Abstract
Thermotherapy is generally considered appropriate for post-stroke patients with spasticity, yet its acute antispastic effects have not been comprehensively investigated. F-wave parameters have been used to demonstrate changes in motor neuron excitability in spasticity and pharmacological antispastic therapy. The present study aimed to confirm the efficacy of thermotherapy for spasticity by evaluating alterations in F-wave parameters in ten male post-stroke patients with spastic hemiparesis (mean age: 49.0±15.0 years) and ten healthy male controls (mean age: 48.7±4.4 years). The subjects were immersed in water at 41°C for 10 min. Recordings were made over the abductor hallucis muscle, and antidromic stimulation was performed on the tibial nerve at the ankle. Twenty F-waves were recorded before, immediately after, and 30 min following thermotherapy for each subject. F-wave amplitude and F-wave/M-response ratio were determined. Changes in body temperature and surface-skin temperature were monitored simultaneously. The mean and maximum values of both F-wave parameters were higher on the affected side before thermotherapy. In the post-stroke patients, the mean and maximum values of both parameters were significantly reduced after thermotherapy (P<0.01). Hence, the antispastic effects of thermotherapy were indicated by decreased F-wave parameters. Body temperature was significantly increased both immediately after and 30 min after thermotherapy in all subjects. This appeared to play an important role in decreased spasticity. Surface-skin temperature increased immediately after thermotherapy in both groups and returned to baseline 30 min later. These findings demonstrate that thermotherapy is an effective nonpharmacological antispastic treatment that might facilitate stroke rehabilitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spasticity or muscle tonus is a hallmark of upper motor neuron lesions, which is easy to identify but difficult to quantify and treat. Spasticity in affected limbs often inhibits the efficacy of physical therapy (physiotherapy) for the treatment of stroke or other central nervous disorders. Many previous studies have investigated spasticity in hemiparetic patients with stroke. Conventional methods for the study of spasticity, such as the H-reflex (Pisano et al. 2000), the T-reflex (Bell and Lehmann 1987) and the modified Ashworth scale (Bohannon and Smith 1987), require specialized technical skills and lack reliability (Pandyan et al. 2001, 2003; Bakheit et al. 2003). The complexity of these methods has made it difficult to observe spastic changes after various load tests.
Magladery and McDougal (1950) were the first to describe the F-wave. This potential was recorded from all of the small hand and foot muscles upon supramaximal electrical stimulation of the appropriate mixed peripheral nerves. Thus, F-waves can be used to study long-pathway nerve conduction and motor neuron excitability. Although the physiology of the F-wave has remained unclear, its presence in deafferented limbs and after myelotomy (Miglietta 1973) has indicated that it depends, in part, on the back-firing of motor neurons. Therefore, the amplitude and duration of the F-wave are considered to reflect the excitability of the motor neuron (Eisen and Odusote 1979; Fox and Hitchcock 1987; Kimura 2001). Additional studies have revealed that the F-wave is a small recurrent discharge of motor neurons that are activated antidromically (Fox and Hitchcock 1987; Kimura 2001).
As discussed above, F-wave size has been suggested as an indicator of motor neuron excitability (Milanov 1999; Fox and Hitchcock 1987) and alterations in F-wave parameters in spasticity have been confirmed in experimental animals (Machida et al. 1983). Furthermore, we have found that the F-wave is ‘easier’ to elicit under spastic conditions. Several authors have reported that F-wave size is increased in spasticity (Eisen and Odusote 1979; Fierro et al. 1993; Milanov 1992a, 1994; Schiller and Stalberg 1978). Indeed, F-wave amplitude has been found to be more sensitive to changes in lower motor neuron excitability during the course of spasticity than both the T-and H-reflexes (Kimura 2001; Hultborn and Nielsen 1995; Milanov 1992a,b; Mastaglia and Carroll 1985). The F-wave/M-response ratio has also been shown to correlate with the degree of muscle tone increase in spasticity due to stroke (Tsai et al. 2001). Thus, even small changes in muscle tone should be represented by changes in F-wave parameters. Precise assessments of F-wave parameters have been used to investigate the effects of physiotherapy (Rosche et al. 1996), drugs (Dressnandt et al. 1995; Milanov and Georgiev 1994), and various physiological conditions (Bell and Lehmann 1987). F-wave amplitude was found to change in parallel with muscle tone after an acute cerebral insult (Milanov 1992b), and was reduced by treatment with tizanidine, baclofen, myolastan, or electroacupuncture in cases of post-stroke hemiparesis (Milanov 1992a; Milanov and Georgiev 1994). Intrathecal application of baclofen reduced the mean F-wave amplitude by approximately 60% (Dressnandt et al. 1995).
