Abstract
To preliminarily assess the acute effects of a single warm-water bath (WWB) on serum adipokine activity, we measured serum adiponectin, leptin and other metabolic profiles before, immediately after and 30 minutes after WWB in seven healthy male volunteers (mean age, 39.7 ± 6.0 years; mean body mass index, 21.6 ± 1.8 kg/m2). The subjects were immersed in tap water at 41°C for 10 minutes. Two weeks later, the same subjects underwent a single WWB with a bath additive that included inorganic salts and carbon dioxide (WWB with ISCO2) by the same protocol as for the first WWB. Leptin levels significantly increased immediately after WWB with tap water and ISCO2 (both P < 0.05), and remained significantly higher than those at baseline even 30 minutes after WWB with tap water (P < 0.05). Adiponectin levels showed a slight, but not significant, increase both immediately after and 30 minutes after WWB with tap water or ISCO2. Some parameters, such as serum total cholesterol, red blood cell count, hemoglobin and hematocrit significantly increased immediately after WWB with tap water or ISCO2 (all P < 0.05), but they all returned to the baseline levels 30 minutes after bathing under both conditions. The sublingual temperature rose significantly after 10 minutes of WWB with tap water (0.96 ± 0.16°C relative to baseline, P < 0.01) and after the same duration of WWB with ISCO2 (1.24 ± 0.34°C relative to baseline, P < 0.01). These findings suggest that a single WWB at 41°C for 10 minutes may modulate leptin and adiponectin profiles in healthy men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adiponectin and leptin are adipocyte-derived hormones that provide an important link between obesity and inflammatory disorders (Han et al. 2007; Kobayashi et al. 2004; Lago et al. 2007; Otero et al. 2006; Peterlin 2009). Adiponectin reduces both the production and the activity of inflammatory cytokines and helps to protect against obesity, vascular inflammation and the development of atherosclerosis. The primary role of leptin is in energy homeostasis, appetite suppression, and the modulation of immune and inflammatory processes. Therefore, adiponectin and leptin are considered to be "good" adipokines (adipocyte-specific or enriched proteins) that seem to play important regulatory roles in a variety of complex processes, including fat metabolism, feeding behavior, hemostasis, vascular tone, energy balance, and insulin sensitivity (Rondinone 2006). The relationships between serum adipokines and lifestyle, such as physical exercise, dietary habits, obesity and smoking, have been investigated, since both obesity and inflammation are closely correlated with the onset of various lifestyle-related diseases, such as type II diabetes mellitus (DM), hyperlipidemia, hypertension, cancer and other diseases (Varady et al. 2010; Vona-Davis and Rose 2007; Yokoyama et al. 2004).
A recent study showed that heat shock directly affects adiponectin and leptin gene expression and secretion in adipocytes in vitro (Bernabucci et al. 2009). Adiponectin mRNA and secretion levels were both higher at 39°C than at 37°C. Further, leptin mRNA and secretion levels in adipocytes were higher at 41°C than at 37°C and 39°C. However, to our knowledge, there have been no investigations of the acute effects of heat shock on the plasma levels of adiponectin or leptin in vivo. In general, warm-water bathing (WWB) in a bathtub or balneotherapy is a popular custom that is associated with a good state of health in Japan; Japanese people usually wash themselves outside the bathtub by showering first, and then enter the bathtub that is filled with hot water, usually between 40°C and 42°C (Hayasaka et al. 2010; Nasermoaddeli and Kagamimori 2005), and 10 minutes of WWB at 41°C has been reported to improve the hemodynamics in patients with chronic heart failure (Tei et al. 1995). Recently, the role of a carbon dioxide (CO2) bath or balneotherapy has received a great deal of attention because of its possible effects on cardiovascular function and for the management of cardiovascular diseases (Pagourelias et al. 2011; Sato et al. 2009; Yamamoto and Hashimoto 2007).
