Abstract
The objective of this research was to evaluate the thermal exchanges, physiological responses, productive performance and carcass yield of Guinea Fowl confined under thermoneutral conditions and under thermal stress. For the experiment, 96 animals were confined in 8 experimental boxes of 1 m2 of area, each, divided in equal numbers and placed inside two distinct climatic chambers, where the birds were distributed in a completely randomized design, with two treatments (air temperatures of 26 and 32 °C, respectively). For the collection of physiological responses and carcass yield 16 birds were evaluated and for the collection of data on feed and water consumption and productive responses, 48 birds per treatment were evaluated. The environmental variables (air temperature (AT), air relative humidity and wind speed), temperature and humidity index (THI), heat exchanges, physiological responses (respiratory rate, surface temperature, cloacal temperature and eyeball temperature), feed (FC) and water (WC) consumption and production responses (weight gain, feed conversion index and carcass yield) of the birds were evaluated. With the elevation of the AT, it could be noticed that the THI went from a thermal comfort condition to an emergency condition, where the birds lost part of their feathers, increased all physiological responses evaluated, and consequently, reduced by 53.5% the amount of heat dissipated in the sensible form and increased by 82.7% the heat losses in the latent form, increasing also the WC. ATs of up to 32 °C did not significantly affect the productive performance and carcass yield of the guinea fowl.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poultry meat has been one of the main protein resources used for human consumption in most regions of the world (Havenstein et al. 2003; Flock et al. 2005). However, high air temperatures place great restrictions on the development of the poultry industry, especially in the tropics and subtropics, which often provide extreme conditions (Khan et al. 2021), resulting in the inability of birds to maintain homeostasis, which can change the biochemical reactions of the birds, reducing feed intake and, consequently, feed efficiency, resulting in lower performance and productivity of these animals (Settar et al. 1999; El-Deeb et al. 2000; Khan et al. 2011).
For the industrial exploitation of animals for zootechnical purposes in these regions, it is often necessary to adopt acclimatization systems, which can, in some cases, make the activity unfeasible due to the increase in production costs. One of the ways to reduce expenses with equipment designed to maintain the temperature and relative humidity of the air in the desired ranges is to select species adapted to the local environment, so that their thermoregulatory tools are sufficient to maintain homeothermia, even in the face of climate adversities.
It is of paramount importance to carry out research that investigates the influence of environmental variables on the thermodynamic aspects involving these animals and their influence on productive performance, in order to enable the intensive exploration of these animals, providing data that can effectively cause significant impacts on the development of local livestock. Furthermore, birds are more sensitive to high air temperatures than other monogastric animals due to their feather cover and the absence of sweat glands (Loyau et al. 2013).
Guinea Fowl are rustic birds, which can withstand the high air temperatures without major physiological and productive damage (Marques et al. 2021), thus standing out as potential animals to be exploited in countries with a tropical climate, as is the case in Brazil. The Brazilian Northeast region has a predominantly semi-arid climate, typically hot and dry, with long droughts, in these regions the air temperatures recorded throughout the day often exceed the upper limit of the thermal comfort zone of most farm animals (Lara and Rostagno 2013; Baracho et al. 2019), which makes it difficult to develop livestock activities in a competitive way, compared to other regions of the country and the world.
The conventional industrial production of broiler chicken is an activity that has high production efficiency, due to technological and genetic advances, consolidating Brazil as one of the largest producers in the world (ABPA 2018), which still does not occur in the rearing of guineafowl, mainly due to the inefficiency of the breeding systems adopted (Marques et al. 2021). One of the ways in which conventional industrial producers have increased production efficiency is through the adoption of adequate confinement systems (Arruda et al. 2021), techniques that can be replicated to enable intensive exploitation of guineafowl.
Thus, this research aimed to evaluate the thermal exchanges, physiological responses, productive performance and carcass yield of Guinea Fowl under thermoneutral conditions and under thermal stress, confined in climatic chambers.
Material and methods
The procedures performed during this research were approved by the Research Ethics Committee of the Federal University of Campina Grande (UFCG), Paraíba, Brazil, Protocol CEP nº 020/2019.
Animals and housing
For the experimentation two climatic chambers were used (EOS®, TR-18, Minas Gerais, Brazil, temperature range of 15.0–50.0 °C and precision of ± 1.0 °C) with dimensions of 3.1 × 2.8 × 2.6 m in width, length and height, respectively, located in the Laboratory of Rural Constructions and Ambience, linked to the Academic Unit of Agricultural Engineering of the UFCG, whose floor plan with the disposal of equipment and experimental boxes is detailed in Fig. 1.
Ninety-six not sexed chicks from Guinea Fowl (Numidia meleagris) at one day of age were acquired from commercial hatcheries in the region, and the birds were vaccinated against Marek's disease, fowl pox, and infectious bronchitis. In the post-hatching phase, the birds were kept in an artificially heated environment, with an average temperature of 33 °C, which was gradually reduced until reaching the experimental air temperature, on the 15th day of life, when the birds were transferred to the experimental boxes, for adaptation to management and experimental diet.
The experimental period began at 21 days of life of the birds, where they were individually weighed and marked with plastic rings with different colors for their respective identifications, extending the experimental period from 06/29/2019 to 09/06/2019, totaling a total trial period of 70 days. During the experiment, birds were exposed to a period of 23 consecutive hours of lighting with one hour of darkness.
The birds were housed in eight experimental boxes with dimensions of 1.0 × 1.0x0.9 m in length, width and height, respectively, which were placed inside the climatic chambers (Fig. 1), where the adopted housing density was 12 birds/m2, as recommended by Nahashon et al. (2009), for guinea fowl. All the boxes were equipped with tubular and pendular drinking fountains and the floor was made of porcelain and covered by a bed of wood shavings, which was replaced whenever necessary.
Experimental design
The birds were distributed in four experimental boxes located inside each climatic chamber, in a completely randomized design, with two treatments (air temperatures), 16 birds (four birds per experimental box) per treatment were evaluated to collect the physiological responses and carcass yield and 48 birds (12 birds per experimental box) to collect data on feed and water consumption and production responses. The air temperatures adopted in the experiment were taken based on the comfort and thermal stress ranges for broiler birds proposed by Macari et al. (2002), namely: 26 °C (within the thermal comfort zone) and 32 °C (above of the thermal comfort zone) with an average relative humidity of 65 ± 5%.
During the entire experimental period, the birds received water and feed ad libitum, and the diets were formulated based on the recommendations of the national research council (NRC 1994) for broilers, where a diet of 3,200 kcal/kg of energy is recommended. metabolizable and approximately 23, 20 and 18% crude protein in the initial, growth and final phases, respectively.
Environmental variables and bioclimatic index
The air temperature inside the climate chambers was controlled through two controllers and digital temperature and relative humidity indicators (Full Gauge Controls®, MT-530 PLUS, Rio Grande do Sul, Brazil, operating ranges from 10 to 85% RH and from -10 to 70 °C) with serial communication to Sitrad® software. The controller received the average data of temperature and relative humidity of the air through a combined temperature and relative humidity sensor (Full Gauge Controls®, SB56, Rio Grande do Sul, Brazil, operating ranges from -10 to 70 °C AT, 10 to 85% RH, accuracy ± 5%), every 15 min, recording and controlling the air temperature so as to always remain in the desired control range (Setpoint), using the equipment detailed in the Fig. 1. The air humidifiers and dehumidifiers were deactivated in the climatic chambers, so as not to interfere in the quantification of the energy balance between the birds and the environment air inside the climatic chambers, came from the evaporative thermal exchanges of the birds.
As environmental data, air temperature (AT), wet-bulb temperature (WBT), relative humidity (RH) and wind speed (WS) were collected. For the collection of AT and WBT, encapsulated temperature sensors DS18B20 (Maxim Integrated™, DS18B20, California, United States, operating temperature range from -55 to 125 ºC, accuracy of ± 0.5 ºC) were used, where for the measurement of WBT the sensor bulb was wrapped with a cotton fabric and part of this fabric was submerged in a container with water to keep it always moist. RH was collected using DHT22 sensors (Guangzhou Aosong Electronic Co., Ltd., AM2302, Guangzhou, China, measuring range 0 to 100% RH, accuracy ± 2.0% RH).
For a better representation of the AT and WBT variables inside the climatic chambers, as a whole, four sets of DS18B20 sensors (with two sensors each set) were distributed in strategic positions, one set being positioned in the geometric center of each experimental box, to the approximate height of the bird's head and another set of sensors located in the geometric center of each climatic chamber (at an approximate height of 1.8 m), the AT and WBT being expressed as the average of the values verified in each position. The data captured by the sensors were read and stored through a board based on an ATmega2560 microcontroller (Arduino®, Mega 2560, Italy).
The wind speed was collected using a Thermo-Higro-Luximeter-Digital Anemometer (Lutron®, LM-8000, Pennsylvania, United States, temperature range measuring range from 0.4 to 30.0 m/s and Accuracy ± 3.0%), which was positioned to read the data in the central region of each of the experimental boxes (at the height of the birds' heads) and also in the geometric center of each of the climatic chambers at a height of 1.8 m, with the WS finally being expressed as the mean arithmetic of these values.
The relative humidity of the air was monitored in two specific positions inside the climatic chambers, with a DHT22 sensor being installed in each chamber near the air conditioning outlet and another sensor of the same model near the exhaust, enabling the recording of the relative humidity of the air at the entrance and exit of the thermodynamic system (as well as the climatic chambers for this study were considered), respectively, to enable the estimation of latent thermal exchanges carried out between the birds and the surrounding environment.
For the estimation of thermal exchanges, the black globe temperature (BGT) was not collected, being replaced in the calculations by the air temperature, because the animals were housed in climate chambers, where the lighting was done with fluorescent lamps (cold) there was also a low air flow and solar radiation was effectively blocked and, in this situation, according to Li et al. (2009) the difference between BGT and AT can be overlooked.
The temperature and humidity index (THI) was calculated to quantify the levels of thermal stress experienced by the animals (24 h a day) for the entire experimental period, according to Eq. 1 proposed by Tao and Xin (2003), for birds. Based on the values found, the THI was classified according to the following ranges: < 27.8 = absence of thermal stress; 27.8–28.9 = moderate thermal stress; 28.9–30.0 = severe heat stress and 30.0 or more = very severe heat stress (Sinkalu et al. 2015).
where,
- THI:
-
temperature and humidity index;
- AT:
-
air temperature (°C);
- WBT:
-
wet bulb temperature (°C).
Thermal exchanges
The energy balance between the animals and the environment inside the climatic chambers was evaluated based on the total thermal energy exchanges (WT) per unit surface area of the birds, resulting from the sum of sensible (radiation and convection) and latent heat exchanges.
Sensitive exchanges
To determine heat exchanges by sensitive means between the surrounding environment and the birds, per unit of body surface area, the model proposed by Turnpenny et al. (2000) was used, according to Eq. 2.
where,
- GS:
-
sensible heat exchanges between the birds and the environment (W/m2);
- CR:
-
heat exchange by convection (W/m2);
- L:
-
heat exchange by radiation (W/m2).
Sensitive exchanges by conduction were not included in Eq. 2 as they are considered negligible (McArthur 1987). Convection heat exchanges were determined by Eq. 3, proposed by McArthur (1987).
where,
- CR:
-
heat exchanges by convection (W/m2);
- ρ :
-
air density (kg/m3);
- Cp:
-
specific heat of air (J/kg K);
- rh:
-
resistance of the boundary layer to convection heat transfer (m2 K/W);
- ST:
-
mean temperature of the bird's body surface (K);
- AT:
-
air temperature (K).
The boundary layer resistance to convective heat transfer was calculated by Eq. 4 (Frank and Nelson 1967).
where,
- rh:
-
resistance of the boundary layer to convection heat transfer (m2 K/W);
- ρ :
-
air density (kg/m3);
- Cp:
-
specific heat of air (J/kg K);
- db:
-
average body diameter of birds (m);
- k :
-
thermal conductivity of air (W/m K);
- Nu:
-
Nusselt's number.
The calculation of the average diameter of birds, for each study period, was estimated by Eq. 5, proposed by Mitchell (1930).
where,
- db:
-
average body diameter of birds (cm);
- P:
-
average weight of birds (g).
The Nusselt number was determined by Eq. 6, when considering the representation of the birds' bodies as spheres.
where,
- Nu:
-
Nusselt's number;
- Re:
-
Reynolds number;
- Pr:
-
number of Prandtl.
The Reynolds number was obtained by Eq. 7.
where,
- Re:
-
Reynolds number;
- WS:
-
wind speed (m/s);
- v :
-
kinematic air viscosity (m2/s);
- db:
-
average body diameter of birds (m).
The thermal exchanges by longwave radiation were calculated using Eq. 8, proposed by McArthur (1987). Only longwave radiation was considered, since there is no incidence of solar radiation inside the chambers.
where,
- L:
-
heat exchanges by radiation (W/m2);
- ρ :
-
air density (kg/m3);
- Cp:
-
specific heat of air (J/kg K);
- Rr:
-
resistance of the limiting layer to heat transfer by radiation (m2 K/W);
- ST:
-
body surface temperature (K);
- \({\overline{\text{T}}}_{\text{r}}\) :
-
mean radiant temperature (K).
The resistance of the boundary layer to heat transfer by radiation was calculated by Eq. 9.
where,
- Rr:
-
resistance of the limiting layer to heat transfer by radiation (m2 K/W);
- ρ :
-
air density (kg/m3);
- Cp:
-
specific heat of air (J/kg K);
- Ɛ S :
-
emissivity of the bird feathers (0.94);
- σ:
-
Stefan-Boltzmann constant (5.67*10–8 W/m2K4);
- \({\overline{T}}_{M}\) :
-
average temperature between ST and \({\overline{\text{T}}}_{\text{r}}\) (K).
The mean radiant temperature was obtained using Eq. 10, proposed by Silva (2000).
where,
- \({\overline{\text{T}}}_{\text{r}}\) :
-
mean radiant temperature (K);
- hc:
-
standard black globe convection coefficient;
- BGT:
-
black globe temperature (K);
- AT:
-
air temperature (K).
The standard black globe convection coefficient was calculated by Eq. 11.
where,
- hc:
-
standard black globe convection coefficient;
- k:
-
thermal conductivity of air (W/m K);
- d:
-
diameter of a standard black globe (0.15 m);
- Re:
-
Reynolds number;
- Pr:
-
number of Prandtl.
Prandtl's number was obtained by Eq. 12.
where,
- Pr:
-
number of Prandtl;
- ρ :
-
air density (kg/m3);
- Cp:
-
specific heat of air (J/kg K);
- v :
-
kinematic air viscosity (m2/s);
- k :
-
thermal conductivity of air (W/m K).
Latent exchanges
The process of loss of thermal energy through the evaporation of water expelled by the respiratory tract of birds was considered as latent exchanges. To quantify the latent thermal energy exchanges, the empirical relationship of Hellickson and Walker (1983) was used, as described in Eq. 13, considering the climatic chamber as a thermodynamic system, where mass and energy cross the boundary.
where,
- E:
-
total heat exchanges by evaporation (W/m2);
- ρ :
-
air density (kg/m3);
- WS:
-
wind speed (m/s);
- Wex:
-
partial vapor pressure of the exhaust air, estimated for the region close to the exhaust (kPa);
- Wa:
-
partial vapor pressure of the inlet air, estimated for the region close to the air conditioning outlet (kPa);
- \({\lambda }_{s}\) :
-
latent heat of vaporization of water at the same temperature as the air on the surface of the respiratory tract of birds (2402 kJ/kg).
The partial pressure of water vapor present in the inlet and outlet air (Wa and Wex, respectively) was calculated by the product between the saturation pressure of the air (Ws) and the relative humidity of the air (RH) in each of the positions, respectively, according to Eq. 14.
where,
- Wa:
-
partial vapor pressure of the inlet air (kPa);
- Wex:
-
exhaust air partial vapor pressure (kPa);
- Ws:
-
saturation pressure of water vapor (kPa);
- RH:
-
relative humidity (%).
The saturation pressure of water vapor was calculated using the Tetens equation (Eq. 15).
where,
- Ws:
-
saturation pressure of water vapor (kPa);
- AT:
-
air temperature (°C).
Characteristic properties of air
The physical characteristics of the air were estimated using models, according to each air temperature evaluated, as suggested by Silva (2000) (Table 1).
Table 2 shows the average values estimated of wind speed kinematic viscosity, density, thermal conductivity, specific heat, partial vapor pressure of the inlet air and of the exhaust, as well as the number Nusselt, Reynolds and Prandtl, for the two evaluated treatments.
Physiological responses
From each of the four birds selected and identified with plastic rings, per experimental box, the following physiological responses were measured weekly: respiratory rate (RR), mean body surface temperature (ST), cloacal temperature (CT) and eyeball temperature (ET).
The quantification of respiratory frequency was based on observation (without direct contact with the birds) and counting of the pectoral movements performed by the birds, for a period of 20 s, and then these values were multiplied by 3, starting to be expressed in movements per minute (mov/min).
To determine the mean temperature of the body surface (ST) of the animals, the temperatures in the regions of the back, below the wing, the head and legs of the birds were taken using an infrared thermometer with a laser sight (Instrutherm®, TI—870, São Paulo, Brazil, measuring range -50 to 550 ºC, reading accuracy ± 2%), positioned at a distance of approximately ten centimeters from the animals. With the surface temperatures of the parts of the animals duly registered, the ST of the birds was determined by Eq. 16, proposed by Richards (1971).
where,
- ST:
-
average body surface temperature of birds (°C);
- Twing:
-
temperature of the region below the wing (°C);
- Thead:
-
head temperature (°C);
- Tleg:
-
temperature of the leg (°C);
- TBack:
-
temperature of the back (°C).
The cloacal temperature of the birds was measured using a clinical thermometer (Incoterm®, Termomed, Rio Grande do Sul, Brazil, temperature range 32.0–43.0 °C and accuracy of ± 0.1 °C), introduced in the animals' cloaca, up to the stabilization of the reading. The temperature of the eyeballs of the animals was monitored by capturing thermograms of the eyeballs, using a thermographic camera (Flir®, TG-165, Oregon, United States, Measuring range: -25 to 380 °C, mediation resolution 0.1 °C, accuracy 1.5 °C), with adopted emissivity of 0.98 (Marques et al. 2021).
Zootechnical performance
During the experimental period, all birds were individually weighed each week. Food and water intakes were recorded daily, given by the difference between the amounts offered and the leftovers, and then the total feed and water consumption (FC and WC, respectively) per bird was calculated, dividing the amount of feed and water consumed in a given period of time by the number of birds in each experimental box, thus obtaining the average intakes per bird.
The birds' body weight gain (WG) was calculated by the difference between the initial and final weight for each week of evaluation. The feed conversion index (FCI) was calculated by dividing the amount of food consumed in a given period of time by the weight gain in the same period, both expressed in the same mass unit.
When the guinea fowl reached the age of 90 days of life, they were submitted to a 24-h fast with free access to water and after that they were weighed, stunned and later slaughtered by cutting the neck, where the four birds pre -selected in each experimental box were separated to obtain carcass yield data.
After 5 min of bleeding, each bird was scalded (at 60 °C for 3 min), plucked, eviscerated and the head, neck, legs and giblets were removed. The carcasses of birds without giblets were weighed (CW) and later expressed as percentages of their live weights (LW), these values being considered as carcass yields (CY%). In addition, the yield of liver (LY%, without gallbladder), gizzard (GY%), heart (HY%), viscera (VY%) and feathers (FY%) were also measured of each bird and, after that, yield of each organ in relation to the live weight of the birds was calculated, which were also expressed as a percentage.
Statistical analysis
For a better presentation and analysis of thermal exchange data, physiological and productive responses of the animals, the experimental period was divided into three phases, namely: F1 (from the fourth to the sixth week of life); F2 (from the seventh to the tenth week of life); and F3 (from the eleventh to the thirteenth weeks of life). The productive responses (FC, WC, WG and FCI) were presented as daily means for each experimental phase evaluated. In addition, the averages of the responses of the birds obtained when considering the entire experimental period were analyzed.
The mean values ± SD of THI, AT and RH were presented in scatter plots. The thermal exchanges, physiological responses, productive responses and carcass yield of the evaluated birds were presented in tables as means ± SD of the data. For the analyses, the ExpDes.pt package (version 1.1.2, Ferreira et al. 2013, Brazil) of the statistical software R version 3.4.1 (R Core Team 2013) was used. The normality of the residuals and the homogeneity of the sample variances were tested using the Shapiro–Wilk and Bartlett tests, respectively. To assess the effects of different air temperatures on the responses evaluated, the ANOVA and the F test were used, according to the statistical model presented in Eq. 17. Tukey's test was used to compare means, with a probability of error of 5% (P < 0.05).
where,
- yij:
-
observed value for the response variable obtained for the i-th treatment (air temperature) in its j-th repetition (animals);
- µ:
-
constant effect (overall mean);
- τi:
-
effect of treatment i on the observed value yij;
- εij:
-
error associated with the i-th treatment in the j-th experimental unit.
Results
Climate variables
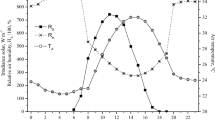
In the two climatic chambers used, the air temperatures were close to the pre-established values for the experiment (Fig. 2), where average ATs of 26.1 ± 0.5 (within the thermal comfort zone) and 31.8 ± 0.6 °C (above the thermal comfort zone) were found, respectively, with small variations, more evident at times close to 6 p.m. The relative humidity of the air in the AT of 32 °C was above the pre-established RH, with an average value of 71.6 ± 2.9% being recorded. At AT of 26 °C the animals were exposed to a mean THI value of 25.0 ± 0.6, this value going to 31.0 ± 0.4, when the animals were subjected to treatment with 32 °C AT, an increase being observed percentage of approximately 24.0% in this index, when comparing the two air temperatures evaluated (Fig. 2).
Thermal exchanges
The increase in air temperature significantly influenced (P < 0.05) the intensity with which the animals exchanged heat in both sensitive and latent forms (Table 3). It is observed that, with the elevation of AT, in the three phases evaluated, there was a significant reduction (P < 0.05) in the amount of heat dissipated by guinea fowl in the sensitive form (reduction of 13.3, 47.1 and 53.0% in phases F1, F2 and F3, respectively), resulting in increased thermal exchanges in the latent form (increase of 73.7, 91.0 and 109.0%, respectively, for each of the evaluated phases). When considering the average values of thermal exchanges, for the entire experimental period, in the two ATs evaluated, it can be noted that the birds reduced the amount of heat dissipated in the sensitive form by 53.5%, and increased heat losses in the sensitive form by 82.7% latent form, when compared the air temperature of 26 °C with that of 32 °C.
Physiological responses
With the increase in air temperature from 26 to 32 °C there was a significant increase (P < 0.05) in respiratory rate (RR) of 23.9, 22.9 and 20.9%, in cloacal temperature (CT) of 0.5, 1.0 and 0.2 °C, in surface temperature (ST) of 5.2, 1.9 and 1.4 °C and in eyeball temperature (ET) of 1.6, 2.1 and 2.0 °C, for the three phases (F1, F2 and F3) evaluated, respectively. When analyzing the mean values of physiological responses of birds during the total experimental period, it was possible to verify that there was also a significant increase (P < 0.05) of 22.9, 1.2, 8.2, 6.0 and 17.9% in RR, CT, ST and ET, respectively, when comparing the air temperature of 26 with that of 32 °C (Table 4).
Productive responses
At the highest air temperature, the birds consumed a greater (P < 0.05) amount of water, with a percentage increase in consumption of 5.6%, when considering the entire experimental period. When comparing the two air temperatures evaluated, there was no significant difference (P > 0.05) in the animal weight, feed consumption, weight gain and feed conversion index responses of the birds. Among the different age groups of the birds, a considerable increase in the feed conversion index can be seen at the two air temperatures tested, with an increase of 138.9% in FCI when compared to phases F2 and F3 at an air temperature of 26° C and 135.3% between phases F2 and F3 at an air temperature of 32 °C (Table 5).
Carcass yield
The increase in air temperature significantly influenced (P < 0.05) only in the percentage of feathers, which was lower in birds subjected to an air temperature of 32 °C (Table 6). The live weight, carcass weight, carcass yield, gizzard yield, heart yield, liver yield and offal yield were not significantly affected (P > 0.05) with increasing air temperature.
Discussion
The fluctuations in the air temperature inside the climatic chambers during the period from 5 to 6 pm, at an air temperature of 26 °C, possibly occurred due to the opening of the chamber for the handling of birds that occurred at that time, facts also found by Marques et al. (2018) in works developed in climatic chambers. At an air temperature of 32 °C, the RH was higher compared to the AT of 26 °C, and this fact is due to changes observed in some physiological processes of animals, such as the increase in respiratory rate, which has one of the consequences, the release of a greater amount of water vapor into the environment and, in addition, in the situation of higher TA, there was greater consumption of water by the birds, making feces and urine more liquefied, and the water contained in these residues when evaporated may also have contributed to the elevation of this variable.
Based on the THI values found in the treatment with AT at 26 °C, the guinea fowl were under thermal comfort environmental conditions, passing to a situation of severe thermal stress when the air temperature was raised to 32 °C, with average values of THI of 25.0 ± 0.6 and 31.0 ± 0.5, respectively, for each AT. According to Sinkalu et al. (2015) THI values equal to or greater than 31 are considered emergency for broilers, as these animals, as well as guinea fowl, have a reduced amount of sweat glands and (Loyau et al. 2013), in this scenario, they need to raise the respiratory rate to dissipate excess heat metabolic, thus reducing the productive efficiency to maintain homeothermia.
