Abstract
Key message
The main achievement of this study is an extension of the existing model of Shigo of the branch–stem junction of coniferous trees by introducing the concept of a sacrificial tissue. This tissue is acting as a predetermined breaking point between branch and stem, and limits fracture and damage to a small and isolated zone within the tree.
Abstract
Shigo developed a macroscopic model of the fibre structure in the vicinity of a branch–stem junction of coniferous trees to explain special physiological functions of the junction. However, abrupt changes in the cell orientations at the vertex of the branch observed on fracture surfaces and micro-cuts of the branch–stem junction interphase demand an extension of the existing model. The recent introduction of the concept of a sacrificial tissue, formed in the upper region of the branch–stem interface, brings more insights into the hierarchical junction microstructure and its mechanical and biological functions. Beyond a critical load, the sacrificial tissue serves as a predetermined crack path of zig–zag morphology originating from the stepwise distribution of transversally loaded cells at the junction. The hierarchical branch–stem junction microstructure, however, secures the stem and branch physiological functions, even when the crack opening is formed along the channel of the sacrificial tissue. Moreover, after the removal of the load, complete closure of the crack can be observed, which is explained by the release of the elastic energy stored in cells of the bent branch with high microfibril angle. The self-repair mechanism of the living branch is based on covering the crack by cell division of a sound cambium in combination with resin deposition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wood exhibits excellent mechanical properties in the fibre direction, but shows poor mechanical performance perpendicular to the grain. This property is a result of the cellulose microfibrils embedded in a lignin–hemicellulose matrix within the wood cell wall, which are primarily oriented in the direction of the cell axis. The orientation of the cellulose chains in the cell wall is due to biological reactions to anisotropic mechanical stimuli induced primarily by environmental and gravitation loads. Tissue deposition, shape and orientation of cells as well as orientation of cellulose chains and microfibrils on the cell level demonstrate that hierarchical wood tissue is optimized for weight and strength (Weinkamer and Fratzl 2011).
For undamaged softwood without any defects or irregularities, the micro-mechanic and macro-mechanic behaviour has been intensively investigated and is quite well understood. However, the mechanical system of branch–tree junctions is much more complex and there are still some open questions with respect to their exact microstructure and mechanical as well as biological functions.
Based on the biological growth characteristics, large fibre deviations can be observed, especially in the vicinity of knots (Meierhofer 1976). From several studies it is known that even small fibre deviations result in significant reduction of strength and stiffness (Eberhardsteiner 2002). Therefore, in the case of sawn timber production, knots and corresponding fibre deviations are the most limiting factors for the usage of wood. On the one hand, better understanding of the fibre arrangement in the vicinity of a branch can help to improve sorting and grading of softwood timber. On the other hand, investigation of this exceptional and powerful structure could also be used in a biomimetic approach to design fibre reinforced composites. Additionally, a better understanding of the branch–stem structure and mechanics might be important in future, because of expected damages due to of climate change and increased storms.
Finite element (FE) models that consider fibre orientation are expected to describe mechanical properties of knotty wood. Several studies have been performed to simulate fibre orientations and to derive valid FE models including the distortion “knot”. Reuschel (1999) applied Computer-Aided Internal Optimization (CAIO) software to study the biomechanics of branch–stem junctions. Further studies used flow models (Fink and Kohler 2011; Jenkel and Kaliske 2014), destructive (Shigo 1985) and non-destructive methods (Kandler et al. 2016) to describe fibre deviation and the wood internal structure around a knot in a softwood tree. However, different approaches (Shigo 1985; Reuschel 1999; Fink and Kohler 2011; Jenkel and Kaliske 2014) suffer from an imprecise three-dimensional description of the fibre structure within these regions (Müller et al. 2015). In particular, the stem–branch interface at the upper side of the junction with the formation of special cells with different morphology, dimension and size, orientation and microstructure is thought to have a significant influence on the physiological and mechanical functions of the branch.
