Abstract
The importance of long-term plant phenological time series is growing in monitoring of climate change impacts worldwide. To detect trends and assess possible influences of climate in Hungary, we studied flowering phenological records for six species (Convallaria majalis, Taraxacum officinale, Syringa vulgaris, Sambucus nigra, Robinia pseudoacacia, Tilia cordata) based on phenological observations from the Hungarian Meteorological Service recorded between 1952 and 2000. Altogether, four from the six examined plant species showed significant advancement in flowering onset with an average rate of 1.9–4.4 days per decade. We found that it was the mean temperature of the 2–3 months immediately preceding the mean flowering date, which most prominently influenced its timing. In addition, several species were affected by the late winter (January–March) values of the North Atlantic Oscillation (NAO) index. We also detected sporadic long-term effects for all species, where climatic variables from earlier months exerted influence with varying sign and little recognizable pattern: the temperature/NAO of the previous autumn (August-December) seems to influence Convallaria, and the temperature/precipitation of the previous spring (February–April) has some effect on Tilia flowering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a worldwide increase in the number of studies, which call attention to the ecological consequences of global climate change. These effects appear at population, community, as well as ecosystem levels by modifying species composition, spatial patterns, and ecosystem functioning (Parmesan 2006; Root et al. 2003; Rosenzweig et al. 2008). Shifts in the timing of recurring life history events (phenophases) of species constitute a major component of the ecological impacts of climate change (Parmesan and Yohe 2003; Walther et al. 2002). Diverging phenological shifts in communities may lead to a breakdown of synchronous species interactions (Buse et al. 2002; Stenseth et al. 2002), which eventually can result in increased risk of extinctions (Memmott et al. 2007). Several studies warn about potentially detrimental impacts of these processes (Both et al. 2009; Visser and Both 2005). Furthermore, phenology also exerts control over many potential feedbacks from vegetation to the climate system by influencing the seasonality of albedo, canopy conductance, fluxes of water and energy, CO2, and biogenic volatile organic compounds (Richardson et al. 2013).
The analysis of long-term plant phenological time series have been in the focus of climate impact research since the early 1990s (Schwartz 1999). Several studies have demonstrated significant advancements in spring phenophases of plants across the northern hemisphere (Menzel et al. 2006; Schleip et al. 2009; Schwartz et al. 2006). Similar tendencies can be seen across several other taxonomical groups, including, e.g., fungi (Kauserud et al. 2010), insects (Robinet and Roques 2010), amphibians (Beebee 2002), and birds (Gordo 2007). These changes are not limited to terrestrial ecosystems but can also be observed in freshwater and marine systems (Thackeray et al. 2010).
Induction of different phenophases along the life cycle of individuals is governed by species-specific environmental thresholds. Alterations in CO2 level, temperature, photoperiod, solar radiation, precipitation, snowmelt, and frost effects exert influence together on plants’ physiological processes (Körner and Basler 2010; Nord and Lynch 2009). There are several conflicting reports in the literature about the relative roles of different drivers and/or explanatory variables of the phenological development (Migliavacca et al. 2012). The best predictors of plant phenology within the temperate zone are local meteorological variables, like air temperature and precipitation, but macroclimatic circulation patterns, such as the North Atlantic Oscillation (NAO) index are also frequently considered as predictors. It is also well known that NAO-induced local weather conditions can affect phenology and this relationship can be different in different parts of Europe (Gordo and Sanz 2010; Scheifinger et al. 2002; Stenseth et al. 2003), as it is expected from this impact system (Hurrel and van Loon 1997; Pauling et al. 2006; Trigo et al. 2002).
Strongly connected to the problem of identifying the appropriate predictor variables is the problem of finding the effective time period, the weather of which most strongly influences the subsequent phenological events. The timing of flowering is controlled by complex mechanisms, which act to ensure that flower emergence occurs in suitable conditions attuned to seasons (Tooke and Battey 2010). Most studies report that the flowering phenophase shows the strongest correlation with the average air temperature of a few months preceding the event, but focusing only on spring temperatures can lead to inaccurate interpretations and predictions for species, because photoperiod and vernalization plays also major roles in spring phenological events (Cook et al. 2012; Körner and Basler 2010; Tooke and Battey 2010).
