Abstract
Long-term assessments of thermal responses of housed Jersey cows raised in tropical conditions were performed to investigate the effect of climate environment on their physiological performance and thermal equilibrium. Twelve Jersey dairy cows with 326.28 ± 30 kg of body weight, 17.66 ± 1.8 of milk yield, and 165.5 ± 6.8 of days in milking were assigned in two 12 × 12 Latin square designs. Air temperature, relative humidity, partial vapor pressure, direct and diffuse short-wave solar radiation and black globe temperature under the shade, and direct sunlight were recorded. Physiological responses as respiratory rate (RR, breaths min−1), ventilation (VE, L s−1), proportion (%) of oxygen (O2) and carbon dioxide (CO2), saturation pressure (PS{TEXH}), and air temperature (TEXH, °C) of the exhaled air were assessed protected from solar radiation and rain. Rectal temperature (TR, °C), skin temperature (TEP, °C), and hair coat surface temperature (TS, °C) were also recorded. The thermal equilibrium was determined from biophysical equations according to the principles of the energy conservation law in a control volume. Exploratory and confirmatory analyses were performed from principal components and by the least square method, respectively. The cows were evaluated under range of ambient air temperature from 26 to 35 °C, relative humidity from 27 to 89%, and short-wave radiation from 0 to 729 W m−2. Exploratory and confirmatory analyses demonstrated that a similar level of nocturnal and diurnal air temperatures evoked distinct (P < 0.05) responses for rectal (TR, °C) and skin (TEP, °C) temperatures, ventilation (VE, L s−1), tidal volume (TV, L breaths−1), and oxygen consumption (∆O2, %) and carbon dioxide output (∆CO2, %), clearly revealing an endogenous rhythm dependence. In conclusion, these findings clarify how the circadian rhythmicity of the thermal environment and animal’s biological clock dictate dynamics of heat generated by metabolism, dissipated to the environment and physiological parameters of the housed Jersey cows raised in tropical condition; therefore, it is fundamental to help us to understand how the Jersey dairy cows under tropics are affected by the climatic conditions, leading to better ways of the environmental management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heat tolerance is determined by the relationship between metabolic heat production and the ability to dissipate body heat (Berman 2011). Cattle are physical systems in which thermal energy is produced continuously by metabolic processes and, at the same time, exchanges with its external environment by sensible and latent pathways (Da Silva and Maia 2013). Studies emphasizing the effect of hot climates on physiological responses and thermal equilibrium of dairy cows are relatively abundant in the literature (Kibler and Brody 1954; Kibler 1960; Gebremedhin et al. 1981; Keren and Olson 2006; Usman et al. 2013; Willians et al. 2016), and most of them aim to understand aspects of morphological traits and physiological and behavioral responses that explain the heat tolerance of dairy cattle, as well as, seeking strategies to mitigate negative effects of the high thermal load on dairy cattle thermoregulation.
Early attempts to import high-yielding dairy cows from temperate regions to be raised in hot climates did not present satisfactory results (Berman 2011; Vilela et al. 2017); these animals show high sensitivity to harsh environmental conditions that may be associated with non-attainment of their genetic potential (Ostojić-Andrić et al. 2017). Nevertheless, over the last century, the dairy cattle populations in tropical regions have acquired phenotypic characteristics that conferred a better thermal tolerance to these environments. Investigations on cutaneous surface traits, physiological responses and balance of heat production, gain, and loss have been performed to better understand the heat tolerance of dairy Holstein cows raised in the tropical conditions (Da Silva 1999, 2000; Da Silva et al. 2003, 2012; Maia et al. 2003, 2005a, b; Santos et al. 2017). However, observing the scenario of the Brazilian milk production chain, other breeds like Jersey have good potential to be managed in tropical conditions due to their genetic, adaptive, and productive characteristics. When compared with the Holstein, the Jersey cattle have a relatively higher relative surface area/mass ratio, better skin protection against ultraviolet solar radiation, and greater sweating capacity (Silva et al. 1988), but there is a lack of findings on thermal equilibrium of Jersey dairy cows.
Furthermore, the majority of findings in the literature highlighting the effect of thermal environment on physiological responses of dairy cattle have been restricted to short measurements across the day. Therefore, the findings of the present study come to supply this information in the literature. The circadian rhythmicity is an adaptive mechanism of the animal, which synchronizes a wide range of physiological and behavioral functions to counter with the possible offensive environmental conditions (Maloney et al. 2013). In this view, the objectives of this study were to perform long-term assessments of thermal responses of the housed Jersey cows raised in tropical conditions and to investigate the effect of climate environment on their physiological performance and thermal equilibrium. Research dealing with these aspects may very well improve our understanding about environmental management requirements and heat tolerance of dairy cows raised in tropical environment.
Materials and methods
Animals, experimental design, and management
The study was carried out at the Paulista Agency for Agribusiness Technology (APTA), Ribeirão Preto, São Paulo State, Brazil (21° 10′ S, 47° 48′ W, 546 m altitude) in September and November 2014. Twelve Jersey dairy cows with 326.28 ± 30 kg of body weight, 17.66 ± 1.8 of milk yield, and 165.5 ± 6.8 days in milking were randomly assigned in two 12 × 12 Latin square (LS) designs. Data were recorded from 08:00 AM to 08:00 PM on LS1 during 12 days; for example, the first animal was evaluated between 08:00 and 09:00 h, the second between 09:00 and 10:00 h, and so until the last evaluation was performed between 07:00 PM and 08:00 h PM. Subsequently, the same animals were randomly assigned on LS2 to perform evaluations from 08:00 PM to 08:00 h AM during 12 different days, totalizing 24 days of records. Between measurements, subjects were housed in a free-stall barn, being fed a total mixed ration of corn silage (70%) and grain (30%) at 06:00 and 17:00 h. Fresh water was provided ad libitum.
Meteorological variables
Meteorological variables as ambient air temperature (TA, °C), relative humidity (HR %), partial vapor pressure (PP{TA}, kPa), black globe temperature under the shade (TG(in), °C), and direct sunlight (TG(out), °C) were registered at regular 10-min intervals with a Data Logger (model HOBO, onset). Furthermore, a portable pyranometer (Model CMP-22, Kipp and Zonen, Delft, Netherlands) was used to measure direct and diffuse short-wave radiation (RS, W m−2; spectral range (λ) = 200–3600 nm) on a horizontal surface.
Physiological responses
The cow was quietly moved to a chute squeeze to perform evaluations protected from solar radiation and rain. Physiological variables as respiratory rate (RR, breaths min−1), ventilation (VE, L s−1), proportion (%) of oxygen (O2) and carbon dioxide (CO2), saturation pressure (PS{TEXH}), and air temperature (TEXH, °C) of the exhaled air were assessed. Rectal temperature (TR, °C), skin temperature (TEP, °C), and hair coat surface temperature (TS, °C) were also recorded. These parameters were obtained using a physiological system for thermal equilibrium evaluation coupled to the face mask adjusted to the animal’s muzzles, developed by the Innovation Group of Animal Biometeorology. Details of the system as operation, mask design, devices used, and temperature sensor resolution were described in previous reports (Maia et al. 2014, 2016; Nascimento et al. 2017; de Melo Costa et al. 2018a). Biophysical equations were used to determine the metabolic heat production (q″met, W m−2), heat exchanges by long-wave radiation (q″RL) and surface convection (q″conv), and latent heat loss by respiratory evaporation (q″er), as proposed by da Silva and Maia (2013) and described by de Melo Costa et al. (2018a).
Statistical analyses
Confirmatory analyses were performed by the least-squares method using the General Linear Models Procedure (PROC GLM) of the Statistical Analysis System (SAS Institute, version 8), according to Littell and Freund (1991). The adjusted means were compared by Tukey post hoc test (P ≤ 0.05). The linear statistical model used to describe physiological variables was:
where Yijklm is the mth observation of the physiological variables; Q is the fixed effect of the ith Latin square (i = 1 and 2); C is the random effect of the jth cow (j = 1,…,12); D is the fixed effect of the kth day within ith Latin square (i = 1 then k = 1,…,12; now i = 2 then k = 13,…,24); H is the fixed effect of the Lth hour class (L = 1,…,24) of the day within ith Latin square (i = 1 then L = 1, from 8 until 9 AM; 2, from 9 until 10 AM; 3, from 10 until 11 AM so on until 12, 7 until 8 AM; if i = 2, then L = 13, from 9 until 10 PM; 14, from 10 until 11 PM so on until 24, from 7 until 8 AM); eijkLm is the residual term, including the random error; and μ is the overall mean. Pearson correlations were tested between all variables.
Based on the physiological responses (TR, TEXH, TEP, TS, RR, VE, ∆O2, and ∆CO2), principal component analyses were performed to verify dissimilarity patterns of the diurnal and nocturnal air temperatures. Note that nocturnal range of air temperatures was taken from 5 AM to 6 PM. Before the analyses, all the variables were standardized for residuals normality criteria (variance = 1; mean = 0). Using the correlation matrix, levels of air temperatures were divided into three groups under the two principal components (Z1 and Z2).
Data availability
Partial data of the present work were previously published in the VII Brazilian Congress of the Biometeorology, Ambience, Behavior and Animal Welfare (VII CBBiomet).
Results
Meteorological variables
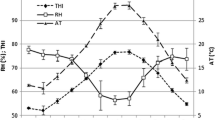
The solar irradiance (RS) ranged between 0 and 729 ± 7.83 W m−2, which from 9 AM to 1 PM, the levels was above 500 W m−2; the high value was recorded at noontime (Fig. 1). The range of solar radiation resulted in a mean ambient air temperature (TA) of 26.14 ± 0.06 °C and thermal amplitude around 10 °C throughout 24 h. Minimal and maximal TA were respectively 22.25 ± 0.06 °C and 32.02 ± 0.07 °C, observed at 4 AM and 3 PM, respectively; between 8 PM and 8 AM, mean of TA varied from 23.80 ± 0.06 to 22.96 ± 0.06 °C and linearly increased until 3 PM. The relative humidity (RH) was lower than 50% from 9 AM to 6 PM, while at all other times, levels were above 80%; minor percentage of humidity (27.48 ± 0.36%) was observed at 3 PM. It is interesting to note that the peak of TA occurred 3 h after the largest level recorded for solar radiation (Fig. 1); under indoor environment, the thermal radiation is absorbed by the external surface of the roof, and subsequently is emitted from long-wave radiation into the facility.
Physiological responses
All the effects considered in the analysis of variance of physiological data were significant (P < 0.05). These results indicate, more importantly, the influence of day/night periods on hair coat surface (TS), skin (TEP), rectal (TR), and exhaled air (TEXH) temperatures; respiratory rate (RR); ventilation (VE); and carbon dioxide (%CO2EXP) and oxygen (%O2EXP) proportions in the exhaled air of Jersey dairy cows (Table 1).
The influence of meteorological variables on the thermal equilibrium of the Jersey cows was corroborated by the principal component analysis. According to the exploratory analyses (Fig. 2), under a thermal oscillation higher than 10 °C throughout 24 h of the day (21 to 33 °C), significant changes of physiological responses were verified (P < 0.05), and consequently affected the thermal equilibrium of Jersey dairy cows.
Bi-plot of the diurnal (1st component < 1.5) and nocturnal (1st component > 1.5) ambient air temperatures and physiological responses versus the two principal components. Note that correlation (from − 1 to 1; represented with arrays ) between variables and the principal components was multiplied by a factor of two. Diurnal ambient air temperatures were taken from 5 AM to 6 PM. Parameters are defined as follows: proportions of oxygen consumed (∆O2,%) and carbon dioxide output (∆O2,%), ventilation (VE, L s−1), respiratory rate (RR, breaths min−1), saturation pressure at the exhaled air temperature (PS{TEXH}, kPa), and rectal (TR, °C), skin (TEP, °C), hair coat surface (TS, °C), and exhaled air (TEXH, °C) body temperatures
Diurnal and nocturnal air temperatures were fitted under the two principal components (Z1 and Z2), in which the Z1 absorbed 81.97% of total variance of the original variables (Fig. 2). The classes of air temperature were separated into three groups, in which the first and second groups had the same air temperatures (i.e., TA = 21–26 °C) recorded during nocturnal and diurnal periods, respectively, and a third group structured by air temperatures above 28 °C (i.e., TA = 29–34 °C). Six physiological responses (i.e., TR = 0.96, TEP = 0.98, RR = 0.93, VE = 0.87, ∆O2 = 0.91, ∆CO2 = 0.93) were highly correlated with Z1. Notably, they were the most relevant parameters to separate the first and second groups of ambient air temperatures. Otherwise, three parameters (i.e., TS = 0.50, TEXH = 0.55, PS{TEXH} = 0.45) presented weak correlations with Z2. Overall, these analyses showed that the same level of nocturnally and diurnally ambient air temperatures evoked different responses for TR, TEP, RR, VE, ∆O2, and ∆CO2.
The metabolic heat production (q″met) was lower at 5 AM and higher at 7 PM (Fig. 3); from 8 PM to 5 AM, the q″met ranged between 136.10 ± 4.71 and 87.42 ± 3.44 W m−2, increasing until 211 ± 4.90 W m−2 at 7 AM; the times of high ambient air temperature (3 PM) were combined with q″met of 194.04 ± 7.68 W m−2 (Fig. 3). The variation of q″met was associated with the patterns of respiratory rate (RR), tidal volume (VT), ventilation (VE), and consequently volumes of oxygen consumption (VO2) and carbon dioxide output (VCO2). From 7 PM to 5 AM, mean of VO2 (117.66 ± 2.74 to 49.93 ± 1.92 L h−1) and VCO2 (146.78 ± 3.52 to 58.12 ± 2.47 L h−1) substantially decreased (P < 0.05). At this interval, the drop on the VO2 and VCO2 and in the metabolic heat production was also linked with the significant alterations of the proportions of O2 (%O2ATM − %O2EXP) and CO2 (%CO2EXP − %CO2ATM) in the exhaled air.
The least square means of the physiological variables depicted as a function of diurnal and nocturnal air temperatures also confirmed the influence of day/night periods (Fig. 4).
The rectal temperature continuously increased from 5 AM to 9 AM (range, 38.57 ± 0.04 to 39.20 ± 0.03 °C) and remained practically stable until 2 PM even with rise of the ambient air temperature; after that, the TR increased again reaching 39.7 ± 0.03 °C at 7 PM (Fig. 5). The peak of the TR did not coincide with the high levels of air temperature, presenting a moderate correlation between them (r = 0.59; P < 0.0001). It seems that the variation of TR was much more linked with the levels of metabolic heat production; as observed with proportion of consumed oxygen and carbon dioxide production, rectal temperature under the same range of nocturnal and diurnal TA evoked different (P < 0.05) responses (Fig. 4). For instance, the least square means calculated for proportions of O2 consumption and CO2 output under classes of ambient air temperature from 22 to 26 °C in nocturnal periods were lower than those observed at the same levels of TA during daytime.
Least square mean (± SEM) of the rectal (TR), exhaled air (TEXH), hair coat surface (TS), and skin (TEP) temperatures; heat exchanges by long-wave radiation (q″RL) and surface convection (q″conv); respiratory rate (RR); saturation pressure of the expired air (PS{TEXH}); and latent heat transferred through the respiratory tract (q″er) of Jersey dairy cows throughout 24 h
The skin temperature (TEP) increased from 33.42 ± 0.12 °C at 5 AM to 35.80 ± 0.09 °C at 8 AM and changed moderately throughout the day until the peak of 36.76 ± 0.07 °C at 6 PM (Fig. 5). The alterations on skin temperature are mainly related to the level of thermal tissue resistance, which is quite responsive to ambient temperature and the content of body thermal energy. We observed minor values of TEP and therefore maximum vasoconstriction under times of lower ambient air temperature and TR (from 4 to 5 AM); on the other hand, the peak of TEP or maximum vasodilatation occurred at times of high levels of TR, but did not coincide with the higher value of TA, even with the high correlation observed between them (TEP − TA = 0.92; P < 0.001). As noted with q″met and TR, confirmatory analyses showed distinct pattern of alterations in the skin temperature as a function of similar diurnal and nocturnal classes of air temperatures (Fig. 4).
The skin and hair coat temperatures (TS) interchanged throughout the day (Fig. 5); from 8 PM to 7 AM, the TEP was below the TS, which the difference between them was close to 0.5 °C. Conversely, at an interval between 8 AM and 6 PM, the TEP was above (± 1 °C) of the TS. Different to those observed on q″met, TR, and TEP, the alterations of TS seem to be highly influenced by the levels of air temperature; the diurnal and nocturnal classes of air temperature evoked similar responses on TS, regardless of the time of the day (Fig. 4). The hair coat surface temperature of the Jersey cows changed moderately over 24 h (range, 34.28 ± 0.12 to 35.59 ± 0.07 °C), but the high thermal amplitude altered substantially the fluxes of the heat exchanges by long-wave radiation and surface convection (Fig. 5). At an interval between 8 PM and 7 AM, the TS varied from 35.54 ± 0.11 to 34.28 ± 0.12 °C following a drop of 6 °C (range = 28.62 ± 0.06 to 22.90 ± 0.06 °C) in the ambient air temperature; over this period, the thermal gradient (∆ = TS − TA) was around 7 °C, reflecting in a range from 95.38 ± 1.20 to 101.23 ± 1.25 W m−2 of sensible heat loss; such fluxes accounted for approximately 90% of total metabolic heat production. In contrast, from 8 AM to 3 PM, as the TA increases (25 to 32 °C), the sensible ways become less significant; when the ambient air temperature was above 28 °C, the heat transfer by long-wave radiation and convection represented close to 30% of the metabolism. Under these circumstances, the moisture vaporization at the cutaneous and respiratory surfaces becomes the principal mean to maintain the thermal equilibrium of Jersey dairy cows.
From 9 PM to 6 AM, the range of respiratory rate (RR; 36.75 ± 0.71 to 30.64 ± 0.72 breaths min−1), saturation pressure (PS{TEXH}; 3.22 ± 0.05 to 2.96 ± 0.05 kPa), and temperature of the exhaled air (TEXH; 30.68 ± 0.10 to 30.75 ± 0.09 °C) coupled with ambient air temperature below 23 °C, and relative humidity above 80% resulted in a mean of respiratory evaporation close to zero (q″er; 3.23 ± 0.33 to 1.58 ± 0.30 W m−2). On the other hand, between 8 AM and 3 PM, as the ambient air temperature increases (22 to 32 °C), an increment on the RR (30.64 ± 0.72 to 39.02 ± 0.20 breaths min−1) and TEXH (30.75 ± 0.09 to 33.05 ± 0.10 °C), but small changes on the PS{TEXH}, has driven the rise of the latent heat transfer from the respiratory system to 20.61 ± 0.61 W m−2, representing close to 10.29% of the metabolism. At this time, sensible heat ways (64.12 ± 1.07 W m−2) still represented 33.68% of the metabolic heat production; then, considering the q″met of 194.04 ± 1.71 W m−2 at 3 PM, it would need to dissipate about 109 W m−2 by cutaneous evaporation to maintain the thermal equilibrium.
In summary, physiological responses of the Jersey dairy cows changed over the circadian period, leading to significant alterations on metabolic heat production and sensible and latent heat loss. Certainly, most of these responses were driven by the changes on ambient air temperature; indeed, most of the physiological variables were highly or moderately correlated with (TA − TR = 0.69; TA − TEP = 0.92; TA − TS = 0.92; TA − TEXH = 0.99; TA − RR = 0.79; TA − VE = − 0.82; TA − VO2 = − 0.87; TA − VCO2 = 0.78).
Discussion
The high levels of solar radiation, ambient air temperature, and daily thermal amplitude are marked features of the regions located in the tropics and certainly pose challenges to the body thermal regulation for dairy cattle managed either indoor or outdoor environments. Furthermore, this condition presents relative constancy throughout the year, being much different when compared with temperate regions (Da Silva et al. 2010, 2012), which has seasonal fluctuations on the meteorological variables.
The present investigation aims to understand how the biological circadian clock and ambient air temperature cycles influence the physiological performance and thermal balance of housed Jersey dairy cows managed in tropical conditions. The cows were assessed under an explicit monophasic circadian rhythm for ambient temperature. To the best of our knowledge, this is the first long-term study on the Jersey dairy breed under tropical conditions. Our study revealed three notable findings. Firstly, analyses of ambient air temperature cycles showed that metabolism, ventilation, tidal volume, and rectal and skin temperatures had apparently biological rhythmicity dependence. Secondly, thermal conditions imposed did not cause any apparent disruption on thermal responses of Jersey cows. Thirdly, based on the significance of ways to eliminate the metabolic heat production by sensible heat transfer under the range of air temperatures from 21 to 34 °C, the upper critical temperature for Jersey dairy cows protected from direct solar radiation is likely close to 28 °C.
The metabolic heat production and body temperatures have robust circadian patterns, which in turn, either directly or indirectly, influence the daily patterns of many physiological responses and heat balance (Todini 2007). Based on the obtained findings herein, the metabolism of Jersey dairy cows followed significant changes of the ambient air temperature cycle. In fact, during the diurnal period (5 AM to 6 PM), means of VO2 and VCO2, proportions of O2 consumption (∆O2) and CO2 output (∆CO2), and metabolic heat production were significantly greater than metabolism during the night (7 PM to 4 AM). Also, these changes were associated with patterns of respiratory ventilation (Fig. 3). Moreover, peaks of metabolic heat production (at 8 AM and 7 PM) during the day occurred 2 h after feeding times. Patterns of circadian changes on metabolism of Jersey cows in the present study were similar to those observed by De Melo Costa et al. (2018) during 24-h assessments with Nellore cattle in tropical conditions. The survey demonstrated lower levels on metabolic heat production during the night period, and two peaks after feeding times. The thermogenic effect after the feeding times has been well discussed in the literature with several other mammals’ species (Hill et al. 2012).