Thermotherapy (i.e., bathing in hot water or using a sauna) plays an important role in the rehabilitation of patients with spasticity. In some cases, it is incorporated into exercise regimes designed to inhibit the spasticity of hemiparetic patients (Lehmann et al. 1970). Spasticity is known to decrease when muscles are heated for a certain length of time within a specific temperature range (Fukui 1991). However, relatively few studies have considered the effects of thermotherapy on the peripheral nerves, spinal cord, or spasticity. In order to confirm whether thermotherapy has clinical value in the treatment of spasticity, a comprehensive assessment of the antispastic effects of warm-water immersion is required. Thus, in the present study, we measured and compared F-wave parameters in spastic hyper-reflexic patients and normal controls to elucidate the effects of thermotherapy. The main aim of this work was to clarify whether the effects of thermotherapeutic treatment in spasticity could be documented using F-wave parameters.
Methods
Subjects
The age-matched subjects comprised ten healthy adult male controls and ten adult male post-stroke patients with spastic hemiparesis. The characteristics of these subjects are summarized in Table 1. The mean age of the control subjects was 48.7±4.4 years (range: 44–56 years) and the mean age of the post-stroke patients was 49.0±15.0 years (range:28–68 years). The control subjects had no neurological disease and showed normal muscle tone. The patients with hemiparesis were recruited from among inpatients admitted to the Kirishima Rehabilitation Center of Kagoshima University, Japan, between 1 September 2002 and 31 March 2004. The diagnosis of stroke was based on a computed tomography (CT) scan or magnetic resonance imaging (MRI), as well as neurological functions. Of the ten post-stroke patients, five were diagnosed with cerebral infarction and five were diagnosed with cerebral hemorrhage. The mean time since onset was 22.1±8.0 weeks (range: 11–36 weeks). The median clinical Brunnstrom stage of the hemiplegic lower limb was 4 (range: 3–5): one patient was stage 3, five patients were stage 4, and four patients were stage 5. Two patients had right hemiplegia and eight had left hemiplegia. All of the patients had increased muscle tone, decreased muscle force, a positive Babinski sign in the affected limb, and showed similar degrees of spasticity.
The study was conducted without altering the existing medication regimes of the patients. None of the patients were receiving either stimulant or relaxant medications (including anti-spasticity medication, anti-convulsion medication, or pharmacological injections). All of the patients were able to walk without assistance, using a T-cane or an ankle-foot orthosis. Only patients with normal latencies of F-waves and M-responses, thus indicating no peripheral nerve injury, were included in this investigation. The following groups were excluded from the analysis: individuals who were older than 71 years; those who had experienced the onset of stroke less than 4 weeks ago; those who had shown an abnormal gait prior to the onset of stroke; those with a medical condition that limited the completion of thermotherapy (such as severe cardiopulmonary disease, joint disability, or peripheral neuropathy); those with severe aphasia that made it impossible to follow verbal instructions; and those with lesions in the bilateral hemispheres, severe sensory disturbance, or dementia that interfered with the outcome assessments. The procedures used in this study complied with the 1975 Declaration of Helsinki, as revised in 1983. Informed consent was obtained from each subject according to the ethical guidelines of the hospital, after they fully understood the purpose and methodology of the study. This work was carried out with the permission of the Ethical Committee of Kagoshima University.
Electromyographic examination
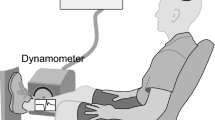
For the electrophysiological studies, the subjects relaxed in a semi-recumbent position in a semi-darkened air-conditioned room with a constant ambient temperature of 25°C. A Sapphire electromyographic system (Medelec, Old Woking, Surrey, UK) with a memory scope was used for the electrophysiological examinations, which were performed before, immediately after, and 30 min after thermotherapy for each subject. The F-waves and M-responses were recorded from the abductor hallucis muscles after supramaximal percutaneous electrostimulation of the posterior tibial nerve at the ankle using bipolar surface electrodes (Eisen et al. 1977). The stimuli were adjusted to 20% above the threshold of the maximum M-response. Twenty F-waves were recorded following supramaximal current pulses at a rate of 0.5/s (0.5 ms duration). A stimulus rate of 0.5 Hz was used to elicit the F-responses, with a total analysis time of 100 ms. The cathode was placed proximally. The measurement of F-waves in the post-stroke patients was carried out with the subject in a semirecumbent position, by fixing an electrode onto the abductor hallucis muscle of the affected side and stimulating a tibial nerve at the poles. This provided a more accurate measure of the total duration of the F-responses. In the control subjects, the F-wave measurements were performed using the same method on the right side of the lower limb. F-wave parameters are known to be bilaterally identical in healthy individuals (Fox and Hitchcock 1987).