When analyzing the behavior of heat exchanges between the guinea fowl and the surrounding environment, in view of the evaluated scenarios, it is observed that the birds subjected to treatment with an air temperature of 26 °C dissipated most of the excess metabolic heat in the sensitive way (71.9%), demonstrating that the animals were in conditions of homeostasis, due to the existence of a thermal gradient between the animals and the surrounding environment, promoting heat loss passively, and, consequently, reducing energy expenditure and need for evaporative cooling, which represented, in this situation, 28.1% of total heat exchanges.
At AT of 32 °C, the animals dissipated 57.8% of the excess metabolic heat in an evaporative way, an intensification that took place through increased panting and gular vibrations, under these conditions the animals began to dissipate a total of 57.2 W/m2 of heat metabolic by the respiratory tract, characterizing a condition of high thermal stress to animals, as this more than doubled the reference value for broiler chickens, which is in the range of 10 to 20 W/m2 (Hutchinson 1954). Thus, it was observed that under conditions of thermal stress the latent heat exchange mechanisms played the most important role in the control of homeothermy in these birds.
When numerically analyzing the behavior of heat exchanges in the guinea fowl, as a function of the age of the birds, it was observed that heat dissipation in the sensitive form tended to increase with age at 26 °C and to decrease when subjected to AT of 32 °C. Possibly this phenomenon occurred because in the first weeks of life the birds need an environment with air temperatures close to 32 °C (which has to be gradually reduced by 2 °C per week of life, until reaching the thermal comfort zone), to remain in thermal comfort situation.
At 32 °C AT, there was an average increase of 22.9% in RR when compared to 26 °C AT, evidencing the intensification of respiratory movements and gular vibrations, as a thermoregulation tool. Panting is a physiological response of birds to adjust their body temperature through heat dissipation by evaporative cooling to maintain thermal homeostasis, under conditions of thermal stress (Oliveira et al. 2006), this situation induces body water imbalance, in addition to changes that accelerate the rate of metabolism, energy expenditure and the depletion of ATP (adenosine TriPhosphate) (Sverdlova et al. 2012; Khan et al. 2021).At the highest air temperature evaluated, the Guinea Fowl showed a significant increase (P < 0.05) in the cloacal and surface temperature in all experimental phases, and this increase was due to the generation of endogenous heat and metabolic reactions, caused by the reduction of the thermal gradient between the temperature of the animals and that of the environment (Ribeiro et al. 2018; Marques et al. 2021). The intensification of respiratory evaporative cooling was not enough to avoid the animals' hyperthermia caused by thermal stress, showing the difficulty that Guinea Fowl had to eliminate excess heat, resulting in a noticeable heating of their body core (Rizzo et al. 2017).It was found that guinea hens significantly increased (P < 0.05) the temperature of the eyeball in all experimental phases with the increase in air temperature and this response is due to the fact that the eye contains many innervated capillary channels, which elevate the blood flow due to activation of heat loss mechanisms by sensitive means to maintain homeothermy (Marques et al. 2018).
Between the two air temperatures evaluated, the variables final weight, feed consumption and weight gain did not differ statistically (P > 0.05), even with the animals showing an increase in respiratory rate, cloacal temperature and surface temperature. This similarity may be due to the fact that birds have adjusted their metabolism to the heat stress condition by physiological and heat exchange mechanisms (Lara and Rostagno 2013). These results infer that these birds are adapted to high air temperatures, being able to minimize the effect of heat stress, due to their slower growth compared to broilers, consequently producing less endogenous heat (Marques et al. 2021). The rapid acclimatization of guinea fowl to the highest AT made it possible to better use the energy resources available for metabolism, even under thermal stress conditions, maintaining their homeotherm, without causing a significant reduction in production (Zaglool et al. 2019).
At the highest air temperature, the birds consumed a greater (P < 0.05) amount of water, with a percentage increase of 5.6% and this increase occurs for the replacement of water lost during the activation and use of evaporative cooling mechanisms, which were most used by birds subjected to treatment with AT of 32 °C, to maintain the hydrodynamic balance and prevent body overheating (Frumkin et al. 1986).
Among the different age groups of the birds, there was a considerable increase in feed conversion, regardless of the treatment to which they were exposed, with an increase of 138.9% between F2 and F3 at AT of 26 °C and 135.3% between F2 and F3 at AT of 32 °C, this possibly happened due to the deceleration in the growth rate, with a reduction in the daily weight gain with advancing the age of the birds, consequently, increasing the feed conversion index (Arruda et al. 2021). Bird growth follows the quadratic pattern, accelerated up to 49 days of age, which is a tipping point where a change in pattern occurs, in which birds reach the highest growth rate, starting to reduce this rate from this threshold (Santos et al. 2005), with a decrease in profitability, therefore, given the results presented in this research, it is intuitive to suggest that Guinea Fowl when reared in confinement should be slaughtered until the age that comprises the beginning of the third experimental phase.
The increase in air temperature did not significantly affect (P > 0.05) the carcass yield (live weight and carcass weight) demonstrating the thermotolerance of guinea fowl to the studied environmental conditions, evidencing its rusticity, even having the need to trigger the evaporative thermoregulatory physiological mechanisms to maintain the thermoneutral situation. In addition, it should be noted that slow-growing bird species such as guinea fowl have high resistance to heat stress (Roushdy et al. 2018), a fact that is also corroborated by the results of the present research regarding organ yields (gizzard, heart, liver and viscera) that were also not affected by the increase in AT.