The circadian changes on animal metabolism are associated with the rhythms of environmental temperature and light, which in turn are related to the alternation activity/rest throughout the day. However, the question that arises is how the ambient air temperature influenced the metabolic heat production of Jersey dairy cows? The present study described distinct metabolic responses at the same level of ambient air temperature occurred in different times throughout the day. The least square means of proportions of O2 consumption and CO2 output under classes of ambient air temperature from 22 to 26 °C in nocturnal periods were lower than those observed at the same levels of TA during daytime (Fig. 4). Furthermore, the metabolic heat production remained practically stable during the day as the air temperature rose from 22 to 34 °C. Similarly, Camerro et al. (2016) observed that the metabolism of Guzerat cattle remained relatively stable, regardless of the variation in the air temperature from 25 to 34 °C. Similar responses were observed for ventilation rate (Fig. 3), and clearly indicate that such changes were much more related to attain oxygen demand than to thermal regulation proposals. Interestingly, the increase on ventilation rate was attained modifying the depth of breath, as observed by significant changes on tidal volume, and stable respiratory rate across the day.
The peaks of body rectal and skin temperatures were not observed at the higher levels of air temperature and were more linked to the levels of metabolic heat production. As noted for proportion of consumed oxygen and carbon dioxide production, these body temperatures under the same range of nocturnal and diurnal TA evoked different responses (Fig. 4). Moreover, our findings showed that the skin temperature (TEP) and hair coat temperature (TS) interchanged throughout the day. From 20:00 to 07:00 h, the TEP was below the TS, which the difference between them was close to 0.5 °C. Conversely, at an interval between 08:00 and 18:00 h, the TEP remained above (± 1 °C) the TS. Nevertheless, different to those observed on q″met, TR, and TEP, the alterations of TS seem to be highly primarily influenced by the levels of air temperature (Fig. 4). The alterations on skin temperature are mainly related to the level of thermal tissue resistance, which is quite responsive to ambient temperature cycle and the content of body thermal energy. The thermal conductance of many mammals changes in a circadian manner, which is higher during the active phase than the inactive phase during the day (Aschoff 1981). Therefore, at night, the combination of lower metabolism and ambient air temperature has driven these adjustments on the skin temperature, which help the animals to avoid the excess of heat loss, while at morning, as the metabolism and ambient air temperature increase, the rise of the TEP could be essential to maintain high rates of sweating, avoiding the overheating of the body (Maia et al. 2005a; Silva and Maia 2011). Despite we did not measure the sweating activity in this study, the skin temperature seems to be the main physiological triggering mechanism for high rates of cutaneous evaporation (Silva and Maia 2011).
Analyzing the influence of ambient air temperature on thermal balance of Jersey dairy cows, and considering the average of metabolic heat production (150 W m−2), findings of the present study demonstrated that an air temperature range between 22 and 27 °C, the majority (> 50%) of the metabolic heat production was dissipated by sensible ways, i.e., long-wave radiation and surface convection (Fig. 6). Above 27 °C, sensible means becomes to represent less than 50% of the heat produced by metabolism, and at 34 °C close to 30%. Under these circumstances, the moisture vaporization at the cutaneous and respiratory surfaces should be the principal mean to maintain the thermal equilibrium of Jersey dairy cows. However, the importance of the respiratory tract as a way to lose latent heat seems minor, ranging for 15 to 25 W m−2 when the air temperature rose between 21 and 34 °C. At a level of air temperature near to 34 °C, sensible heat ways and latent heat eliminated by respiratory surface represented 30%; thus, it would need to dissipate about 100 W m−2 from cutaneous evaporation to maintain the thermal equilibrium. In fact, Silva et al. (1988) recorded values near to 125 g m−2 h−1 for sweating rate or 100 W m−2 of energy transferred by moisture evaporation on the skin surface of Jersey dairy cows protected from solar radiation and exposed to a similar level of ambient air temperature.
Sensible heat flow by convection and long-wave radiation (q″RL + q″conv, W m−2), and evaporative heat loss by respiration (q″er, W m−2) of Jersey dairy cows as a function of diurnal (D) and nocturnal (N) air temperatures. Note that diurnal ambient air temperatures were taken from 5 AM to 6 PM. Data are presented as mean ± SEM.
Kibler and Brody (1954) also described that the portion of the sensible heat loss of Jersey dairy cows managed under controlled environment significantly decreased when the ambient air temperature exceeded 26 °C, representing close to 28% of the thermal energy produced by metabolism; the remaining was dissipated by latent ways, which the cutaneous evaporation presented the largest significance. These authors suggested that the upper critical temperature for Jersey dairy cows should be between 25 and 27 °C. Similarly, Maia et al. (2005a) investigating the thermal equilibrium of Holstein cows managed under field in a tropical environment observed that when the air temperature was above 26 °C, the body thermal regulation was mainly governed by the cutaneous evaporation. In this study, the Jersey dairy cows were able to maintain the thermal equilibrium mainly by sensible ways when the range of air temperature was between 22 and 27 °C; above 27 °C, thermoregulation was mainly governed by the moisture evaporation at the skin surface, and in a minor percentage from the respiratory surfaces. This thermal condition was generally observed from 09:00 to 19:00 h. At this time, the potential for means to heat stress relief increases, especially if the relative humidity is below 50% (Berman 1985). Our findings help to understand how the dairy cows under tropics are affected by the climatic conditions, leading to better ways of the environmental management.