Therefore, the primary objective of the present before–after study was to evaluate whether a single 10-minute WWB at 41°C could modulate plasma levels of adiponectin or leptin in a single group of healthy men. In this pilot study, we also assessed the difference in leptin and adiponectin responses to WWB under two conditions: WWB using tap water (WWB with tap water) and WWB using a bath additive that included inorganic salts and CO2 (WWB with ISCO2).
Methods
Subjects
Seven healthy male volunteers aged 32 to 50 years (39.7 ± 6.0 years, mean ± SD) participated in this study. Inclusion criteria were as follows: male; age 30–50 years; free of cardiovascular disease; not taking medications or supplements. Women were excluded to avoid the confounding effects of the menstrual cycle on the measurements. Their body mass indices (BMIs) ranged from 17.9 to 27.7 kg/m2 (21.6 ± 1.8 kg/m2, mean ± SD). The procedures complied with the 1975 Declaration of Helsinki, as revised in 1983. This study was approved by the Ethics Committee of Kagoshima University Hospital and written informed consent was obtained from all of the subjects.
Protocol
We performed this study from 9 a.m. to 10 a.m. from November to December 2009. Bathing was performed after a 12 h overnight fast and the subjects drank only 300 ml of tap water at 7 a.m. on the day of the study. None of the subjects bathed within 12 hours before the study.
We performed WWB as described by Tei et al. (1995) and Matsumoto et al. (2006). The room temperature was maintained at 25 ± 0.5°C. After 20 minutes of rest in the supine position, we measured stable baseline values. Each subject, who was wearing only underwear, reclined on a stretcher at an angle of 36 degrees. A bathtub that could be raised and lowered automatically (Elevale CEL-700; SAKAI Medical Co., Ltd., Tokyo) was then raised, and the subject was immersed up to the subclavicular level. The subject rested for 10 minutes in a semirecumbent position with the bath temperature regulated at 41°C. After the completion of WWB, the bathtub was automatically lowered and the subject was dried thoroughly with a towel. The subject was then kept warm wrapped in blankets on a stretcher for 30 minutes. First, we performed this protocol using tap water (i.e. WWB with tap water). After a 2-week interval, all of the subjects underwent the same protocol of WWB but with ISCO2, which was made by dissolving a bath additive, Kikiyu®-Magnesium and Carbon dioxide (BATHCLIN Co., Ltd, Tokyo, Japan), in tap water warmed to 41°C, 5 minutes before the start of bathing. CO2 was generated by dissolving 247.5 g of a bath additive containing organic acid, sodium carbonate, sodium bicarbonate, magnesium sulfate, and sodium sulfate in 330 L of tap water; the resulting concentration of CO2 was 160–180 ppm and that of inorganic salts was about 64 ppm. The subjects were blinded to the product name and the composition of the bath additive.
Blood sampling and biochemical measurements
Fasting venous blood (10 ml) was collected from the median cubital vein of all subjects at 5 minutes before, immediately after and 30 minutes after bathing. The blood samples were distributed into individual tubes, and each sample was subjected to centrifugation at 2000 G (3600 rpm) for 5 minutes. Assays for total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride and glucose were performed using enzymatic methods, and insulin was measured with an electrochemiluminescence immunoassay that is routinely used in clinical laboratories at the university hospital. Samples for evaluating hormones including adipokines were immediately frozen at −30°C and stored until measurement. Assays for adiponectin, leptin and free fatty acid (FFA) were performed in a commercial laboratory (Clinical Pathology Laboratory Inc., Kagoshima, Japan). Adiponectin was measured by the latex immunology turbidimetric method, using a human adiponectin latex kit (Mitsubishi Chemical Medience Corp., Tokyo), and the leptin concentration was determined by radioimmunoassay (RIA), using a human leptin RIA kit (Millipore Corp., Billerica, MA). Full blood counts were also performed.
Measurement of sublingual temperature and hemodynamics
To monitor the physical changes during this protocol, the body temperature and hemodynamics of the subject were also measured three times (before, immediately after, and 30 minutes after each bath) for each subject.
Systolic blood pressure, diastolic blood pressure and pulse rate were measured using a digital blood pressure monitor (HEM-907, Omron, Tokyo, Japan) on the left arm in the supine position. Cutaneous blood flow at the forehead and breast were measured as peripheral blood flow with a laser-Doppler flow meter using a type-C probe (ALF21D; ADVANCE Co., Ltd., Tokyo). The sublingual temperature was measured, as the deep body temperature, with a digital electric thermometer (CTM-303; Terumo, Tokyo) by placing a probe under the tongue.
Statistics
All values are given as the mean ± standard deviation (SD). The effects of “time” (i.e. before bath, immediately after bath, and 30 minutes after bath) were determined using repeated-measures analysis of variance (ANOVA) followed by a post hoc test (Dunnett’s test) when the F value was significant. The effects of “condition” (i.e., WWB with tap water vs. WWB with ISCO2) were determined using two-way ANOVA with repeated measures; independent variables were “condition”, dependent variables were “time”, and covariates were baseline (before bath) values for each measurement. P values <0.05 were considered to be statistically significant.
Results
All seven subjects completed the WWB with tap water and WWB with ISCO2. None of the subjects experienced obvious side effects that were related to bathing in either condition. Table 1 summarizes the changes in the laboratory parameters in blood taken before, immediately after, and 30 minutes after WWB with tap water or WWB with ISCO2. While there were no significant differences in the adiponectin levels of the subjects during the study period, the adiponectin level tended to increase immediately after WWB under both conditions and this tendency persisted for 30 minutes after WWB. Among the seven subjects, adiponectin levels were increased immediately after WWB with ISCO2 in six and at 30 minutes after WWB with ISCO2 in five, compared to those before WWB. Leptin levels were significantly increased immediately after WWB under both conditions (both P < 0.05) and remained higher than those at baseline 30 minutes after WWB with tap water (P < 0.05), but not with ISCO2. Two-way ANOVA revealed no significant time and condition interaction for both the adiponectin and leptin levels (Table 1).
Total cholesterol levels and LDL cholesterol and HDL cholesterol levels were increased immediately after WWB and returned to nearly the same levels as those at baseline 30 minutes after WWB (Table 1). There was no significant time and condition interaction for these three cholesterol levels (Table 1). Throughout the study period there were no significant changes in triglyceride or FFA for WWB with tap water (Table 1). However, for WWB with ISCO2, significant increases in FFA and decreases in triglyceride were observed immediately after WWB (P < 0.05) and 30 minutes after WWB (P < 0.05), respectively. There was a significant time and condition interaction for the triglyceride level (P < 0.05), but not the FFA level (Table 1).
Fasting blood glucose levels for WWB with tap water were not significantly different during the study period, but fasting blood glucose levels for WWB with ISCO2 were significantly increased 30 minutes after WWB (P < 0.05) (Table 1). While the insulin level showed no significant changes for WWB with tap water, the insulin level significantly increased immediately after WWB with ISCO2 (P < 0.01) and then returned to nearly the same level as that at baseline. There was no significant time and condition interaction for either fasting blood glucose or insulin levels (Table 1).
According to the results of the hemogram, similar tendencies were observed under both conditions of WWB (Table 1). The red blood cell count, hemoglobin and hematocrit were significantly increased immediately after WWB (all P < 0.01), and returned to nearly the same level as those at baseline 30 minutes after WWB. On the other hand, the leukocyte count increased immediately after WWB and remained higher than that at baseline 30 minutes after WWB under both conditions (with ISCO2, P < 0.01; with tap water, P < 0.05). There were no significant differences in the platelet count during the study period in both conditions. In addition, there was no significant time and condition interaction for all measurements in the hemogram (Table 1).
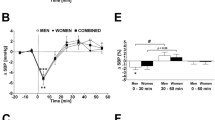
Table 2 shows a summary of the physiological measurements taken before, immediately after, and 30 minutes after warm-water bathing using tap water or with ISCO2. Deep body temperature, as reflected by sublingual temperature, rose significantly after 10 minutes of WWB with tap water (0.96 ± 0.16°C relative to baseline, P < 0.01) and after the same duration of WWB with ISCO2 (1.24 ± 0.34°C relative to baseline, P < 0.01). Under both conditions, the significant elevation of temperature from baseline persisted even 30 minutes after WWB [WWB with tap water, +0.34 ± 0.16°C, (P < 0.01); WWB with ISCO2, +0.59 ± 0.23°C (P < 0.01)]. The mean changes in temperature from baseline were greater after WWB with ISCO2 than after WWB with tap water, but there was no significant time and condition interaction. The pulse rate immediately after WWB was significantly greater than that at baseline under both conditions (with ISCO2, P < 0.01; with tap water, P < 0.05). Although the pulse rate decreased 30 minutes after WWB, it still remained higher than that at baseline, especially after WWB with ISCO2 (P < 0.01). Systolic blood pressure showed no significant change after WWB under either condition. However, diastolic pressure was significantly reduced immediately after WWB with ISCO2 and then decreased further 30 minutes after WWB (both P < 0.01), but there were no significant changes throughout the study period under WWB with tap water. Cutaneous blood flow at the forehead was significantly increased immediately after WWB with ISCO2 (P < 0.01) and returned to nearly the same level as that at baseline 30 minutes after WWB (Table 2), while there was no significant change throughout the study period under WWB with tap water (Table 2). Cutaneous blood flow at the breast was significantly increased immediately after WWB in both conditions (both P < 0.01), and tended to remain greater than the respective values at baseline 30 minutes after WWB, but not significantly. There was no significant time and condition interaction for any of the physiological measurements (Table 2).
Discussion
In the present pilot study in healthy men, serum levels of leptin were significantly increased immediately after a single 10-minute WWB at 41°C with tap water or ISCO2 and remained significantly higher than those at baseline even 30 minutes after WWB with tap water. Serum levels of adiponectin showed a slight, but not significant, increase immediately and 30 minutes after a single WWB under both conditions. Some parameters, such as serum total cholesterol, red blood cell count, hemoglobin and hematocrit, significantly increased immediately after WWB with tap water or ISCO2, but returned to the respective control levels 30 minutes after bathing under both conditions. Therefore, these results suggest that even a single WWB, i.e., head-out immersion in 41°C water for 10 minutes, may modulate the adipokine profile regardless of hemoconcentration (dehydration) after the hyperthermic action.
The present study does not clarify whether the extent (amount and duration) of the increase in adipokines observed after WWB is clinically beneficial. Hooper (1999) reported that 3 weeks of therapy using a hot tub for 30 minutes a day significantly decreased fasting plasma glucose and glycosylated hemoglobin levels in patients with type II DM. Biro et al. (2003) applied sauna therapy, a type of thermal therapy, to lifestyle-related diseases and found that repeated sauna therapy improves vascular endothelial function and reduces body weight. The plasma level of adiponectin is inversely related to the body mass index (BMI) and to measures of insulin resistance (Weyer et al. 2001), and decreased serum adiponectin is an independent risk factor for progression to type II DM (Daimon et al. 2003). Further, leptin regulates energy metabolism and balance. Therefore, an increase in leptin or adiponectin after WWB may improve glucose or lipid homeostasis in type II DM or other lifestyle-related diseases.
Recently, Fioravanti et al. (2011) assessed the plasma levels of leptin and adiponectin in patients with knee osteoarthritis treated by 2 weeks of spa therapy, which combined the application of mud-packs to both knees for 20 minutes at an initial temperature of 45°C with a bicarbonate–sulfate mineral water bath at 38°C for 15 minutes, once a day for 6 days a week for 2 weeks. Their data showed a slight, but not significant, increase in plasma leptin concentrations and a significant decrease in serum adiponectin levels at the end of the mud-bath therapy cycle. The increase they observed in the leptin level, but not the change in the adiponectin level, might be consistent with the results in the present acute evaluation after WWB. The difference might be due to the difference in subjects: we included only relatively younger, lean and male subjects without apparent OA in the present study. In addition, the difference could be due to a difference in modalities or agents, such as the dose (duration) of thermal stimulation and the substances used in the mineral water. On the other hand, after lean, healthy male volunteers were exposed to cold, plasma adiponectin levels were acutely and significantly decreased, with no specific changes in leptin levels (Iwen et al. 2011). In that study, norepinephrine plasma levels increased after cold exposure. Thus, while a change in temperature might influence adipokine profiles, it might be important to avoid stimulating the sympathetic nervous system during thermal stimulation.
According to a proposal by Gutenbrunner et al. (2010), hydrotherapy refers to WWB with tap water, as in the present study: core element, use of plain water; modality, head-out immersion; agent, thermotherapy. In contrast, WWB with ISCO2 is a type of “Balneotherapy”: core element, use of water that contains inorganic salts and CO2; modality, bathing (head-out immersion); agents, inorganic salts and CO2, and thermotherapy. In general, Balneotherapy requires specific geological, geographic and meteorological preconditions, and regional conditions strongly influence the use of these treatments (Gutenbrunner et al. 2010). Accordingly, in this study, we used an artificial bath additive, rather than natural mineral water, to create a representative form of “Balneotherapy”. The composition of the bath additive was also based on the following considerations: CO2 bath has been reported to induce peripheral vasodilation, increase peripheral blood flow and activate parasympathetic nerve activity; and magnesium and sodium ions are found in most spa/hot springs for the application of Balneotherapy or Spa therapy, and have also been reported to help the body retain heat after bathing (Gutenbrunner et al. 2010; Hashimoto and Yamamoto 2004; Ito et al. 1982; Nasermoaddeli and Kagamimori 2005; Pagourelias et al. 2011; Sato et al. 2009). In the current study, while no significant differences were observed in the changes in adipokine levels between WWB with tap water and WWB with ISCO2, differences were found in the changes in triglyceride and FFA levels, and in other parameters, such as sugar metabolism and cutaneous blood flow. This difference may be due to a difference in the increase in deep body temperature and/or the direct effect of the bath additive that included inorganic salts and CO2. In fact, CO2 bathing has been reported to have beneficial effects on peripheral vascular occlusive diseases (Pagourelias et al. 2011).
This study has some limitations. First, since it is only a pilot study, it has a small sample size. Second, we used WWB, and it is not clear whether the changes in serum adipokine levels were caused by heat shock alone or heat shock in combination with hydrostatic pressure (Tei et al. 1995). Further, it is still unknown whether WWB would have similar effects in patients with lifestyle-related diseases, since all of the subjects in the present study were healthy men. Therefore, the optimal dose (repeating) and long-term effects of WWB remain to be determined for adipokine profiles that are beneficial for preventing or controlling lifestyle-related diseases.
In conclusion, in our group of healthy men, a single warm-water bath was shown to have the potential to increase serum adiponectin and leptin levels. Further evaluations with larger and longer-term randomized double-blind placebo-controlled trials based on the present study are needed.