The aim of our study is to evaluate the shift in the flowering phenologies of six spring-flowering plant species in Hungary during the second half of the last century (1952–2000). In spite of the number of phenological time series available, Hungary is a relatively unexplored country in central Europe in terms of phenological studies. Similarly to other parts of western and central Europe, Hungary is relatively warm and springs tend to be drier during positive NAO periods, while wetter than average during negative NAO periods (Bartholy et al. 2009a). In this study, we have set two main goals: (i) to test whether trends similar to those observed in other parts of Europe can also be detected within Hungarian spring phenological changes, and (ii) to determine which month’s climate exerts the greatest effect on the flowering onset of different species.
Materials and methods
Phenological data
To seek for potential shifts in spring phenological events, we studied the beginning of flowering (BF) of six angiosperm species: lily of the valley (Convallaria majalis L.), common dandelion (Taraxacum officinale (L.) Weber), common lilac (Syringa vulgaris L.), black elder (Sambucus nigra L.), black locust (Robinia pseudoacacia L.), and small-leaved lime (Tilia cordata Mill.) during the period 1952–2000 in Hungary. Historical data on plant phenological observations were obtained from the Hungarian Meteorological Service (Table 1). Our selection of species and observing stations was seriously limited by the extreme fluctuation in the number of stations, observers, and observed species which characterized the phenology observations of the Hungarian Meteorological Service (Dunay 1984; Szalai et al. 2008). During 1952–1961, the observation network of the Meteorological Service consisted of ∼100 stations performing phenological observations on ∼100 phenological events of 37 plant species. The number of stations decreased to ∼60 with an increasingly uneven geographical coverage during 1961–1981 (Table 1). After a 1-year disruption in 1982, the network was reorganized with a drastically reduced list of observed species and phenological events (shifting from wild plant species to crops), and a reduced number of stations with a more even coverage (Dunay 1984; Szalai et al. 2008). This reorganized network, which was hosted by the regional Pest Control and Agrochemistry stations of the Agriculture and Food Ministry, operated in consistent and reliable way from 1983 until 2000, when the entire network fall apart due to lack of funding. As a consequence of these recurrent reorganizations phenological time series long enough for climate impact analysis can only be studied for a few phenological events, which were observed both prior to and after 1982, and even for these events the number of complete time series is very limited (Table 1). Luckily, the definition of phenological events did not change during the reorganizations, so the beginning of flowering phenological event was consequently defined as “the appearance of the first flowers producing pollens on at least 50 % of the observed plants” (Dunay 1984), which equals the event 61, according to the BBCH scale (Meier 1997).
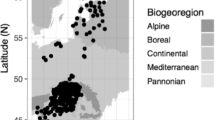
We converted all dates given in months and days to day of the year (DOY) values. To exclude potential coding and typing mistakes we first checked for outliers using the extremevalues R package (van der Loo 2010) assuming a normal distribution. In order to cope with the fluctuations and inhomogeneity in the distribution of stations, we grouped the observations according to the six geographical macroregions of Hungary (Dövényi 2010). For each region, we only kept observation time series of at least 10 years of continuous data. As an additional criterion, we only analyzed data for those regions where there were at least two stations with at least 10 years of continuous data both before and after the reorganization of 1982. This way, we compiled regional datasets for three geographical macroregions (Alföld—Great Hungarian Plain, r1; Nyugat-magyarországi peremvidék—West Hungary, r3; and Dunántúli-középhegység—Transdanubian Mountains, r5) consisting altogether of 133 local data series of the studied species (see Fig. 1). To deal with uneven data coverage, we used linear mixed models to derive single homogenized time series as described by Shaber and Badeck (2002). Accordingly, for each species and region, we created time series using a fixed effect of year, with a station-level random effect with the help of the pheno R package (Schaber 2012). Accordingly, the analyses were carried out on 18 homogenized time series for the six species and the three regions (r1, r3, r5).
The location of the study regions and the data sources. Local observational stations used in our analysis: squares: phenological stations; crosses: meteorological stations. r1 Alföld (Great Hungarian Plain), r3 Nyugat-magyarországi Peremvidék (West Hungary), r5 Dunántúli-középhegység (Transdanubian Mountains). Phenological stations outside the three study regions are also shown in this map, but they were not used in the analysis
Climate data
To determine any correlations between the macroclimatic conditions and the flowering phenology of the selected species, we generated a large number of climatic variables based on air temperature, precipitation, and NAO indiex data. As a starting point for generating air temperature and precipitation variables, we used monthly records from the Hungarian Meteorological Service. After check for outliers similar to what was done in case of the phenological data, we used the same linear mixed model procedure (Shaber and Badeck 2002) to generate a single homogenized macroclimatic signal for each region (see Fig. 1).