Conclusion
The present study sheds basic evidences on how the circadian rhythmicity of the thermal environment and animal’s biological clock dictate dynamics of heat generated by metabolism, dissipation to the environment, and physiological parameters of housed Jersey cows raised in tropical conditions. These findings bear substantial evidences that the circadian cycle of the metabolism and body rectal and skin temperatures of housed Jersey dairy cows are primarily regulated by the endogenous rhythms. The thermal condition imposed did not cause any apparent disruption on thermal responses of housed Jersey cows.
References
Aschoff J (1981) Thermal conductance in mammals and birds: its dependence on body size and circadian phase. Comp Biochem Physiol 69:611–619
Berman A, Folman Y, Kaim M, Mamen M, Herz Z, Wolfenson D, Arieli A, Graber Y (1985) Upper critical temperatures and forced ventilation effects for high-yielding dairy cows in a subtropical climate. J Dairy Sci 68:1488–1495
Berman A (2011) Invited review: are adaptations present to support dairy cattle productivity in warm climates? J Dairy Sci 94:2147–2158
Camerro LZ, Maia ASC, Neto MC, Costa CCM, Castro PA (2016) Thermal equilibrium responses in Guzerat cattle raised under tropical conditions. J Therm Biol 60:213–221
da Silva RG (1999) Estimativa do balanço térmico por radiação em vacas Holandesas expostas ao sol e à sombra em ambiente tropical. Revista Bras Zootec 28(6):1403–1411
da Silva RG (2000) Um modelo para a determinação do equilíbrio térmico de bovinos em ambientes tropicais. Revista Bras Zootec 29(4):1244–1252
Da Silva RG, Guilhermino MM, Morais DAEF (2010) Thermal radiation absorbed by dairy cows in pasture. Int J Biometeorol 54:5–11. https://doi.org/10.1007/s00484-009-0244-1
Da Silva RG, La Scala JRN, Lima-Filho AE, Catharin MC (2002) Respiratory heat loss in the sheep: a comprehensive model. Int J Biometeorol, Berlin 46:136–140
Da Silva RG, La Scala N, Tonhati H (2003) Radiative properties of the skin and hair coat of cattle and other animals. Trans ASABE 46(3):913–918
Da Silva RG, Maia ASC (2013) Principles of animal biometeorology. Springer, New York (Ed. 1)
Da Silva RG, Maia ASC, Costa LLM, Queiroz JPAF.(2012) Latent heat loss of dairy cows in an equatorial semi-arid environment. Int J Biometeorol (Print) , 56: 927–932
De Melo Costa CC, Maia ASC, Nascimento ST, Nascimento CCN, Fonsêca VFN, Chiquitelli Neto M (2017) Thermal balance of Nellore cattle. Int J Biometeorol 74:317–324. https://doi.org/10.1016/j.jtherbio.2018.04.014
da Silva RG, Maia ASC, de Macedo Costa LL, de Queiroz JPAF (2012) Latent heat loss of dairy cows in an equatorial semi-arid environment. Int J Biometeorol 56(5):927–932
De Melo Costa CC, Maia ASC, Nascimento ST, Nascimento CCN, Fonsêca VFN, Chiquitelli Neto M (2018a) Thermal balance of Nellore cattle. Int J Biometeorol 74:317–324. https://doi.org/10.1016/j.jtherbio.2018.04.014
De Melo Costa CC, Maia ASC, Brown-Brand M; Chiquitelli Neto M, Fonsêca VFN (2018) Thermal equilibrium of Nellore cattle in tropical conditions: an investigation of circadian pattern Int. J Biometeorol: https://doi.org/10.1007/s00484-0171349-6
Gebremedhin KG, Cramer CO, Porter WP (1981) Predictions and measurements of heat production and food and water requirements of Holstein calves in different environments. Trans of ASAE 24(3):0715–0720. https://doi.org/10.13031/2013.34326
Hill JO, Wyatt HR, Peters JC (2012) Energy balance and obesity. Circulation 126:126–132. https://doi.org/10.1161/CIRCULATIONAHA.111.087213
Keren EN, Olson BE (2006) Thermal balance of cattle grazing winter range: model application. J Anim Sci 84(5):1238–1247. https://doi.org/10.2527/2006.8451238x
Kibler HH (1960) Oxygen consumption in cattle in relation to rate of increase in environmental temperature. Nature 186:972–973
Kibler HH, Brody S (1954) Influence of diurnal temperature cycles on heat production and cardiorespiratory activities in Holstein and Jersey cows. Research bulletin 601, University of Missouri College of Agriculture Agricultural Experiment Station, February
Littell R C, Freund R J (1991) Spector, P. C. SAS® System for linear models, Third Edition, Cary, NC: SAS Institute Inc., 329p
Maia ASC, da Silva RG, Bertipaglia ECA (2003) Características do pelame de vacas Holandesas em ambiente tropical: um estudo genético e adaptativo. Revista Bras Zootec 32(4):843–853
Maia ASC, da Silva RG, Loureiro CMB (2005a) Sensible and latent heat loss from the body surface of Holstein cows in a tropical environment. Int J Biometeorol 50:17–22. https://doi.org/10.1007/s00484-005-0267-1
Maia ASC, da Silva RG, Loureiro CMB (2005b) Respiratory heat loss of Holstein cows in a tropical environment. Int J Biometeorol 49:332–336. https://doi.org/10.1007/s00484-004-0244-0
Maia ASC, da Silva RG, Nascimento ST, Nascimento CCN, Pedroza HP, Domingos HGT (2014) Thermoregulatory responses of goats in hot environments. Int J Biometeorol 59:1025–1033
Maia ASC, Nascimento ST, Nascimento CCN, Gebremedhin KG (2016) Thermal equilibrium of goats. J Therm Biol 58:43–49. https://doi.org/10.1016/j.jtherbio.2016.03.012
Maloney SK, Meyer LR, Blache D, Fuller A (2013) Energy intake and the circadian rhythm of core body temperature in sheep. Physiol Rep 1:01–09
Ostojić-Andrić D, Petrović MM, Pantelić V, Petrović VC, Nikśić D, Lazarević M, Marinković M (2017) Production performance of Holstein Friesan cattle under breeding-selection program in Central Serbia. Proceedings of Scientific Conference with International Participation “Animal Science - Challenges and Innovations”, Sofia, Bulgaria
Santos SGCG, Saraiva EP, Pimenta Filho EC, Gonzaga Neto S, Fonsêca VFC, Pinheiro A d C, Almeida MEV, de Amorim MLCM (2017) The use of simple physiological and environmental measures to estimate the latent heat transfer in crossbred Holstein cows. Int J Biometeorol 61(2):217–225
Silva RG (2008). Biofísica ambiental – os animais e seu ambiente. FUNEP, Jaboticabal, SP, Brazil
Silva RG, Arantes-Neto JG, Holtz Filho SV (1988) Genetic aspects of the variation of the sweating rate and coat characteristics of Jersey cattle. Braz J Genet (11):335–347
Silva RG, Maia ASC (2011) Evaporative cooling and cutaneous surface temperature of Holstein cows in tropical conditions. R Bras Zootec 40:1143–1147
Todini L (2007) Thyroid hormones in small ruminants: effects of endogenous, environmental and nutritional factors. Animal 1(7):997–1008. https://doi.org/10.1017/S1751731107000262
Usman T, Qureshi MS, Yu Y, Wang Y (2013) Influence of various environmental factors on dairy production and adaptability of Holstein cattle maintained under tropical and subtropical conditions. Adv Environ Biol 7(2):366–372
Vilela D, Resende JCD, Leite JB, Alves E (2017) A evolução do leite no Brasil em cinco décadas. Revista de Política Agrícola, v. 26, n. 1, p 5–24
Williams R, Scholtz MM, Neser FWC (2016) Geographical influence of heat stress on milk production of Holstein dairy cattle on pasture in South Africa under current and future climatic conditions. South African Journal of Animal Science 46(4):441
Acknowledgments
We would like to show our gratitude to the Agência Paulista de Tecnologia dos Agronegócios – APTA of Ribeirão Preto, SP, Brazil, for sharing their animals and the structure so that it was possible to carry out the research.
Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), process numbers 2011/17388-6 and 2014/09639-7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nascimento, S.T., Maia, A.S.C., de França Carvalho Fonsêca, V. et al. Physiological responses and thermal equilibrium of Jersey dairy cows in tropical environment. Int J Biometeorol 63, 1487–1496 (2019). https://doi.org/10.1007/s00484-019-01734-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-019-01734-w