References
Bernabucci U, Basiricò L, Morera P, Lacetera N, Ronchi B, Nardone A (2009) Heat shock modulates adipokines expression in 3T3-L1 adipocytes. J Mol Endocrinol 42:139–147
Biro S, Masuda A, Kihara T, Tei C (2003) Clinical implications of thermal therapy in lifestyle-related diseases. Exp Biol Med Maywood 228:1245–1249
Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, Yamaguchi H, Ohnuma H, Igarashi M, Tominaga M, Kato T (2003) Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese population: the Funagata study. Diabetes Care 26:2015–2020
Fioravanti A, Cantarini L, Bacarelli MR, de Lalla A, Ceccatelli L, Blardi P (2011) Effects of Spa therapy on serum leptin and adiponectin levels in patients with knee osteoarthritis. Rheumatol Int 31:879–882
Gutenbrunner C, Bender T, Cantista P, Karagülle Z (2010) A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int J Biometeorol 54:495–507
Han SH, Quon MJ, Kim JA, Koh KK (2007) Adiponectin and cardiovascular disease: response to therapeutic interventions. J Am Coll Cardiol 49:531–538
Hashimoto M, Yamamoto N (2004) Decrease in heart rates by artificial CO2 hot spring bathing is inhibited by beta1-adrenoceptor blockade in anesthetized rats. J Appl Physiol 96:226–232
Hayasaka S, Shibata Y, Goto Y, Noda T, Ojima T (2010) Bathing in a bathtub and health status: a cross-sectional study. Complement Ther Clin Pract 16:219–221
Hooper PL (1999) Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med 341:924–925
Ito M, Fujiwara T, Amano K, Hirano S (1982) Studies on the thermal preservability of bath preparations. J Jpn Cosmet Sci Soc 6:175–180 (in Japanese)
Iwen KA, Wenzel ET, Ott V, Perwitz N, Wellhöner P, Lehnert H, Dodt C, Klein J (2011) Cold-induced alteration of adipokine profile in humans. Metabolism 60:430–437
Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y (2004) Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res 94:e27–e31
Lago F, Dieguez C, Gómez-Reino J, Gualillo O (2007) The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev 18:313–325
Matsumoto S, Kawahira K, Etoh S, Ikeda S, Tanaka N (2006) Short-term effects of thermotherapy for spasticity on tibial nerve F-waves in post-stroke patients. Int J Biometeorol 50:243–250
Nasermoaddeli A, Kagamimori S (2005) Balneotherapy in medicine: a review. Env Health Prev Med 10:171–179
Otero M, Lago R, Gomez R, Dieguez C, Lago F, Gómez-Reino J, Gualillo O (2006) Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatol Oxford 45:944–950
Pagourelias ED, Zorou PG, Tsaligopoulos M, Athyros VG, Karagiannis A, Efthimiadis GK (2011) Carbon dioxide balneotherapy and cardiovascular disease. Int J Biometeorol 55:657–663
Peterlin BL (2009) The role of the adipocytokines adiponectin and leptin in migraine. J Am Osteopath Assoc 109:314–317
Rondinone CM (2006) Adipocyte-derived hormones, cytokines, and mediators. Endocrine 29:81–90
Sato M, Kanikowska D, Iwase S, Nishimura N, Shimizu Y, Belin de Chantemele E, Matsumoto T, Inukai Y, Taniguchi Y, Ogata A, Sugenoya J (2009) Effects of immersion in water containing high concentrations of CO2 (CO2-water) at thermoneutral on thermoregulation and heart rate variability in humans. Int J Biometeorol 53:25–30
Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, Tanaka N (1995) Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 91:2582–2590
Varady KA, Bhutani S, Church EC, Phillips SA (2010) Adipokine responses to acute resistance exercise in trained and untrained men. Med Sci Sport Exer 42:456–462
Vona-Davis L, Rose DP (2007) Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 14:189–206
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA (2001) Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocr Metab 86:1930–1935
Yamamoto N, Hashimoto M (2007) Immersion in CO2-rich water containing NaCl diminishes blood pressure fluctuation in anesthetized rats. Int J Biometeorol 52:109–116
Yokoyama H, Emoto M, Araki T, Fujiwara S, Motoyama K, Morioka T, Koyama H, Shoji T, Okuno Y, Nishizawa Y (2004) Effect of aerobic exercise on plasma adiponectin levels and insulin resistance in type 2 diabetes. Diabetes Care 27:1756–1758
Acknowledgements
The authors are grateful to Mr. Y. Fukudome for his excellent technical assistance throughout this study.
Disclosures
This work was supported in part by BATHCLIN Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimodozono, M., Matsumoto, S., Ninomiya, K. et al. Acute effects of a single warm-water bath on serum adiponectin and leptin levels in healthy men: A pilot study. Int J Biometeorol 56, 933–939 (2012). https://doi.org/10.1007/s00484-011-0502-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-011-0502-x