Peak-to-peak measurements were made of the M-response amplitude, the largest of the 20 F-responses (F max) and the amplitude of the 20 averaged F-responses (F mean). The procedure was performed for each leg both before and after thermotherapy. The F-wave variables recorded for evaluation were the F-wave amplitudes (mean and maximum) and the M-response amplitude. The following values were calculated: F mean, F max, the ratio of the F mean to the M-response amplitude (F mean/M ratio), and the ratio of the F max to the M-response amplitude (F max/M ratio).
The same observer measured the F-wave parameters for all subjects. The amplitude of the maximum and the mean F-waves recorded from a given individual showed satisfactory reproducibility.
Physiological examination
The following physiological parameters were monitored to determine the mechanism of thermotherapeutic effectiveness and the side effects of thermotherapy: body temperature, surface-skin temperature, intermittent blood pressure, and heart rate. Measurements were made using an automatic sphygmomanometer based on the oscillometric measurement (GP303S: Paramatek, Fukuoka, Japan). A probe (2 mm in diameter) for a deep-body thermometer (electric thermistor; CTM303; Terumo, Tokyo, Japan) was fixed with tape onto the hypoglottis (under the tongue). A surface-skin temperature probe (1.5 cm in diameter) was fixed in the same way onto the quadriceps muscle of the thigh on both sides. Changes in temperature were monitored for 30 min after thermotherapy. The temperature of the subject was also measured three times (before, immediately after, and 30 min after thermotherapy) for each subject.
Procedure
Thermotherapy was performed according to the guidelines of the Japanese Association of Physical Medicine, Balneology and Climatology. Before administering thermotherapy to post-stroke patients with spasticity, we conducted studies using healthy volunteers in order to determine the most appropriate temperature and duration of warm-water bathing. The results were used to develop the protocol for the present study. Bathing in warm water at 41°C is customary in Japan. Thus, in order to reduce spasticity through changes of body temperature, the subjects were immersed in warm water at 41°C for 10 min, which was comfortable and did not induce sympathetic hypertonia or dehydration.
All subjects participated in the thermotherapy at the same time of day (1400–1600 hours), after they had eaten a meal. The subjects initially rested for 30 min in a head-out semi-recumbent position with the lower limb extended on a lift-bath stretcher. The subjects reclined on the stretcher at an angle of 30°. Each subject wore only underwear during the rest periods, but was kept warm using two blankets. The bath jet was switched off during this period. Physiological parameters were monitored before, during, and after the bathing session. Once stable baseline F-wave measurements had been obtained and a physiological examination had been performed, a bathtub (450 l volume, 65 cm width, 170 cm length and 42 cm depth) with automatic vertical motion (Elevele; Sakai, Japan) was prepared, and the subject immersed in water up to the subclavicular level. A thermostat was used to maintain the temperature at 41±0.2°C. Each bathing session was 10 min in length, during which time the subject was kept at rest in a semirecumbent position. After completion of the warm-water bathing, the bathtub was automatically lowered and the subject was dried thoroughly with a towel. Measurements of F-waves and a physiological examination were carried out immediately (within 5 min) after the bathing session, and again 30 min later, while the subject remained wrapped in blankets on the lift-bath stretcher.
Data analysis
All values are given as the mean ± standard deviation (SD). Statistical analyses were performed using the paired Wilcoxon test or analysis of variance (ANOVA). Probability (P) values below 0.05 were considered statistically significant. All analyses were performed using STAT view 5 software (SAS Institute, Cary, N.C.).
Results
None of the subjects experienced discomfort before, during, or after the warm-water bathing. The physiological examination was completed safely in all subjects. Table 2 shows the changes in F-wave parameters before and after thermotherapy. Table 3 presents a summary of the physiological measurements taken before, immediately after, and 30 min after thermotherapy.
F-wave amplitude
In the control subjects, the F max was observed infrequently among the 20 responses, while the amplitude and the duration of the F mean were reproducible across consecutive runs. In repeated studies of one control individual, the F mean values were 184±43 μV on the right (1.1±0.2% of the M-response) and 179±42 μV on the left (1.0±0.1% of the M-response); the F max values were 784±150 and 738±280 μV on the right and left, respectively (4.2±0.6 and 3.9±0.6% of the M-response, respectively). The amplitudes of the largest and the average F-waves recorded for each individual showed satisfactory reproducibility and did not differ significantly between the two sides.
In post-stroke patients, the F-wave on the unaffected side was recorded as a biphasic (or occasionally triphasic) potential with low amplitude and short duration. On the affected side, the F-wave was usually a polyphasic potential with increased amplitude and duration.
The F mean values for the controls before, immediately after, and 30 min after thermotherapy were 218±105, 198±98, and 201±101 μV, respectively. The F mean values for the post-stroke patients on the affected side before, immediately after, and 30 min after thermotherapy were 794±185, 536±101, and 557±104 μV, respectively. Before the thermotherapy, the F mean values for the post-stroke patients on the affected side were significantly greater than those for the controls (P<0.01). The F mean values for the post-stroke patients decreased markedly below the baseline immediately after thermotherapy in ten legs (P<0.01). Although the F mean values showed a tendency to return towards the baseline over time, they remained significantly lower than the baseline, even 30 min after thermotherapy (Table 2; Fig. 1).
Percentage changes from baseline in mean F-wave amplitude ( F mean) and maximum F-wave amplitude ( F max) following thermotherapy in healthy controls (○) and post-stroke patients (▪). All values are given as mean ±SD. Significant differences (healthy controls vs post-stroke patients): *P<0.01, **P<0.05. Note that in the patients, F mean and F max decreased significantly after thermotherapy compared with the controls, and the decrease persisted 30 min after thermotherapy
The F max values for the controls before, immediately after, and 30 min after thermotherapy were 754±219, 779±166, and 745±103 μV, respectively. The F max values for the post-stroke patients on the affected side before, immediately after, and 30 min thermotherapy were 1,126±256, 734±198, and 740±89 μV, respectively. Before thermotherapy, the F max values for the post-stroke patients on the affected side were significantly higher than those of the controls (P<0.01). The F max values for these patients decreased markedly below the baseline immediately after thermotherapy in nine legs (P<0.01). Although the F max values showed a tendency to return towards the baseline over time, they remained significantly lower than the baseline, even 30 min after thermotherapy (Table 2; Fig. 1).
These results reveal significantly increased values for the average and largest F-wave amplitudes on the spastic side prior to thermotherapy (P<0.01). Moreover, there was a significant decrease in the F-wave amplitudes after thermotherapy on the affected side; i.e., F mean and F max values were clearly reduced after thermotherapy. This decrease was even more significant when the relationship between F-waves and M-responses was considered.
F-wave/M-response ratio
In the controls, the F mean/M ratios before, immediately after, and 30 min after thermotherapy were 1.2±0.4, 1.1±0.2, and 1.1±0.3%, respectively. The F mean/M ratios for post-stroke patients before, immediately after, and 30 min after thermotherapy were 4.2±0.4, 2.9±0.3, and 3.1±0.4%, respectively, on the affected side. Before thermotherapy, the F mean/M ratios for post-stroke patients were significantly higher on the affected side than those for the controls (P<0.01). The F mean/M ratios for these patients decreased markedly below the baseline after thermotherapy in ten legs (P<0.01). Although the F mean/M ratios showed a tendency to return towards the baseline over time, they remained significantly lower than the baseline, even 30 min after thermotherapy (Table 2; Fig. 2).
Percentage changes from baseline in F mean/M ratio and F max/M ratio following thermotherapy in healthy controls (○) and post-stroke patients (▪). All values are given as mean ±SD. Significant differences (healthy controls vs post-stroke patients): *P<0.01, **P<0.05. Note that in the patients, F mean/M ratio and F max/M ratio decreased significantly compared with the controls both immediately after and 30 min after thermotherapy
The F max/M ratios for the controls before, immediately after, and 30 min after thermotherapy were 4.2±1.1, 4.3±0.9, and 4.1±0.8%, respectively. The F max/M ratios for post-stroke patients before, immediately after, and 30 min after thermotherapy were 6.2±1.3, 3.9±0.9, and 4.0±0.9%, respectively, on the affected side. Before thermotherapy, the F max/M ratios for the post-stroke patients on the affected side were significantly higher than those for the controls (P<0.01); the amplitude of the F mean and F max were significantly greater, both in absolute terms and as a percentage of the M-response. The F max/M ratios for these patients decreased markedly below the baseline following thermotherapy in ten legs (P<0.01). Although the F max/ M ratios tended to return towards the baseline over time, they remained significantly lower than the baseline, even 30 min after thermotherapy (Table 2; Fig. 2).