Finally, another answer that further evidenced the ability of guinea fowls to adapt to thermally stressful environments was the significant loss (P < 0.05) of the percentage of feathers in relation to the live weight of birds kept in an environment with air temperature of 32 °C, with the feathers acting as a thermal buffer between the animals and the surrounding environment, and this reduction can accelerate the convective and radiative heat exchanges from the animals' skin surface to the environment (Wolf and Walsberg 2015), reflecting the acclimatization efficiency that these birds have, given a potential heat stress situation.
Conclusions
When guinea fowls were subjected to an air temperature of 32 °C, they needed to activate thermoregulation mechanisms, a fact reflected by the increase observed in all physiological responses evaluated and, in addition, the birds lost a significant part of their feathers as a way to facilitate thermal exchanges. The birds reduced by 53.5% the amount of heat dissipated in the sensible form, and increased the loss of heat in the latent form by 82.7%, when compared the air temperatures of 26 and of 32 °C. Although the birds have redirected part of their metabolic energies to thermoregulation in a situation of thermal stress, this did not affect their productive results nor their carcass and organ yield, showing that the guinea fowls have rusticity at high air temperatures and, in addition, they can be explored totally confined and without the need to use environmental variable control equipment, in regions where the air temperature reaches 32 °C.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
ABPA. Brazilian Animal Protein Association (2018) Annual Report.: http://abpa-br.com.br/storage/files/relatorio-anual-2018.pdf. Accessed 26 August 2021
Arruda AS, Marques JI, Leite PG, Furtado DA (2021) Productive and hematologic responses of country poultry subjected to different housing densities and water salinity levels. Poult Sci 100:1–10. https://doi.org/10.1016/j.psj.2021.101070
Baracho MS, Nääs IA, Lima NDS, Cordeiro AFS, Moura DJ (2019) Factors Affecting Broiler Production: A Meta-Analysis. Braz J Poultry Sci 21:1–9. https://doi.org/10.1590/1806-9061-2019-1052
El-Deeb MA, Sharara HH, Makled MN (2000) Enhance calcium and phosphorus utilization by enzyme phytase supplemented to broiler diet contained rice bran. Egypt Poult Sci 20:545–566
Ferreira EB, Cavalcanti PP, Nogueira DA (2013) ExpDes.pt: Experimental Designs pacakge (Portuguese). R package version 1.1.2
Flock DK, Laughlin KF, Bentley J (2005) Minimizing losses in poultry breeding and production: How breeding companies contribute to poultry welfare. World’s Poult Sci J 61:227–237. https://doi.org/10.1079/WPS200560
Frank W, Nelson GL (1967) Nonevaporative convective heat transfer from the surface of a bovine. Transactions of the ASABE 10:733–0737. https://doi.org/10.13031/2013.39773
Frumkin R, Pinshow B, Weinstein Y (1986) Metabolic heat production and evaporative heat loss in desert phasianids: chukar and sand partridge. Physiol Zoology 6:592–605
Havenstein GB, Ferket PR, Qureshi MA (2003) Growth, livability and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult Sci 82:1500–1508. https://doi.org/10.1093/ps/82.10.1500
Hellickson MA, Walker JN (1983) Ventilation of Agricultural Structures. St. Joseph: ASABE, 23
Hutchinson JCD (1954) Evaporative cooling in fowls. J Agric Sci 45:48–59. https://doi.org/10.1017/S0021859600045780
Khan RU, Naz S, Nikousefat Z, Tufarelli V, Javdani M, Rana N, Laudadio V (2011) Effect of vitamin E in heat-stressed poultry. World’s Poult Sci J 67:469–478. https://doi.org/10.1017/S0043933911000511
Khan RU, Naz S, Ullah H, Ullah Q, Laudadio V, Qudratullah BG, Tufarelli V (2021) Physiological dynamics in broiler chickens under heat stress and possible mitigation strategies. Anim Biotechnol 32:1–10. https://doi.org/10.1080/10495398.2021.1972005
Lara LJ, Rostagno MH (2013) Impact of heat stress on poultry production. Animals 3:356–369. https://doi.org/10.3390/ani3020356
Li S, Gebremedhin KG, Lee CN, Collier RJ (2009) Evaluation of Thermal Stress Indices for Cattle. ASABE, St. Joseph, MI, 2009 Reno, Nevada, June 21-June 24, 2009. https://doi.org/10.13031/2013.27441
Loyau T, Berri C, Bedrani L, Métayer-Coustard S, Praud C, Duclos MJ, Tesseraud S, Rideau N, Everaert N, Yahav S, Mignon-Grasteau S, Collin A (2013) Thermal manipulation of the embryo modifies the physiology and body composition of broiler chickens reared in floor pens without affecting breast meat processing quality. J Anim Sci 8:3674–3685. https://doi.org/10.2527/jas.2013-6445
Macari M, Furlan RL, Gonzales E (2002) Fisiologia aviária aplicada a frangos de corte. 2nd ed. Jaboticabal: FUNEP/UNESP. 375
Marques JI, Lopes Neto JP, Nascimento JWB, Talieri IC, Medeiros GR, Furtado DA (2018) Pupillary dilation a termal stress indicator in boer crossbred goats maintained in a climate chamber. Small Rumin Res 158:26–29. https://doi.org/10.1016/j.smallrumres.2017.11.013
Marques JI, Leite PG, Furtado DA, Oliveira AG (2021) Evaluation of Heat Stress Through Temperature and Pupillary Dilatation of the Guinea Fowl (NumidaMeleagris) in a Controlled Environment. Braz J Poultry Sci 23:1–6. https://doi.org/10.1590/1806-9061-2020-1409
McArthur AJ (1987) Thermal interaction between animal and microclimate: a comprehensive model. J Theor Biol 126:203–238. https://doi.org/10.1016/S0022-5193(87)80229-1
Mitchell HH (1930) The surface area of single comb white leghorn chickens. J Nutrit 2:443–449. https://doi.org/10.1093/jn/2.5.443
Nahashon SN, Adefope N, Amenyenu A, Tyus J, Wright D (2009) The effect of floor density on growth performance and carcass characteristics of French guinea broilers. Poult Sci 88:2461–2467. https://doi.org/10.3382/ps.2008-00514
NRC. National Research Council (1994) Nutrient Requirements of Poultry (9th, rev. National Academy Press, Washington, DC
Oliveira RFM, Donzele JL, Abreu MLT, Ferreira RA, Vaz RGMV, Cella OS (2006) Efeitos da temperatura e da umidade relativa sobre o desempenho e o rendimento de cortes nobre de frangos de corte de 1 a 49 dias de idade. Rev Bras Zootec 35:797–803. https://doi.org/10.1590/S1516-35982006000300023
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ribeiro NL, Costa RG, Filho ECP, Ribeiro MN, Bozzi R (2018) Effects of the dry and the rainy season on endocrine and physiologic profiles of goats in the Brazilian semi-arid region. Ital J Anim Sci 17:454–461. https://doi.org/10.1080/1828051X.2017.1393320
Richards SA (1971) The significance of changes in the temperature of the skin and body core of the chicken in the regulation of heat loss. J Physiol 216:1–10. https://doi.org/10.1113/jphysiol.1971.sp009505
Rizzo M, Arfuso F, Alberghina D, Giudice E, Gianesella M, Piccione G (2017) Monitoring changes in body surface temperature associated with treadmill exercise in dogs by use of infrared methodology. J Therm Biol 69:64–68. https://doi.org/10.1016/j.jtherbio.2017.06.007
Roushdy EM, Zaglool AW, El-Tarabany MS (2018) Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J Therm Biol 74:337–343. https://doi.org/10.1016/j.jtherbio.2018.04.009
Santos AL, Sakomura NK, Freitas ER, Fortes MLS, Carrilho ENVM, Fernandes JBK (2005) Estudo do Crescimento, Desempenho, Rendimento de Carcaça e Qualidade de Carne de Três Linhagens de Frango de Corte. Braz J Anim Sci 34:1589–1598. https://doi.org/10.1590/S1516-35982005000500020
Settar P, Yalcin S, Turkmut L, Ozkan S, Cahanar A (1999) Season by genotype interaction related to broiler growth rate and heat tolerance. Poult Sci 78:1353–1358. https://doi.org/10.1093/ps/78.10.1353
Silva RG (2000) A heat balance model for cattle in tropical environments. Braz J Anim Sci 29:1244–1252. https://doi.org/10.1590/S1516-35982000000400039
Sinkalu VO, Ayo JO, Adelaiye AB, Hambolu JO (2015) Ameliorative effects of melatonin administration and photoperiods on diurnal fluctuations in cloacal temperature of Marshall broiler chickens during the hot dry season. Int J Biometeorol 59:79–87. https://doi.org/10.1007/s00484-014-0826-4
Sverdlova NS, Lambertz M, Witzel U, Perry SF (2012) Boundary Conditions for Heat Transfer and Evaporative Cooling in the Trachea and Air Sac System of the Domestic Fowl: A TwoDimensional CFD Analysis. PLoS ONE 7:1–9. https://doi.org/10.1371/journal.pone.0045315
Tao X, Xin H (2003) Acute synergistic effects of air temperature, humidity, and velocity on homeostasis of market-size broiler. Trans Am Soc Agric Eng 46:491–497. https://doi.org/10.13031/2013.12971
Turnpenny JR, McArthur AJ, Clark JA, Wathes CM (2000) Thermal balance of livestock: 1. A Parsimonious Model Agric for Meteorol 101:15–27. https://doi.org/10.1016/S0168-1923(99)00159-8
Wolf BO, Walsberg GE (2015) The Role of the Plumage in Heat Transfer Processes of Birds. Amer Zool 40:575–584. https://doi.org/10.1093/icb/40.4.575
Zaglool AW, Roushdy EM, El-Tarabany MS (2019) Impact of strain and duration of thermal stress on carcass yield, metabolic hormones, immunological indices and the expression of HSP90 and Myogenin genes in broilers. Res Vet Sci 12:193–199. https://doi.org/10.1016/j.rvsc.2018.11.027
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marques, J.I., Leite, P.G., Furtado, D.A. et al. Thermal exchanges, physiological responses and productive performance of Guinea Fowl (Numidia meleagris) subjected to different air temperatures. Int J Biometeorol 67, 1237–1249 (2023). https://doi.org/10.1007/s00484-023-02492-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-023-02492-6