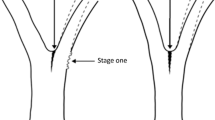
The model of Shigo (1985) considers the flow pattern of the tracheids, where the fibres of the stem are simply deviated by the branch, building a collar. Several papers describing the fibre deviations around knots for finite element modelling follow this concept of flow pattern (Hu et al. 2018). Regarding the lower side of the branch, Shigo’s model (1985) gives an explanation of the fibre formation, where branch tracheids merge directly into the stem body. However, at the upper side the deviated fibres create a corner. Shigo model gives no description of fibre morphology within this corner. Also, other authors (e.g. Jenkel and Kaliske 2014) tried to describe fibre orientation around knots using flow lines neglected the special pattern of fibres in the corner. Nevertheless, the fibres within this corner form a channel of a special wood tissue, having a dedicated mechanical function, explaining the high ductility of the branch–stem junction. Using the terminology of Müller et al. (2015), we refer to this tissue as “sacrificial tissue”. This tissue is acting as a predetermined breaking point between branch and stem and limits fracture and damage to a small and isolated zone within the tree.
In an earlier publication, the authors compared strains and failure of a mechanically loaded Norway spruce (Picea abies (L.) Karst.) branch–stem junction with a polyester cast of identical shape. Deformation measurements by means of electronic speckle pattern analysis showed that the biological structure is characterized by small and very homogeneous distribution of cross-sectional strains at branch–stem junctions, whereas pronounced strain concentrations can be observed for the polyester cast at the same load level (Müller et al. 2006). The optimized morphology of the branch–stem junction of coniferous trees was discussed also by Mattheck and Kubler (1997), Mattheck and Bethge (1998), and Mattheck (1998). Studies on comparable structures to softwood branch–stem junctions, namely on tree forks and branches of hardwood species, can be found in works of Neely (1991), Slater and Harbinson (2010), Slater and Ennos (2013, 2015a, b). Moreover, Müller et al. (2015) delivered a simplified model which suggests that all hierarchical levels of a tree contribute to the mechanical response of branch–stem junction. However, those recent works on hardwood species do not consider the complex hierarchical nature of the junction. In particular, no 3D microscopic studies of these structures showing the varying orientation of cells or nano-scale investigations on cellulose microfibrils and microfibril angle variation in the different tissues in the vicinity of branch have been provided in the literature. Additionally, those recent studies concentrated on hardwoods and their morphological properties, whereas softwood species and their hierarchical microstructure as well as functional properties are the focus of this report.
The branch base is surrounded by a collar of trunk tissue and is ingrained in the stem structure, which results in a structurally and functionally unified arrangement. This arrangement gives the branch–trunk junction its unique properties of strength, flexibility, and resiliency (Shigo 1985). Since the fibres show a complicated arrangement in the neighbourhood of knots, the anatomy and cell structure of branch wood are clearly distinguishable from normal wood (Shigo 1985). Branches of coniferous trees are characterized by higher density, small or partly missing annual rings, a high proportion of compression wood with highly increased microfibril angle and lignin content, and decreased fibre length (Trendelenburg 1955; Timell 1986; Shigo 1985, 1990).
The special features of knots and surrounding tissue were recently described by Müller et al. (2015), showing the synergy of multi-scale toughening and protective mechanisms at branch–stem interfaces. The key element of the biological design concept is based on the formation of the sacrificial tissue, formed in the upper region of the branch–stem interface of coniferous trees. The mechanism of forming sacrificial tissue has to be understood on the different hierarchical levels of the wood structure. The concept of the sacrificial tissue explains the predefined crack path, which is based on the specific arrangement of the tracheids. Overloading of the branch results in a disconnection of the branch from the stem in a controlled way by maintaining the stem and branch integrity. The crack front grows along a narrow predefined region of transversally loaded tracheids which are interwoven with wood rays. Fibre bridging of tracheid bundles and crossing ray cells explains the high fracture toughness of the branch–stem junction. