Abstract
Long-term changes of plant phenological phases determined by complex interactions of environmental factors are in the focus of recent climate impact research. There is a lack of studies on the comparison of biogeographical regions in Europe in terms of plant responses to climate. We examined the flowering phenology of plant species to identify the spatio-temporal patterns in their responses to environmental variables over the period 1970–2010. Data were collected from 12 countries along a 3000-km-long, North–South transect from northern to eastern Central Europe.
Biogeographical regions of Europe were covered from Finland to Macedonia. Robust statistical methods were used to determine the most influential factors driving the changes of the beginning of flowering dates. Significant species-specific advancements in plant flowering onsets within the Continental (3 to 8.3 days), Alpine (2 to 3.8 days) and by highest magnitude in the Boreal biogeographical regions (2.2 to 9.6 days per decades) were found, while less pronounced responses were detected in the Pannonian and Mediterranean regions. While most of the other studies only use mean temperature in the models, we show that also the distribution of minimum and maximum temperatures are reasonable to consider as explanatory variable. Not just local (e.g. temperature) but large scale (e.g. North Atlantic Oscillation) climate factors, as well as altitude and latitude play significant role in the timing of flowering across biogeographical regions of Europe. Our analysis gave evidences that species show a delay in the timing of flowering with an increase in latitude (between the geographical coordinates of 40.9 and 67.9), and an advance with changing climate. The woody species (black locust and small-leaved lime) showed stronger advancements in their timing of flowering than the herbaceous species (dandelion, lily of the valley). In later decades (1991–2010), more pronounced phenological change was detected than during the earlier years (1970–1990), which indicates the increased influence of human induced higher spring temperatures in the late twentieth century.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The scientific understanding of the causes of observed changes in the climate system has been increasing according to the report of the Intergovernmental Panel on Climate Change (Stocker et al. 2013). Climate model projections indicate that, regarding temperature and precipitation changes, similar tendencies are likely to continue over the coming century; however, future changes will vary across regions (Stocker et al. 2013). These evidences also call ecologists’s attention to phenomena in natural ecosystems’s shifting in time related to global warming (Walther et al. 2002; Parmesan 2006; IPCC 2007; Franks 2015).
Phenology is the study of periodically repeating stages in the life cycle of animals and plants as influenced by environmental conditions (Demarèe and Rutishauser 2009). The likelihood of species occurrence in a certain area depends on survival and reproduction, which are both depending on the species’ phenology and thus intimately linked to climate (Cleland et al. 2007). Observational (Menzel et al. 2006; Koch et al. 2009; Schleip et al. 2009), field experimental (Wolkovich et al. 2012), predicted (Aguilera et al. 2015) and remotely sensed (White et al. 2005) data suggest that the timing of several plant phenological phases advance and/or delay across the globe, from the Northern (Schwartz et al. 2006) to the Southern Hemisphere (Chambers et al. 2013) due to climatic changes. Several studies demonstrate significant advancements in phenological phases of plants across Europe (Menzel and Fabian 1999; Chmielewski and Rotzer 2001; Schleip et al. 2009). These changes in central Eastern Europe have so far been documented to be less marked than in western and central Europe (Askeyev et al. 2010).
Climate factors, phenophases and their timing play the most important role in such changes. The main causes depend on the climatic region(s) from Mediterranean to high latitudes. The most influential variables are temperature (Rutishauser et al. 2009), precipitation (Penuelas et al. 2004), photoperiod (Körner and Basler 2010), the North Atlantic Oscillation (Scheifinger et al. 2002), as well as cold or warm spells (Menzel et al. 2011) and edaphic factors (Wielgolaski 2001).
Biogeographical regions are useful geographical reference units when describing habitat types and species living under similar conditions in different countries (Roekaerts 2002). The establishment of plant phenology across regions of Europe is a first important step towards providing a general overview, still covering a wide spatial window. The purpose of this study was (i) to compare different biogeographical regions (Boreal, Continental, Alpine, Pannonian and Mediterranean), and test whether the areas experienced any trends in flowering time, (ii) to evaluate the possible factors that influence phenological shifts and (iii) to discover phenological patterns along various latitudes and periods (1970–1980, 1981–1990, 1991–2000 vs 2001–2010).
Materials and methods
Phenological data of plants
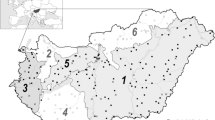
Phenological data we analysed were collected from 12 countries (Finland, Estonia, Latvia, Lithuania, Poland, Slovakia, Hungary, Slovenia, Croatia, Bosnia and Herzegovina, Montenegro, Macedonia) in northern to eastern Central Europe for the period 1970–2010 (Fig. 1). The data comprise phenological records on the first flowering date of six plant species: lily of the valley (Convallaria majalis L.), common dandelion (Taraxacum officinale L.), common lilac (Syringa vulgaris L.), black elder (Sambucus nigra L.), black locust (Robinia pseudoacaica L.) and small-leaved lime (Tilia cordata Mill.). Even though the datasets include observations originating from different phenological networks (Table 1), the studied beginning of flowering (BF) event was consequently defined as “the appearance of the first flowers producing pollen on at least 10 % of the observed plants visible”. This phenophase equals the event 61, according to the BBCH (Biologische Bundesanstalt, Bundessortenamt and Chemical Industry) code (see Meier2001). The observations provided coverage for twelve North- and East-Central European countries along the geographical coordinates of 40.9–67.9 latitudes ranging to the 13.6–32.1 longitudes (Fig. 1). The aim of the phenological site selection was to provide the best temporal and spatial coverage as possible. To reach this, the selection criteria were as follows: (1) the site has at least 10 years of continuous records; (2) there are at least five sites within one biogeographical region. This criteria set resulted in the North–South phenological (NS-Pheno) database (see also Templ et al. 2016) that included different numbers of observations per biogeographical region.
The indicative map of European Biogeographical Regions was first defined in practice of the conservation of natural habitats, wild fauna and flora (Roekaerts 2002; ETCBD 2006). The dataset of biogeographical regions was taken from the European Environment Agency web page.Footnote 1 We merged these data sets with the phenological time series in order to compare the following biogeographical macroregions: Boreal, Continental, Alpine, Pannonian and Mediterranean.
The Boreal region is the largest biogeographical region of Europe. Its climate is cool and mainly continental, its vegetation is dominated by coniferous forests, while the biodiversity is relatively low. The Continental region is characterised by clear continental climate, especially across the central and eastern parts. Widespread grasslands are decreasing due to intensification of agriculture and afforestation. The region shows increasing fragmentation of habitats due to dense and increasing infrastructure within urban areas. The Alpine region is determined by vertical zonality induced by the exposition of mountain slopes and advecting air masses. In this way, different ecological conditions are represented at different altitudes resulting in various vegetation types. The Pannonian region, situated in the lowland areas of the Carpathian Basin, used to be dominated by a mosaic of deciduous forests and forest steppes, which are mostly turned into agricultural fields by now. The Mediterranean region is characterised by a climate where warm, moist winters alternate with hot, dry summers. The region is dominated by evergreen forests and shrublands.
Environmental data
Climatic variables based on air temperature (daily arithmetic mean, minimum, maximum), precipitation and the indices of the North Atlantic Oscillation (NAO) were obtained from different databases. Daily data (January to May) of air temperature and precipitation were used from the E-OBS high-resolution gridded dataset developed by the ENSEMBLES EU-FP6 projectFootnote 2 with a 0.25 ∘ spatial resolution (Haylock et al. 2008).
As a descriptor of the frequency distribution, the quartiles at 0.25 (Q.25), 0.5 (median), 0.75 (Q.75) level and the skewness of climate data series were determined. Additionally to raw climate data, the motivation behind using such characteristics of predictors was to also take into account the spread of values, in terms of the interquartile distance. This is often done using the classical standard deviation. However, since squared distances to the mean are taken into account, outliers have a large influence on this estimate.
We created a set of environmental predictors (a) for monthly temperature (namely, the 0.25, 0.5, 0.75 quartiles and skewness of the minimum-, mean-, maximum- temperature datasets) and (b) for precipitation (0.25, 0.5, 0.75 quartiles and skewness of the monthly precipitation).
Additionally, monthly indices (January to May) of the NAO (Hurrel 1995) were used from the database provided by the Climatic Research Unit (CRU) of the University of East Anglia.
Furthermore, metadata information regarding the locations, namely latitude, longitude and altitude of phenological sites, were also used in the models to consider spatial differences.
Data analysis
Data pre-processing
Dates of the phenological observations— flowering data—were converted to days of the year (doy), starting with first of January and considering leap years. Each phenological station (shown in Fig. 1) was assigned to the closest grid cell.
Calculation of trends
The obtained time series were assigned to five biogeographical regions (Fig. 1), based on the code list of the European Environmental Agency. Accordingly, trend analyses were carried out on long-term (1970–2010) data series of (a) monthly climate data (Fig. 2) and (b) flowering onset (Fig. 3) for each biogeographical region. We found that the data contain outliers, therefore a robust regression method, namely MM-type estimators for linear regression (see Maronna et al. 2006) were applied to calculate trends. The reason for using this method was that least squares estimates for regression models are highly sensitive to outliers. Outliers are observations which do not follow the pattern of the other observations. Robust techniques reduce the influence of outliers (without removing them from the data series), but approximately give the same results as if no outliers were presented in the dataset (see more details in Todorov and Filzmoser 2009). Finally, significant trends were found at the level of significance p < 0.05 using the Mann Kendall test (Mann 1945).
Comparison of decades
In order to compare various decades, phenological time series were divided into four decadal-long periods: 1970–1980, 1981–1990, 1991–2000 and 2001–2010. As it was found to be a highly influential factor, the differences in flowering onset dates (station-wise) according to latitudes (N) were analysed. Trends in the timing of flowering dates were illustrated with regression lines using locally weighted scatterplot smoothing (loess) (Cleveland 1979) for all decades (Fig. 4).
Influence of environmental variables
To describe the influence of the environmental variables on the timing of flowering, we again used robust MM-type estimators for linear regression (see Maronna et al. 2006) on each plant species for each biogeographical region. The difference between the models was only given by the applied explanatory variables. Namely, the climatological data sets preceding the timing of flowering and metadata information about the station locations were included in the models, fitted by the lmrob function of the R package robustbase (Rousseeuw et al. 2015). For better interpretability, the predictors were standardized to zero mean and unit variance. The predictor expressing the first quartile of precipitation was excluded from the models because these first quartiles were mostly zero. The estimated regression coefficients obtained with robust methods were visualized on heatmaps (Fig. 5). On the heatmaps, we distinguish between cells including values of corresponding regression coefficients and empty (white or grey) cells. The colour key of the heatmaps expresses the values of the regression coefficients. Namely, the darker the colour the stronger the effect, which is either negative or positive. Naturally, the maximum and minimum of the coefficients vary depending on each heatmap. For better comparison, the colour range was restricted to −1 and 1, thus any coefficient larger or smaller than this range was assigned to black colour. Non-significant regression coefficients were suppressed to reduce the amount of information to gain a better overview about the important values. Thus, for any empty white-coloured cell, the null hypothesis (regression coefficient equals zero) cannot be rejected (no effect). The empty grey-coloured cells report that no data were available in some biogeographical regions for certain species.
Regression coefficients given for each plant and explanatory variable within different biogeographical regions of Europe. Cases of grey cells without values report that no data were available in those biogeographical regions. White cells indicate that non-significant influence was found. Negative values indicate negative influence on flowering time (i.e. advancement), while positive values express positive effect
All statistical analyses were performed using R (R Development Core Team 2016).
Results
Trends in climatic variables
Regarding the climatological variables, we found that the monthly mean (Fig. 2), minimum, maximum temperatures preceding the flowering onset dates showed significant warming trends (1970–2010) across the Alpine and Continental regions calculated by the Mann-Kendall trend test. Temperature has been increased significantly during April (1970–2010) across the Mediterranean and the Pannonian region. Over the studied 41 years, the Boreal region did not show significant changes in temperature. Furthermore, we did not detect any significant long-term changes in case of precipitation and NAO.
Temporal characteristics of flowering
According to climatological trends, this section provides an overview about flowering trends (1970–2010) over biogeographical regions of Europe, with special interest on the North–South transect, drawn by latitude. As expected, the flowering time starts earlier across the warmer Mediterranean and Pannonian regions, i.e. along the lower latitudes, while it starts later across the cooler Boreal and Alpine regions. From 23 studied cases, 17 showed significant flowering trends (Fig. 3). All of these phenological changes were related to earlier appearance—indicated by negative regression coefficients (Table 2). Most species showed significant trends in the Continental and Alpine regions (Table 2), according to significant temperature increase (Fig. 2). Less significant phenological shifts were found across the Pannonian region. However, data availability does not allow us to give such general statements about phenological changes in the Mediterranean region. Still it is noticeable that all coefficients were negative (except for S. vulgaris in the Boreal region and C. majalis in the Pannonian region) indicating advancements in flowering time (Table 2). The strongest advancement was found for R. pseudoacacia (3.8–9.6 days earlier shift per decade) and T. cordata (3.2–9.5 days per decade), while less pronounced responses were given by the herbaceous T. officinale and C. majalis (Table 2).
Furthermore, we detected differences in the mean flowering onset date between decades (Fig. 4). Differences were detected in mean flowering date (doy) along a North–South transect as we evaluated the time series over different time periods. Accordingly, the flowering of different species generally starts earlier in the latest period (1991–2010) compared to the earlier years (1970–1990). This applies especially to R. pseudoacacia, S. vulgaris and S. nigra over the whole range of latitudes. For other species, like T. officinale, this is only true along some ranges of latitudes, especially in the southern part of Northern Europe. However, because of missing data problems (no values for the North during the period 2001–2010) not much can be concluded from the current database for T. cordata.
Spatial patterns in flowering phenology across Europe
In order to explain the causes of phenological changes, the effect of climatic variables and geographical information on flowering dates were analysed (1970–2010). In Fig. 5, we illustrate the robust regression coefficients for the six species in each biogeographical region of Europe using heatmaps. In most cases, the effects of latitude and altitude were significantly positive. Thus, the species living in northern or higher habitats were characterised by later dates of flowering onset (see Fig. 4).
On the contrary, the effect of longitude was rather negative or non-explainable (70 % of the cases). This indicates that the species living in more eastern parts of Europe were characterised by earlier dates of flowering.
Regarding climatological variables, the clearest pattern arises from the effect of NAO. All species revealed a significantly negative relationship to the index of NAO (Fig. 5). From our study, the effect of mean temperature seems to give the most vague information. Earlier flowering in response to increased temperatures is better visible when looking at the minimum and maximum temperatures and thus it is easier to interpret the flowering dates with the distribution of minimum and maximum temperatures. It can be seen that in most cases, the quartiles of the minimum temperature have negative effect on the timing of flowering. Namely, the higher the minimum temperature the earlier the flowering time—except in the Alpine region. The second (median) and third quartiles of the maximum temperature distribution also show negative effects (again except for the Alpine region). The effect of precipitation did not show a general pattern among species and biogeographical regions, but about half of the the studied cases indicated significant influence.
Discussion
The term of phenological pattern has mainly been associated with plant communities (e.g. Pilar and Gabriel1998; Martìnkovà et al. 2002); in other cases, areas at different scales were compared to describe phenological patterns of areas. Studies have described phenological changes in timing of various spring plant phenophases across hemispheres (Schwartz et al. 2006; Chambers et al. 2013), continents (Menzel and Fabian 1999), along countries (Ahas and Aasa 2006; Kalvane et al. 2009; Szabò et al. 2016) and zones (Karyieva et al. 2012) related to climate driven mechanisms and recent human induced climatic changes. Phenological events of plants across biogeographical regions are particularly poorly documented, except the efforts done by Rodriguez-Galiano et al. (2015) and Templ et al. (2016).