To identify the combination of climatic variables and effective periods, which effectively influence the spring phenologies of the studied species, we used the methodology of Gordo and Sanz (2010). Accordingly, we defined a series of monthly, bimonthly, and trimonthly means (temperature) and sums (precipitation) of climate variables, which could potentially influence the actual phenological event. We created 18 monthly mean temperature (T1) variables relative to the current year: from the previous year’s January (T101) to current year’s June (T118). Similarly, we defined seventeen bimonthly (T2) variables, with T201 meaning the mean temperature of previous year’s January–February, T202 being previous year’s February–March, and T217 being current year’s May–June. Trimonthly temperature data series (T3) were also created in a similar way, ranging from T301 (previous year’s January–March) to T316 (current year’s April–June). We defined precipitation variables the same way as T, namely 18 monthly (P101–P118), 17 bimonthly (P201–P217), and 16 trimonthly (P301–P316) precipitation sum variables were created.
To check for potential effects of large-scale circulation patterns on the timing of the studied phenological events, we also defined predictor variables based on the values of the NAO index. Based on the monthly NAO values taken from the NOAA Climatic Research Unit homepage (http://www.cru.uea.ac.uk/cru/data/nao/), we defined 18 variables for monthly (N101–N118), 17 variables for bimonthly (N201–N217), and 16 variables of trimonthly (N301–N316) mean NAO values, exactly following the structure of the other climatological variables. This way we created a set of 144 strongly correlated climatic predictors (51 for temperature, 51 for precipitation, and 51 for NAO). However, unlike mean temperature and sum of precipitation data (which were constructed for each individual region separately), there was a single common NAO data series for all of the studied regions.
Data analysis
We first computed linear regression coefficients for the time series to assess the magnitude and the significance of the trends in flowering date over time for each species and within each region (Sneyers 1990).
As a next step, we looked for statistical relationships between the phenological data and the climatic variables. To this end, we first removed temporal trends from all of the time series, taking the residuals of the univariate linear regressions fitted in the previous step as “detrended” phenological variables. After this detrending step, we created separate linear regression models for each species and region. As the set of predictors was still highly intercorrelated, we used forward stepwise selection to sequentially add terms from the set of potential predictors until reaching minimal Akaike’s information criterion (AIC), a metric commonly applied to compare and rank multiple competing models (Johnson and Omland 2004). We checked the significance of the parameters from the best models with univariate F tests, adding the terms sequentially to the model in the same order they were found by the stepwise algorithm. We used Bonferroni correction to avoid a potential proliferation of type I errors (Abdi 2007). All data preparation and statistical analysis steps were performed in the R statistical environment (R Developmnet Core Team 2013), using the add-on packages reshape2 (Wickham 2007), extremevalues (van der Loo 2010), and pheno (Schaber 2012).
Results
We found significant shifts in flowering onset dates for four species (lily of the valley, common dandelion, black elder, and small-leaved lime) in the examined period (1952–2000). All of the trends (even the nonsignificant ones) were negative (onset of flowering gradually shifting earlier). The strongest advancements (2.8–4.4 days per decade) were found for small-leaved lime. Two of the examined woody species (common lilac and black locust) did not show significant trends. Altogether, 7 of the 18 studied cases (species × regions) showed significant advancement in their beginning of flowering (Table 2).
Altogether, we found 45 significant relationships between the timing of the flowering events and the meteorological variables studied (Table 3, Fig. 2). The majority (33) of these relationships were linked to temperature, whereas NAO and precipitation were found in 9 and 3 relationships, respectively. For almost all of the studied cases, the most influential predictor was the temperature of the 2 or 3 months immediately preceding the typical date of the phenological event studied (henceforward called “short-term” relationships, Table 3). We also found 15 significant “long-term” connections documenting delayed influence of past meteorological conditions. These long-term relationships were however much weaker, involving all three climatic elements (temperature, precipitation, and NAO) with very little pattern recognizable. The coefficients for “short-term” temperature and NAO variables were generally negative, indicating that warm years and positive NAO anomalies generally induced earlier flowering onset. On the other hand, coefficients for long-term effects greatly varied among species and seasons (Table 3, Fig. 2).
Periods of the climatic predictors significantly influencing the flowering onset of the studied species (Taraxacum officinale, Syringa vulgaris, Convallaria majalis, Robinia pseudoacacia, Sambucus nigra, Tilia cordata). Periods are grouped by species along the vertical axis and grouped by color according to climatic variable (a), the direction of the relationship (b), and significance level (c). Triangles indicate the mean date of the flowering onset in Hungary
There were only 27 out of the 144 climatic predictors studied that were included in any of the significant relationships identified. The most influential meteorological predictors seemed to be March-April temperature (T215: dandelion, common lilac, lily of the valley), April–May temperature (T216: black locust, lime) and March–May temperature (T315: black locust, black elder). There is also a weak recurrent pattern in the case of NAO, with an effective period at the end of the winter (January–March, N313), which seems to influence several species (Syringa, Convallaria, Robinia). As for long-term relationships, the temperature/NAO of the previous autumn seems to influence lily-of-the valley, and the temperature/precipitation of the previous early spring (February-April) has some effect on lime flowering (Table 3, Fig. 2).
Discussion
Shifts in spring plant phenological events in the Carpathian Basin (Central Europe) are particularly poorly documented, with a few exceptions coming from the works of Keresztes (1984), Walkovszky (1998), Schieber et al. (2009), Molnár et al. (2012), Varga et al. (2012), and Lehoczky et al. (submitted). Based on datasets for these six plant species, the current paper presents the most in-depth study so far of phenological trends and their environmental drivers among Hungary.
Throughout Europe, the vast majority of spring phenological time series ending after 1988 reveal advancing trends (Dose and Menzel 2004). We also detected significantly advancing trends in 7 of the studied 18 cases for 4 of the 6 examined species during the second half of the twentieth century in Hungary. This ratio is considerably smaller than the findings of Parmesan and Yohe (2003) for the entire Northern Hemisphere, where 87 % of the terrestrial datasets exhibited responses coherent with global warming. Askeyev et al. (2010) pointed out that phenological changes have been less marked in the eastern part of Europe, than in western and central Europe. This can be one of the possible reasons for not finding significant flowering trends in all of the studied regions and species. Nevertheless, our results also show shifts very similar to the tendencies experienced in other parts of Europe (2.2–2.5-day advance per decade) (Chmielewski and Rötzer 2001; Menzel et al. 2006).
Similarly to most of the studies exploring the relationship between climatic variables and spring phenological events (IPCC 2007), we have found temperature to be the most influential determinant of the timing of flowering in Hungary. All of the most significant (p < 0.001) relationships found belong to temperature from the January–May period (Fig. 2). In most cases, the effective periods are the 2–3 months containing or immediately preceding the mean month of flowering. This observation is highly supported by other studies as well (e.g., Estrella et al. 2007; Fitter and Fitter 2002; Sparks et al. 2000).
Beyond question, there are no other meteorological variables and effective periods, which could match the significance of short-term air temperature in shaping the spring phenologies of the studied species. The only exception is NAO, with a weak effective period at the end of the winter (January–March, N313), which seems to influence a broad range of plants in Hungary, including earlier and later flowering species (Syringa, Convallaria, Robinia). In many parts of the world, teleconnection indices, like NAO, describing large-scale circulation patterns are considered as a useful proxy for precipitation anomalies (López-Moreno et al. 2011). In Hungary, winter NAO is known to be strongly correlated to both temperature (positively) and precipitation (negatively, Zsilinszki 2014). It is important to note, however, that being an abstract teleconnection index, NAO cannot directly influence plant physiological processes, probably just indirectly through further unidentified meteorological variables. As we have explicitly added, both mean temperature and precipitation to our set of predictors; thus, the significant influence of NAO cannot be attributed to either of them as an underlying factor in our case. Consequently, we can assume that there is still a significant, yet unknown meteorological factor (e.g., temperature or precipitation extremities, solar radiation, wind, etc.—see also Gordo and Sanz 2010) influencing the flowering phenologies of a broad range of taxa, for which January–March winter NAO values act as a proxy in Hungary.
Even though spring phenologies are known to be highly rainfall-sensitive in many parts of the world (Peñuelas et al. 2004), we could not find any clear relationships for precipitation. On the other hand, it is known (Fu et al. 2014) that at higher latitudes, the effect of GDD requirement, chilling, and precipitation influence the spring vegetation green-up in complex interaction. The simple linear modeling technique applied by us might not be appropriate for capturing such complex responses in general, whereas the few weak but significant long-term relationships involving precipitation (and NAO) might be seen as legacies testifying to the presence of such complex nonlinear relationships.
Common dandelion shows significant phenological trends in 2 of the 3 studied regions for the 1952–2000 period. Dandelion exhibits a strong response to the temperature (February–April) of the current year, with sporadic additional long-term effects (N109, P104, T201). At the European level, dandelion shows a stronger response to temperature in warmer than in colder countries (Menzel et al. 2006). We also documented a relatively high-speed advancement for this species in Hungary (−3.4 to –3.9 days per decade), which fits well into this broader European picture (Menzel et al. 2006; Jatczak and Walawender 2009).
In the case of common lilac, we did not find any significant shifts, but we demonstrated a temperature (February–April, T314) and a NAO (January–March, N313) sensitivity of the flowering onset. There is a known temperature sensitivity of this species across Europe (−2 to –4 days/°C for the mean temperature of the previous months) exhibiting a stronger response to temperature in warmer than in colder countries (Menzel et al. 2006). There are several studies that documented significant shifts of 4–6 days per decade in the flowering of common lilac both in Europe (Sparks et al. 2011; Jatczak and Walawender 2009) and in North America (Schwartz and Reiter 2000).
Lily of the valley is one of the species in Europe, which is not really well represented in phenological studies (Nekovár et al. 2008). We have detected a significant trend for this species in West Hungary, and we also found that the temperature of the 60 days preceding flowering (March–April, T216) is strongly related to the timing of lily of the valley flowering. A similar relationship has already been demonstrated between the emergence of lily of the valley shoots and the February–April temperature by Sparks et al. (2011) in western Poland. Furthermore, we have also found a weak but noteworthy memory effect: several temperature and NAO variables from the previous autumn (T108, T111, T310, N309) seemed to influence the beginning of flowering of this species. Such impact of the previous autumn’s weather has already been documented for other herbaceous species (Crimmins et al. 2010). At semi-arid environments, autumn conditions are more important to low-elevation species and spring variables found to play greater role in flowering of high-elevation species (Crimmins et al. 2010).
One of the most explored species in Hungary in terms of shifts in flowering phenology is black locust. Using a long-term data series of 144 years (1851–1994) Walkovszky (1998) showed 3–8-day advancement in the flowering date of black locust, relating the event to the mean temperature of March–May. Our results confirm the findings of temperature sensitivity of this species (with an effective period of (March–)April–May, T216 and T315), but we did not find any significant shifts in flowering during our study period (1952–2000). At the European level, the flowering of black locust shows stronger response to temperature in colder countries (Menzel et al. 2006). In contrast to the response of other species (Menzel et al. 2006), black locust has negative regression coefficients of the temperature sensitivity against flowering. In Poland, Jatczak and Walawender (2009) has found an advancement of 2.7 days per decade for this species during the second half of the twentieth century.
Elder flowering in Europe is shifting in average ∼2.8 days earlier per decade (Menzel 2000). There is also clear documented temperature sensitivity for this species: Sparks et al. (2000) have found a strong relationship with March and April mean temperatures. This influence of the spring temperatures are clearly supported also with our results showing an unambiguous and highly significant negative relationship between the timing of flowering and the trimonthly temperature of the current year’s March–May period (T315). And additionally, we also documented a relatively clear influence of NAO for almost the same period (N216) in the only macroregion lacking a significant temporal trend (r5).
Late spring temperature values (April–June, T117, T216, T316) were the most influential ones also in case of small-leaved lime, but we also documented a relatively clear long-term influence of previous year’s early spring temperature and precipitation as well (February–April, T302, P302). Schleip et al. (2009) has also found that April–June temperatures influence the flowering of the closely related Tilia platyphyllos, even though the strength of this relationship (temperature weights) was weaker than for most of the other studied tree species.
The connections between flowering phenologies and climate identified in this paper offer some insight into the future potential consequences of climate change in this region. As the temperature increase expected in Hungary (Krüzselyi et al. 2011) may considerably exceed the global rate of warming (Bartholy et al. 2009b; Pieczka et al. 2010), future advancement of spring flowering is to be expected. Further understanding of the relationship between plant phenologies and climate in Central and Eastern Europe may be gained from future studies on more species and phenophases. There is still a lot of data to analyze in Hungary, as well as in other European countries (Szabó et al. 2014). For detecting climate change impacts the best solution would be a coordinated renaissance of the national phenological observation networks in this region, supported by citizen science.
References
Abdi H (2007) Bonferroni and Šidák corrections for multiple comparisons. In: Salkind N (ed) Encyclopedia of measurement and statistics. Sage, Thousand Oaks, CA
Askeyev OV, Sparks TH, Askeyev IV, Tishin DV, Tryjanowski P (2010) East versus West: contrasts in phenological patterns? Glob Ecol Biogeogr 19:783–793
Bartholy J, Pongrácz R, Gelybó G (2009a) Climate signals of the North Atlantic Oscillation detected in the Carpathian basin. Appl Ecol Environ Res 7:229–240
Bartholy J, Pongrácz R, Torma C, Pieczka I, Kardos P, Hunyady A (2009b) Analysis of regional climate change modelling experiments for the Carpathian Basin. Int J Glob Warming 1:238–252
Beebee TJC (2002) Amphibian phenology and climate change. Conserv Biol 16:1454–1454
Both C, Van Asch M, Bijlsma RG, Van Den Burg AB, Visser ME (2009) Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? J Anim Ecol 78:73–83
Buse A, Dury SJ, Woodburn RJW, Perrins CM, Good JEG (2002) Effects of elevated temperature on multi-species interactions: the case of Pedunculate Oak, Winter Moth and Tits. Funct Ecol 13:74–82
Chmielewski FM, Rötzer T (2001) Response of tree phenology to climate change across Europe. Agric For Meteorol 108:101–112
Cook BI, Wolkovich EM, Parmesan C (2012) Divergent responses to spring and winter warming drive community level flowering trends. Proc Natonal Acad Sci U S A 109:9000–9005
Crimmins TM, Crimmins MA, Bertelsen D (2010) Complex responses to climate drivers in onset of spring flowering across a semi-arid elevation gradient. J Ecol 98:1042–1051
Dose V, Menzel A (2004) Bayesian analysis of climate change impacts in phenology. Glob Chang Biol 10:259–272
Dövényi Z (2010) Magyarország kistájainak katasztere. MTA FKI, Budapest (in hungarian)
Dunay S (1984) Növényfenológiai megfigyelések Magyarországon. Légkör 29:2–9 (in hungarian)
Estrella N, Sparks TH, Menzel A (2007) Trends and temperature response in the phenology of crops in Germany. Glob Chang Biol 13:1737–1747
Fitter AH, Fitter RSR (2002) Rapid changes in flowering time in British plants. Science 296:1689–1691
Fu YH, Piao S, Zhao H, Jeong S-J, Wang X, Vitasse Y, Ciais P, Janssens IA (2014) Unexpected role of winter precipitation in determining heat requirement for spring vegetation green-up at northern middle and high latitudes. Glob Chang Biol 12:3743–3755
Gordo O (2007) Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim Res 35:37–58
Gordo O, Sanz J (2010) Impact of climate change on plant phenology in Mediterranean ecosystems. Glob Chang Biol 16:1082–1106
Hurrel JW, van Loon H (1997) Decadal variations in climate associated with the North Atlantic Oscillation. Clim Chang 36:301–326
IPCC (2007) Climate Change 2007 - Impacts, Adaptation and Vulnerability: Contribution of Working Group II to the Fourth Assessment Report of the IPCC. Cambridge University Press, Cambridge
Jatczak K, Walawender J (2009) Average rate of phenological changes in Poland according to climatic changes—evaluation and mapping. Adv Sci Res 3:127–131