The mean values of the F mean/M and F max/M ratios were also significantly higher on the spastic side before thermotherapy (P<0.01). The F mean/M and F max/M ratios were significantly reduced after thermotherapy. Thus, all F-wave parameters for the post-stroke patients were lower after thermotherapy than before this treatment.
M-response
The amplitudes of the M-responses for the controls before, immediately after, and 30 min after thermotherapy were 18.3±5.4, 18.1±9.1, and 18.1±2.8 mV, respectively. These values were not significantly different for the post-stroke patients. The amplitudes of the M-responses for the post-stroke patients on the affected side before, immediately after, and 30 min after thermotherapy were 18.3±3.3, 18.3±2.9, and 18.3±3.1 mV, respectively. The M-response was not altered following thermotherapy in either group, despite the marked change in F-wave values.
Physiological examination
Table 3 shows the physiological measurements of the two groups, which were recorded before and after thermotherapy at the same sites. Both body temperature (measured under the tongue) and surface-skin temperature (measured on the belly of the quadriceps muscle of the thigh) demonstrated an increase following thermotherapy. Body temperature rose significantly to 37.7±0.4°C in the controls and to 37.9±0.4°C in the post-stroke patients immediately after the 10-min warm-water bathing session. Body temperature was 37.0±0.4°C in the controls and 37.2±0.4°C in the patients 30 min after thermotherapy. In both groups, body temperature remained significantly higher than the baseline, even 30 min after bathing, and the changes were of similar magnitude. The surface-skin temperature rose significantly to 35.7±0.5°C in the controls and 35.5±0.6°C in the post-stroke patients immediately after thermotherapy. These values returned to near-baseline levels 30 min after thermotherapy, with no significant differences between the two groups. In healthy individuals, similar patterns of change, of a magnitude comparable to those of patients, were observed in both body temperature and surface-skin temperature.
Although there was no significant change in systolic blood pressure, diastolic blood pressure decreased significantly following thermotherapy (P<0.01). The average decrease from the baseline was approximately 10 mmHg, with no significant differences between the two groups. Heart rate increased significantly above the baseline immediately after thermotherapy in both groups and returned to near-baseline levels 30 min later, with no significant differences between the two groups (Table 3). No side effects of thermotherapy were observed during physiological examination.
Discussion
In the present study, spasticity was assessed using F-wave parameters. The baseline F-wave values for both the control subjects and the post-stroke patients were in agreement with those reported previously (Schiller and Stalberg 1978; Milanov 1992b; Tsai et al. 2001). Briefly, before thermotherapy, the F-wave amplitude and F/M ratio were higher in the post-stroke patients than in the controls. All of the F-wave parameters (F mean, F max, F mean/M ratio and F max/M ratio) decreased significantly after thermotherapy and remained below the baseline, even after 30 min.
A hyper-reflexic state with spasticity is found in the majority of post-stroke patients. It has been suggested that the responses to thermotherapy by patients with this condition (i.e., these tonic derangements) might differ from the responses of normal subjects. There are several competing thermotherapeutic techniques for the treatment of spasticity; an electrophysiological evaluation of thermotherapeutic effects might therefore be helpful in deciding which technique will be most effective for a particular patient.
To our knowledge, the present study is the first investigation into the antispastic effects of thermotherapy in post-stroke patients. We confirmed that warm-water bathing decreased spasticity and increased body temperature after transient thermal exposure, as indicated by a decrease in the F-wave parameters. One major reason for the use of thermotherapy in post-stroke patients with spasticity is the suggestion that it decreases muscle tone and facilitates neuromuscular function. However, care must be taken when applying this method, because some patients can experience sensory disturbance in their affected side or various other complications, including difficulties or contraindications in a few cases. Therefore, in the present study, the effects of thermally induced sympathetic hypertonia and various other bath-related activities were minimized: the temperature of the warm-water bath was maintained at a comfortable 41°C, a bathtub with automatic motion was used, and physical activity was minimized by aiding the subjects when entering or leaving the bath. The indifferent temperature of Japanese subjects is 1 or 2°C higher than those of European and American individuals. Warm-water bathing is usually performed at 38–42°C for 15 min in combination with exercise (Yorizumi and Maeda 1998). The present study used a similar method to that applied in clinical settings; 41°C was chosen as the experimental temperature as Japanese subjects prefer a warm bath and no physiological changes have been reported at this temperature (Tei et al. 1995, 1996; Miwa and Iwase 1993; Agishi 1985; Ohtsuka et al. 1994). Tei et al. (1995) reported both warm-water bathing (at 41°C for 10 min) and sauna bathing to be safe for patients with congestive heart failure without chest symptoms, and the increase in oxygen consumption during these activities was only 0.3 METs. None of our subjects complained of excessive heat stimulation. Thus, we believe that using a comfortable temperature and optimizing the duration of warm-water bathing results in less physiological stimulation by heat stress.