We aimed to discover phenological patterns across biogeographical regions of Europe between various time windows of the period 1970–2010. Besides the established NS-Pheno database, the novelty in our study is that we described flowering patterns along a 3000-km-long North–South transect of Europe. Our NS-Pheno database allowed us to test, if the species living in the northern latitudes show more pronounced response to climate change than those living in southern biogeographical regions. In the Boreal region, the intensity of the phenological response to temperature increases from South to North across Finland (Pudas et al. 2008). The reason is that the observed (1847-2013) warming in Finland is almost twice as high as the global temperature increase (Mikkonen et al. 2015). Our results seem to be contradictory to the findings of Mikkonen et al. (2015), because we did not show significant temperature increase in this region (see Fig. 2.). In contrast to Mikkonen et al. (2015), we studied shorter time series (1970–2010) and applied robust methods, which both may have influence on the results. Similarly to Lappalainen et al. (2008), we experienced the most pronounced advancing flowering trends (Table 1) at a species level within this subarctic climate zone. There is also a known phenological sensitivity of species in other boreal countries such as in Estonia (Ahas and Aasa 2006), Latvia and Lithuania (Kalvane et al. 2009). Furthermore, our results confirm the observed climate variability patterns and trends in the Alpine region (Auer et al. 2007; Gobiet et al. 2014). Namely, the region has been facing a significant temperature increase (Fig. 2), which resulted in significant phenological shifts in the area (see Table 2). As we move from the northern areas to temperate and cool climate zones, phenological responses of plants to warmer environment are strong (Menzel et al. 2006; Jatczak and Walawender 2009). The territory of Hungary covers 80–85 % of the drier Pannonian region. For this region, Szabò et al. (2016) already showed that plant species advanced their flowering time (1952–2000) by 1.9–4.4 days per decade. This tendency is confirmed in our study, but only for two out of five species significantly (Table 2), which can originate from the different lengths of the study periods.
It is known that the annual pattern of phenological seasons across Europe is related to the North Atlantic Oscillation (Menzel et al. 2005). Similarly to most of the studies, we have also found temperature to be an influential determinant for the timing of flowering (Stocker et al. 2013). Our results highlighted that not just the mean temperature but the distribution of minimum and maximum temperatures are reasonable to consider as explanatory variables when explaining flowering times. The importance of rainfall and water availability is pronounced by Penuelas et al. (2004) as complex drivers of phenological shifts. We showed (Fig. 5) a significant influence of precipitation on the beginning of flowering in approximately half of the studied cases.
Our main focus was not only to test the effect of climatic variables, but also others such as latitude. We addressed the question: Which patterns can we draw when comparing northern biogeographical regions to southern ones? Are they similiar to the patterns shown for the territory of China (Ge et al. 2015) and the findings of a meta-analysis conducted by Root et al. (2003)?
It is known that over the past half century the temperature along higher latitudes has increased more than along lower latitudes (Stocker et al. 2013). Accordingly, Root et al. (2003) showed that the estimated phenological shifts from 32.0 N to 49.9 N latitude are smaller than between the 50.0 N to 72.0 N latitude band. Our observations confirm these findings for Europe, since we noticed the most significant plant responses within the Boreal biogeographical region (approximately between 54.0 N and 67.0 N), which was followed by the Continental and Alpine regions (from 40.0 N in 55.0 N). But, is only the latitude responsible for this pattern? Ge et al. (2015) investigated the 20.0–50.0 latitudes in China and found significant phenological advancements; however, they could only explain 9 % of the overall variance in spring phenological trends. Previously, Estrella et al. (2009) stated that the geographic coordinates (latitude and longitude) have only a modest influence on the mean onset of the groups of phenophases; however, inclusion of altitude can improve models for some cases. In our study, not only the effects of latitude (Fig. 4), but also altitude were found to have a significantly positive effect on the beginning of flowering (Fig. 5). These findings indicate that although we experience a similar pattern (stronger response at higher latitudes) among continents, the drivers of these changes cannot be described simply. We showed that among biogeographical regions of Europe, the effect of longitude was mostly non-significant. This can be explained by the longitudinal extent of our study window, which probably was too narrow (13.6–32.1 longitudes) to show any West–East oriented flowering pattern. Therefore, our results cannot support the findings of Askeyev et al. (2010) who demonstrated less marked phenological changes at the eastern edge of Europe.
According to our results, more pronounced phenological changes occur in the latest (1991–2010) than in the earliest (1970–1990) study periods, as the effect of climate change is more and more influential since the industrial era (Stocker et al. 2013).
References
Aguilera F, Fornaciari M, Ruiz-Valenzuela L, Galàn C, Msallem M, Dhiab A B, Dìaz-de la Guardia C, del Mar Trigo M, Bonofiglio T, Orlandi F (2015) Phenological models to predict the main flowering phases of olive (Olea europaea L.) along a latitudinal and longitudinal gradient across the Mediterranean region. Int J Biometeorol 95:629–641
Ahas R, Aasa A (2006) The effects of climate change on the phenology of selected Estonian plant, bird and fish populations. Int J Biometeorol 51:17–26
Askeyev O V, Sparks T H, Askeyev I V, Tishin D V, Tryjanowski P (2010) East versus West: contrasts in phenological patterns? Glob Ecol Biogeogr 19:783–793
Auer I, Böhm R, Jurkovic A, Lipa W, Orlik A, Potzmann R, Schöner W, Ungersböck M, Matulla C, Briffa K, Jones P, Efthymiadis D, Brunetti M, Nanni T, Maugeri M, Mercalli L, Mestre O, Moisselin J M, Begert M, Müller-Westermeier G, Kveton V, Bochnicek O, Stastny P, Lapin M, Szalai S, Szentimrey T, Cegnar T, Dolinar M, Gajic-Capka M, Zaninovic K, Majstorovic Z, Nieplova E (2007) Histalp—historical instrumental climatological surface time series of the Greater Alpine Region. Int J Climatol 27:17–46
Chambers L E, Altwegg R, Barbraud C, Barnard P, Beaumont L J, Crawford R J M, Durant J M, Hughes L, Keatley M R, Low M, Morellato P C, Poloczanska E S, Ruoppolo V, Vanstreels R E T, Woehler E J, Wolfaardt A C (2013) Phenological changes in the southern hemisphere. PLoS ONE e75514:8
Chmielewski F M, Rotzer T (2001) Response of tree phenology to climate change across Europe. Agric For Meteorol 108:101–112
Cleland E, Chuine I, Menzel A, Mooney H A, Schwartz M D (2007) Shifting plant phenology in response to global change. Trends Ecol Evol 22:357–365
Cleveland W (1979) Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 74:829–836
Crepinsek Z, Zrnec C, Susnik A, Zust A (2008) Slovenian phenological observations. In: Nekovar J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski F E (eds) COST Action 725—the history and current status of plant phenology in Europe. COST Office, Brussels
Demarèe G R, Rutishauser T (2009) Origins of the word “Phenology”. EOS Trans Am Geophys Union 90:291–291
Estrella N, Sparks T, Menzel A (2009) Effects of temperature, phase type and timing, location, and human density on plant phenological responses in Europe. Clim Res 39:235–248
ETCBD (2006) The indicative map of European biogeographical regions: methodology and development. Technical report European Topic Centre on Biological Diversity. National Museum of Natural History, France
Franks S J (2015) The unique and multifaceted importance of the timing of flowering. Am J Bot 102:1401–1402
Ge Q, Wang H, Rutishauser T, Dai J (2015) Phenological response to climate change in China: a meta-analysis. Glob Chang Biol 21:265–274
Gobiet A, Kotlarski K, Beniston M, Heinrich G, Rajczak J, Stoffel M (2014) 21st century climate change in the European Alps—a review. Sci Total Environ 493:1138–1151
Grisule G, Briede E (2008) The history and current status of phenological recording in Latvia. In: Nekovar J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski F E (eds) COST Action 725 – the history and current status of plant phenology in Europe. COST Office, Brussels
Haylock M R, Hofstra N, Klein Tank A M G, Klok E J, Jones P D, New M (2008) A European daily high-resolution gridded data set of surface temperature and precipitation for 1950–2006. J Geophys Res - Atmos 113, 10.1029/2008JD010201
Hodzic S, Voljevica A (2008) Phenology in Bosnia and Hercegovina. In: Nekovar J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski F E (eds) COST Action 725—the history and current status of plant phenology in Europe. COST Office, Brussels
Hurrel J W (1995) Decadal trends in the North Atlantic Oscillation regional temperatures and precipitation. Science 269:676–679
IPCC (2007) Technical summary. Cambridge University Press , Cambridge
Jatczak K, Walawender J (2009) Average rate of phenological changes in Poland according to climatic changes. Adv Sci Res 3 :127–131
Kalvane G, Romanovskaja D, Briede A, Baksiene E (2009) Influence of climate change on phenological phases in Latvia and Lithuania. Clim Res 39:209–210
Karyieva J, van Leeuven W, Woodhouse C (2012) Impacts of climate gradients on the vegetation phenology of major land use types in Central Asia (1981–2008). Front Earth Sci 6:206–225
Koch E, Dittmann E, Lippa W, Menzel A, Nekovar J, Sparks T, van Vliet AJH (2009) COST725—Establishing a European phenological data platform for climatological applications: major results. Adv Sci Res 3:119–122
Körner C, Basler D (2010) Phenology under global warming. Science 327:1461–1462
Kubin E, Kotilainen E, Poikolainen J, Hokkanen T, Nevalainen S, Pouttu A, Karhu J, Pasanen J (2007) Monitoring instructions of the finnish national phenological network. Technical report Finnish Forest Research Institute, Muhos Research Unit , Metla
Lappalainen H, Linkosalo T, Venalainen A (2008) Long-term trends in spring phenology in a boreal forest in central Finland. Boreal Environ Res 13:303–318
Mann H B (1945) Nonparametric tests against trend. Econometrica 13:245–259
Maronna R, Martin R, Yohai V (2006) Robust statistics: theory and methods. Wiley, New York. ISBN:978-0-470-01092-1
Martìnkovà J, Smilauer P, Mihulka S (2002) Phenological pattern of grassland species: relation to the ecological and morphological traits. Flora 197:290–302
Meier U (2001) BBCH-Monograph: growth stages of mono- and dicotyledonous plants. Technical Report, 2 Edn. Federal Biological Research Centre for Agriculture and Forestry
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397:659
Menzel A, Seifert H, Estrella N (2005) SSW to NNE—North Atlantic Oscillation affects the progress of seasons across Europe. Glob Chang Biol 11:909–918
Menzel A, Seifert H, Estrella N (2011) Effects of recent warm and cold spells on European plant phenology. Int J Biometeorol 55:921–932
Menzel A, Sparks T H, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kuebler K, Bissollii P, Braslavska O, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl A, Defila C, Donelly A, Filella Y, Jatczak K, Mage F, Mestre A, Nordli A, Penuelas J, Pirinen P, Remisova V, Scheifinger H, Striz M, Susnik A, Van Vliet AJH, Wielgolaski FE, Zach S, Zust A (2006) European phenological response to climate change matches the warming pattern. Glob Chang Biol 12:1969–1976
Mikkonen S, Laine M, Mäkelä H, Gregow H, Tuomenvirta H, Lahtinen M, Laaksonen A (2015) Trends in the average temperature in Finland, 1847–2013. Stoch Env Res Risk A 29:1521– 1529
Nekovàr J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski F E (2008) COST ACtion 725—The history and current status of plant phenology in Europe Technical report. COST Office , Brussels
Niedz̀wiedz̀ T, Jatczak K (2008) History of phenology in Poland. In: Nekovar J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski F E (eds) COST Action 725—the history and current status of plant phenology in Europe. COST Office, Brussels
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annual review of ecology. Evol Syst 37:637– 669
Penuelas J, Filella I, Zhang X, Llorens L, Ogaya R, Lloret F, Comas P, Estiarte M, Terradas J (2004) Complex spatiotemporal phenological shifts as a response to rainfall changes. New Phytol 161 :837–846
Pilar C, Gabriel M (1998) Phenological pattern of fifteen Mediterranean phanaerophytes from Quercus ilex communities of NE-Spain. Plant Ecol 139:103–112
Popovic T, Drljevic M (2008) History and current status of phenology in Montenegro. In: Nekovar J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski F E (eds) COST Action 725—the history and current status of plant phenology in Europe. COST Office, Brussels
Pudas E, Leppala M, Tolvanen A, Poikolainen J, Venalainen A, Kubin E (2008) Trends in phenology of Betula pubescens across the boreal zone in Finland. Int J Biometeorol 52:251–259
R Development Core Team (2016) R: a language and environment for statistical computing r foundation for statistical computing. Vienna, Austria. http://www.R-project.org
Remisovà T, Nejedlik P (2008) History and present observations in Slovak plant phenology. In: Nekovar J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski F E (eds) COST Action 725—the history and current status of plant phenology in Europe. COST Office, Brussels
Rodriguez-Galiano V, Dash J, Atkinson P (2015) Characterising the land surface phenology of Europe using decadal MERIS data. Remote Sens 7:9390–9409
Roekaerts M (2002) The biogeographical regions map of Europe: basic principles of its creation and overview of its development Technical report. European Topic Centre Nature Protection and Biodiversity, European Environment Agency