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
Kauserud H, Heegaard E, Semenov MA, Boddy L, Halvorsen R, Stige LC, Sparks TH, Gange AC, Stenseth NC (2010) Climate change and spring-fruiting fungi. Proc R Soc Biol 277:1169–1177
Keresztes B (1984) Az akác (Robinia pseudoacacia L). Akadémia Kiadó, Budapest (in hungarian)
Körner C, Basler D (2010) Phenology under global warming. Science 327:1461–1462
Krüzselyi I, Bartholy J, Horányi A, Pieczka I, Pongrácz R, Szabó P, Szépszó G, Cs T (2011) The future climate characteristics of the Carpathian Basin based on a regional climate model mini-ensemble. Adv Sci Res 6:69–73
López-Moreno JI, Vicente-Serrano SM, Morán-Tejeda E, Lorenzo-Lacruz J, Kenawy A, Beniston M (2011) Effects of the North Atlantic Oscillation (NAO) on combined temperature and precipitation winter modes in the Mediterranean mountains: Observed relationships and projections for the 21st century. Glob Planet Chang 77:62–76
Meier U (1997) BBCH-Monograph: Growth stages of mono- and dicotyledonous plants. Blackwell Wissenschafts-Verlag, Berlin
Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant–pollinator interactions. Ecol Lett 10:710–717
Menzel A (2000) Trends in phenological phases in Europe between 1951 and 1996. Int J Biometeorol 44:76–81
Menzel A, Sparks T, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kubler K, Bissolli P, Braslavska O, Briede A et al (2006) European phenological response to climate change matches the warming pattern. Glob Chang Biol 12:1969–1976
Migliavacca M, Sonnentag O, Keenan TF, Cescatti A, O’Keefe J, Richardson AD (2012) On the uncertainty of phenological responses to climate change, and implications for a terrestrial biosphere model. Biogeosciences 9:2063–2083
Molnár A, Tökölyi J, Végvári Z, Sramkó G, Sulyok J, Barta Z (2012) Pollination mode predicts phenological response to climate change in terrestrial orchids: a case study from central Europe. J Ecol 100:1141–1152
Nekovár J, Koch E, Kubin E, Nejedlik P, Sparks T, Wielgolaski FE (2008) COST Action 725 - The history and current status of plant phenology in Europe. COST Office, Brussels
Nord EA, Lynch JP (2009) Plant phenology: a critical controller of soil resource acquisition. J Exp Bot 60:1927–1937
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Pauling A, Luterbacher J, Casty C, Wanner H (2006) Five hundred years of gridded high-resolution precipitation reconstruction over Europe and the connection to large-scale circulation. Clim Dyn 26:387–405
Peñuelas J, Filella I, Zhang I, Llorens L, Ogaya R, Lloret F, Comas P, Estiarte M, Terradas J (2004) Complex spatiotemporal phenological shifts as a response to rainfall changes. New Phytol 161:837–846
Pieczka I, Bartholy J, Pongrácz R, Hunyady A (2010) Climate change scenarios for Hungary based on numerical simulations with a dynamical climate model. In: Lirkov I, Margenov S, Waśniewski J (eds) Large-scale scientific computing. Springer, Berlin Heidelberg, pp 613–620
R Developmnet Core Team (2013) R: A Language and Environment for Statistical Computing. R foundation for Statistical Computing, Vienna, Austria, URL http://www.R-project.org
Richardson AD, Keenan TF, Migliavacca M, Ryu Y, Sonnentag O, Toomey M (2013) Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric For Meteorol 169:156–173
Robinet C, Roques A (2010) Direct impacts of recent climate warming on insect populations. Integr Zool 5:132–142
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Rosenzweig C, Karoly D, Vicarelli M, Neofotis P, Wu Q, Casassa G, Menzel A, Root TL, Estrella N, Seguin B et al (2008) Attributing physical and biological impacts to anthropogenic climate change. Nature 453:353–357
Schaber J (2012) pheno: Auxiliary functions for phenological data analysis. R package version 1.6.
