D, Djulbegovic B, Goode MJ, Jakubowski AA, Lee SJ, Miller CB, Rarick MU, Regan DH, Browman GP, Gordon MS (2002) Use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. Blood 100:2303–2320
Rizzo JD, Somerfield MR, Hagerty KL, Seidenfeld J, Bohlius J, Bennett CL, Cella DF, Djulbegovic B, Goode MJ, Jakubowski AA, Rarick MU, Regan DH, Lichtin AE (2008) Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update. Blood 111:25–41
Rodgers G, Cella D, Chanan-Khan A, Chesney C, Cleeland C, Coccia P, et al (2007) National comprehensive cancer network clinical practice guidelines in oncology. Cancer and treatment related anemia (V.3.2007). http://wwwnccnorg/professionals/physician_gls/f_guidelinesasp
Shord SS, Hamilton JM Jr, Cuellar S (2008) Parenteral iron with erythropoiesis-stimulating agents for chemotherapy-induced anemia. J Oncol Pharm Pract 14:5–22
Steinmetz T, Hellmich M, Neise M, Aldaud A, Lerchenmuller C, Tsamaloukas A, Fandel F, Weiligmann C, Totzke U, Schmitz S (2007) Prediction of the responsiveness to treatment with erythropoiesis-stimulating factors: a prospective clinical study in patients with solid tumors. Oncologist 12:748–755
Thomas C, Kirschbaum A, Boehm D, Thomas L (2006) The diagnostic plot: a concept for identifying different states of iron deficiency and monitoring the response to epoetin therapy. Med Oncol 23:23–36
Thomas C, Thomas L (2002) Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem 48:1066–1076
Thomas C, Thomas L (2005) Anemia of chronic disease: pathophysiology and laboratory diagnosis. Lab Hematol 11:14–23
Tonelli M, Hemmelgarn B, Reiman T, Manns B, Reaume MN, Lloyd A, Wiebe N, Klarenbach S (2009) Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. Cmaj 180:E62–E71
Weiss G, Goodnough LT (2005) Anemia of chronic disease. N Engl J Med 352:1011–1023
Acknowledgements
The study was supported by Roche-Pharma AG, Grenzach-Wyhlen, Germany. The authors would like to thank Uwe Totzke (Totzke Scientific, Geneva, Switzerland) for revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steinmetz, H.T., Tsamaloukas, A., Schmitz, S. et al. A new concept for the differential diagnosis and therapy of anaemia in cancer patients. Support Care Cancer 19, 261–269 (2011). https://doi.org/10.1007/s00520-010-0812-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-010-0812-2