The development of validated and reliable outcome measures for spasticity rehabilitation has been hampered by the difficulties involved in quantifying functionally important parameters, including pain, ease of care, and mobility (Pierson 1997). Nonetheless, a combination of measures designed to assess technical and functional outcomes, patient satisfaction, and the cost-effectiveness of treatment can be used together to evaluate status and track changes in spasticity management, including treatment programs involving botulinum toxin. Recently, F-wave parameters (F-wave amplitude and F/M ratio) were proposed as a precise method of evaluating changes in (segmental) motor neuron excitability in spasticity. The F mean recorded at rest from the normal abductor hallucis muscle was about 1–2% of the M-response amplitude, which was the same order of magnitude as that reported for hand muscles (Upton et al. 1971). The small ratio recorded using surface electrodes might be due to repeated activation of a few specific neurons or infrequent responses of most anterior horn cells (Schiller and Stalberg 1978). The largest F max in our control subjects was usually less than 5% of the M-response amplitude, indicating that a large portion of the motor neuron pool could not be excited at any given moment. The F max was larger and more persistent in the post-stroke patients on the affected side; as a result, F mean approached F max (Table 2). Fisher et al. (1994) published normal values for F-wave amplitudes, with a mean F-wave/M-response ratio of 2.2±0.7% (range: 1.2–3.5%). Therefore, the F mean/M ratio in our patients was clearly increased before thermotherapy and remained slightly elevated thereafter. Eisen and Odusote (1979) reported a significant correlation between the size of the mean F-wave and M-response (r=0.53), where the mean F-wave amplitude was 7.27 and the M-response amplitude was +79.1. They also found that the F max correlated positively with the M-response amplitude (r=0.559), where the F max was 19 and the M-response amplitude was +473. On the affected side, ten (100%) of the post-stroke patients in the present study were abnormal, in that the values for the mean F-wave amplitude were too high relative to the respective M-response amplitudes before thermotherapy (Table 2).
Milanov (1994) proposed that alpha-neuron-excitability, as measured by F-wave amplitudes, usually develops as a secondary effect after the alteration of some other segmental mechanism in spasticity (for example, increased gamma-motor neuron activity, altered inter-neuron activity, or decreased presynaptic inhibition). Thus, several different mechanisms might have led to the alteration of F-wave amplitudes observed in the present study. Our results clearly showed a marked reduction in both F-wave amplitude (both mean and maximum) and the F/M ratio after thermotherapy. Thermotherapy might not have influenced all of these parameters, which could explain why a decrease in F-wave amplitude after thermotherapy was not observed in every treated leg. Nonetheless, our results showed that high F-wave parameters in spasticity could be influenced by thermotherapy. Spasticity was judged to have decreased as attenuation was observed in the F-wave measurements, which are an objective index of spasticity. In this experiment, both F-wave amplitude and F/M ratio were decreased after thermal stress, indicating a reduction in motor neuron excitability. Therefore, the antispastic effect of thermotherapy can be documented by recording F-waves. Spasticity associated with a chronic hemiparesis was present to a similar degree in all of the patients before thermotherapy, and muscle tone decreased significantly after the treatment. When the subjects were immersed in warm water at 41°C, a continuous increase in body temperature was observed. This effect was probably caused either by heat transportation through the blood circulation or by direct heat conduction. Body temperature was elevated by thermotherapy and thermal comfort appeared to be related to changes in spasticity status. The mechanisms by which increased body temperature alter F-wave parameters are likely to be complex. Increased body temperature appeared to play an important role in the decrease of spasticity. Thermotherapy is thought to lower the activities of gamma-afferent fibers through a nervous system response, in addition to strengthening and relaxing of muscular and soft tissues. This process is believed to cause a decrease in impulses from the muscle spindles to the afferent fibers, which consequently inhibits impulses to the alpha fibers. This could explain the observed changes in F-wave parameters. To our knowledge, this is the first report of a correlation between body temperature and F-wave parameters during thermotherapy. Further studies will be necessary to determine whether thermotherapy changes F-wave parameters according to the degree and type of spasticity.
As expected, the results of this investigation revealed decreased values of the F mean, F max, F mean/M ratio, and F max/M ratio in response to thermotherapy. Some controversy surrounds the subject of alterations in F-wave parameters in spasticity. Most authors have reported the participation of greater numbers of larger motor neurons in the discharge, and have suggested that this is the cause of the increased F-wave amplitudes (Eisen and Odusote 1979; Fisher et al. 1994). However, as shown by the results presented here, F-wave-parameter alterations in spasticity are complex. The changes in F-wave parameters caused by thermotherapy might be a result of decreased motor neuron excitability (Schiller and Stalberg 1978). Previously, T-and H-reflexes, despite their limitations, have generally been used for the assessment of motor neuron excitability. However, F-waves are easily recorded in both upper and lower extremities. Electrophysiological techniques have not yet proved useful in assessing spasticity (Garcia-Mullin and Mayer 1972). However, this approach has potential applications in documenting both spasticity and responses to therapy, and might provide an objective measure of spasticity in cases where it is clinically uncertain. Further studies on the effects of local thermotherapy (Lehmann et al. 1970) and sauna baths (Tei et al. 1995, 1996) in the treatment of spasticity of hemiparetic limbs might contribute to stroke rehabilitation. In the present study, acute antispastic changes in post-stroke patients were examined soon after thermotherapy. The long-term effects of repeated bathing remain a subject of interest. Since our initial study, a number of patients have shown positive responses to several months of daily bathing in warm water (unpublished data). We believe that repeated thermotherapy improves the quality of life of patients, by permitting an increase in daily activities and improving their gait stability. However, further investigations will be necessary to confirm the clinical applicability of warm-water bathing as a nonpharmacological and economical antispastic therapy for post-stroke patients with spasticity. The correlation between the thermotherapeutic antispastic effect and different temperatures could also provide an interesting topic for further study.
Conclusions
The effects of thermotherapy for spasticity were studied in ten male post-stroke patients and ten healthy male subjects by recording F-waves. Increases in the F mean, F max, F mean/M ratio and F max/M ratio were observed in the post-stroke patients on the affected side before thermotherapy. Spasticity improved after thermotherapy in post-stroke patients on the affected side and all F-wave factors were lower post-treatment. Increased body temperature appeared to be the cause of the decreased F-wave parameters. Our results confirm that thermotherapy, when performed appropriately, can be applied as a nonpharmacological antispastic treatment with little risk. The long-term benefits of this intervention warrant further investigation.
References
Agishi Y (1985) Endocrine and metabolic aspects of balneotherapy. Int J Biometeorol 29:89–103
Bakheit AM, Maynard VA, Curnow J, Hudson N, Kodapala S (2003) The relation between Ashworth scale scores and the excitability of the alpha motor neurones in patients with post-stroke muscle spasticity. J Neurol Neurosurg Psychiatry 74:646–648
Bell KR, Lehmann JF (1987) Effect of cooling on H-and T-reflexes in normal subjects. Arch Phys Med Rehabil 68:490–493
Bohannon RW, Smith MB (1987) Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67:206–207
Dressnandt J, Auer C, Conrad B (1995) Influence of baclofen upon the alpha-motoneuron in spasticity by means of F-wave analysis. Muscle Nerve 18:103–107
Eisen A, Odusote K (1979) Amplitude of the F-wave: a potential means of documenting spasticity. Neurology 29:1306–1309
Eisen A, Schomer D, Melmed C (1977) An electrophysiological method for examining lumbosacral root compression. Can J Neurol Sci 4:117–123
Fierro B, Raimondo D, Modica A (1993) F-wave study at different stimulation rates in upper motoneurone lesions. Electromyogr Clin Neurophysiol 33:27–31
Fisher MA, Hoffen B, Hultman CH (1994) Normative F-wave values and the number of recorded F-waves. Muscle Nerve 17:1185–1189
Fox JE, Hitchcock ER (1987) F-wave size as a monitor of motor neuron excitability: the effect of deafferentation. J Neurol Neurosurg Psychiatry 50:453–459
Fukui K (1991) Thermotherapy: physical therapy. Ishiyaku, Tokyo, pp 10–11
Garcia-Mullin R, Mayer RF (1972) H reflexes in acute and chronic hemiplegia. Brain 95:559–572
Hultborn H, Nielsen J (1995) H-reflexes and F-responses are not equally sensitive to changes in motoneuronal excitability. Muscle Nerve 18:1471–1474
Kimura J (2001) Electrodiagnosis in diseases of nerve and muscle. Oxford University Press, Oxford, pp 439–465
Lehmann JF et al (1970) Effect of therapeutic temperature on tendon extensibility. Arch Phys Med Rehab 51:481
Machida M, Sato K, Asai T, Okada A (1983) An experimental study of the F-wave in the dog. Effects of spasticity and central muscle relaxant. Electromyogr Clin Neurophysiol 23:353–360
Magladery JW, McDougal DB (1950) Electrophysiological studies of nerve and reflex activity in normal man 1. Identification of certain reflexes in electromyogram and the conduction velocity of peripheral nerve fibres. Bull Johns Hopkins Hosp 86:265–290
Mastaglia FL, Carroll WM (1985) The effects of conditioning stimuli on the F-response. J Neurol Neurosurg Psychiatry 48:182–184
Miglietta OE (1973) The F response after transverse myelotomy. In: Desmedt JE (ed) New developments in electromyography and clinical neurophysiology, vol 3. Karger, Basel, pp 323–327
Milanov GI (1992a) A comparison of methods to assess the excitability of lower motoneurones. Can J Neurol Sci 19:64–68
Milanov IG (1992b) F-wave for assessment of segmental motoneuron excitability. Electromyogr Clin Neurophysiol 32:11–15
Milanov I (1994) Examination of the segmental pathophysiological mechanisms of spasticity. Electromyogr Clin Neurophysiol 34:73–79
Milanov I (1999) Clinical and neurophysiological correlations of spasticity. Funct Neurol 14:193–201
Milanov I, Georgiev D (1994) Mechanisms of tizanidine action on spasticity. Acta Neurol Scand 89:274–279
Miwa C, Iwase S (1993) Effects of bathing at 40°C on thermoregulatory function in humans. Environ Med 37:207–210
Ohtsuka Y, Yabunaka N, Fujisawa H, Watanabe I, Agishi Y (1994) Effect of thermal stress on glutathione metabolism in human erythrocytes. Eur J Appl Physiol Occup Physiol 68:87–91
Pandyan AD, Price CI, Rodgers H, Barnes MP, Johnson GR (2001) Biomechanical examination of a commonly used measure of spasticity. Clin Biomech 16:859–865
Pandyan AD, Price CI, Barnes MP, Johnson GR (2003) A biomechanical investigation into the validity of the modified Ashworth Scale as a measure of elbow spasticity. Clin Rehabil 17:290–293
Pierson SH (1997) Outcome measures in spasticity management. Muscle Nerve 6:S36–S60
Pisano F, Miscio G, Del Conte C, Pianca D, Candeloro E, Colombo R (2000) Quantitative measures of spasticity in post-stroke patients. Clin Neurophysiol 111:1015–1022
Rosche J, Rub K, Niemann-Delius B, Mauch E, Kornhuber HH (1996) Effects of physiotherapy on F-wave-amplitudes in spasticity. Electromyogr Clin Neurophysiol 36:509–511
Schiller HH, Stalberg E (1978) F-responses studied with single fibre EMG in normal subjects and spastic patients. J Neurol Neurosurg Psychiatry 41:45–53
Tei C et al (1995) Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 91:2582–2590
Tei C et al (1996) Thermal vasodilation as a treatment of cognitive heart failure: a novel approach. J Cardiol 27:29–30
Tsai KH, Yeh CY, Chang HY, Chen JJ (2001) Effects of a single session of prolonged muscle stretch on spastic muscle of stroke patients. Proc Natl Sci Counc Repub China B 25:76–81
Upton ARM, McComas AJ, Sica REP (1971) Potentiation of “late” responses evoked in muscles during effort. J Neurol Neurosurg Psychiatry 34:699–711
Yorizumi K, Maeda M (1998) Clinical management of spasticity (in Japanese). Rigaku Ryoho 15:693–700
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, S., Kawahira, K., Etoh, S. et al. Short-term effects of thermotherapy for spasticity on tibial nerve F-waves in post-stroke patients. Int J Biometeorol 50, 243–250 (2006). https://doi.org/10.1007/s00484-005-0009-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-005-0009-4