Romanovskaja D, Baksiene E Nekovar J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski F E (eds) (2008) Phenological investigations in Lithuania. COST Office, Brussels
Root T L, Price J T, Hall K R, Schneider S H, Rosenzweig C, Pounds J A (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Rousseeuw P, Croux C, Todorov V, Ruckstuhl A, Salibian-Barrera M, Verbeke T, Koller M, Maechler M (2015) Robustbase: basic Robust Statistics. R Foundation for Statistical Computing, package version 0.92-3
Rutishauser T, Schleip C, Sparks T H, Nordli O, Menzel A, Wanner H, Jeanneret F, Luterbacher J (2009) Temperature sensitivity of Swiss and British plant phenology from 1753 to 1958. Clim Res 39 :179–190
Scheifinger H, Menzel A, Koch E, Peter C, Ahas R (2002) Atmospheric mechanisms governing the spatial and temporal variability of phenological phases in Central Europe. Int J Climatol 22:1739–1755
Schleip C, Sparks T H, Estrella N, Menzel A (2009) Spatial variation in onset dates and trends in phenology across Europe. Clim Res 39:249–260
Schwartz M D, Ahas R, Aasa A (2006) Onset of spring starting earlier across the Northern Hemisphere. Glob Chang Biol 12:343–351
Stocker T, Qin D, Plattner G K, Tignor M, Allen S K, Boschung J, Nauels A, Xia Y, Bex V, Midgley P M (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Szabò B, Vincze E, Czùcz B (2016) Flowering phenological changes in relation to climate change in Hungary. Int J Biometeorol 60:1347–1356
Szalai S, Bella S, Nèmeth A, Dunay S (2008) History of Hungarian phenological observations. In: Nekovar J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski F (eds) COST Action 725—the history and current status of plant phenology in Europe. COST Office, Brussels
Templ B, Fleck s, Templ M (2016) Change of plant phenophases explained by survival modelling. Int J Biometeorol, doi:10.1007/s00484-016-1267-z 10.1007/s00484-016-1267-z
Todorov V, Filzmoser P (2009) Multivariate robust statistics: methods and computation. Südwestdeutscher Verlag für Hochschulschriften, Saarbrücken
Vucetic V, Vucetic M, Loncar Z (2008) History and present observations in Croatian plant phenology. In: Nekovàr J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski F E (eds) COST Action 725—the history and current status of plant phenology in Europe. COST Office, Brussels
Walther G R, Post E, Convey P, Menzel A, Parmesan C, Beebee T J C, Fromentin J M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
White M A, Hoffman F, Hargrove W W, Nemani R R (2005) A global framework for monitoring phenological responses to climate change. Geophys Res Lett 32:L04705
Wielgolaski F (2001) Phenological modifications in plants by various edaphic factors. Int J Biometeorol 45:196–202
Wolkovich E, Cook B, Allen J M, Crimmins T M, Betancourt J L, Travers S E, Pau S, Regetz J, Davies T J, Kraft N J B, Ault T R, Bolmgren K, Mazer S J, McCabe G J, McGill B J, Parmesan C, Salamin N, Schwartz M D, Cleland E E (2012) Warming experiments underpredict plant phenological responses to climate change. Nature 485:494–497
Acknowledgments
We acknowledge the E-OBS dataset from the EU-FP6 project ENSEMBLES (http://ensembles-eu.metoffice.com) and the data providers in the ECA&D project (http://www.ecad.eu). We are very grateful to all institutes and scientists who provided data for the North–South phenological database. We would especially like to emphasize our gratefulness to those data contributors who did not participate as authors in the writing of this manuscript: K. Jatczak (Centre for Polands̀ Climate Monitoring), P. Nejedlik (Slovak Hydrometeorological Institute), T. Niedz̀wiedz̀ (University of Silesia), T. Popovic (Hydrometeorological Institute of Montenegro), H. Simola (Finnish Meteorological Institute), Z. Snopkovà (Slovak Hydrometeorological Institute) and S. Stevkova (Hydrometeorological Institute of Macedonia). Additionally, we would like to pay respect to J. Terhivuo (Finnish Museum of Natural History) who unfortunately could not see these results published. And finally, thanks to F. Szentkirályi for inspiritaion during the project planning.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Templ, B., Templ, M., Filzmoser, P. et al. Phenological patterns of flowering across biogeographical regions of Europe. Int J Biometeorol 61, 1347–1358 (2017). https://doi.org/10.1007/s00484-017-1312-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-017-1312-6