Scheifinger H, Menzel A, Koch E, Peter C, Ahas R (2002) Atmospheric mechanisms governing the spatial and temporal variability of phenological phases in central Europe. Int J Climatol 22:1739–1755
Schieber B, Janík R, Snopková Z (2009) Phenology of four broad-leaved forest trees in a submountain beech forest. J For Sci 55:15–22
Schleip C, Sparks TH, Estrella N, Menzel A (2009) Spatial variation in onset dates and trends in phenology across Europe. Clim Res 39:249–260
Schwartz MD (1999) Advancing to full bloom: planning phenological research for the 21st century. Int J Biometeorol 42:113–118
Schwartz MD, Reiter BE (2000) Changes in North American spring. Int J Climatol 20:929–932
Schwartz MD, Ahas R, Aasa A (2006) Onset of spring starting earlier across the Northern Hemisphere. Glob Chang Biol 12:343–351
Shaber J, Badeck FW (2002) Evaluation of methods for the combination of phenological time series and outlier detection. Tree Physiol 22:973–982
Sneyers R (1990) On the statistical analysis of series of observations. World Meteorological Organization, Geneva
Sparks TH, Jeffree EP, Jeffree CE (2000) An examination of the relationship between flowering times and temperature at the national scale using long-term phenological records from the UK. Int J Biometeorol 44:82–87
Sparks TH, Górska-Zajaczkowska M, Wojtowicz W, Tryjanowski P (2011) Phenological changes and reduced seasonal synchrony in western Poland. Int J Biometeorol 55:447–453
Stenseth NC, Mysterud A, Ottersen G, Hurrell JW, Chan K-S, Lima M (2002) Ecological Effects of Climate Fluctuations. Science 297:1292–1296
Stenseth NC, Ottersen G, Hurrell JW, Mysterud A, Lima M, Chan KS, Yoccoz NG, Adlandsvik B (2003) Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Nino Southern Oscillation and beyond. Proc R Soc Lond B Biol Sci 270:2087–2096
Szabó B, Lehoczky A, Filzmoser P, Templ M, Szentkirályi F, Pongrácz R, Ortner T, Mert C, Czúcz B and the SN Pheno Team (2014) From South to North: flowering phenological responses at different geographical latitudes in 12 European countries. In: European Geosciences Union (EGU) General Assembly, Vienna, pp EGU2014-9489.
Szalai S, Bella S, Németh Á, Dunay S (2008) History of Hungarian phenological observations. In: Nekovar J et al (eds) COST Action 725—the history and current status of plant phenology in Europe. COST Office, Brussels, pp 174–182
Thackeray SJ, Sparks TH, Frederiksen M, Burthe S, Bacon PJ, Bell JR, Botham MS, Brereton TM, Bright PW, Carvalho L et al (2010) Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob Chang Biol 16:3304–3313
Tooke F, Battey NH (2010) Temperate flowering phenology. J Exp Bot 61:2853–2862
Trigo RM, Osborn TJ, Corte-Real JM (2002) The North Atlantic Oscillation influence on Europe: climate impacts and associated physical mechanisms. Clim Res 20:9–17
van der Loo MPJ (2010) Distribution based outlier detection in univariate data. Statistics Netherlands, The Hague/Heerlen
Varga Z, Varga-Haszonits Z, Enzsolne Gerencser E, Zs L, Milics G (2012) Bioclimatological analysis of the development of lilac (Robinia pseudoacacia L.). Acta Agronomica Ovariensis 54:35–52 (in hungarian)
Visser ME, Both C (2005) Shifts in phenology due to global climate change: the need for a yardstick. Proc R Soc Biol 272:2561–2569
Walkovszky A (1998) Changes in phenology of the locust tree (Robinia pseudoacacia L.) in Hungary. Int J Biometeorol 41:155–160
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Wickham H (2007) Reshaping data with the reshape Package. J Stat Softw 21:1–20
Zsilinszki A (2014) A 2012-13-as tél szinoptikus sajátosságainak értékelése NAO, AO távkapcsolat elemzések bevonásával. Dissertation in hungarian, Loránd Eötvös University
Acknowledgments
This research was supported by the grant of the Hungarian Scientific Research Fund (OTKA 81979), project of “Establishing the method of satellite phenological observations for ecosystems response to global change detection.” Thanks to Prof. Filzmoser, Annamária Lehoczky, and Ákos Németh for providing valuable comments and suggestions on the manuscript. The work of Bálint Czúcz was supported by the János Bolyai research fellowship of the Hungarian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szabó, B., Vincze, E. & Czúcz, B. Flowering phenological changes in relation to climate change in Hungary. Int J Biometeorol 60, 1347–1356 (2016). https://doi.org/10.1007/s00484-015-1128-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-015-1128-1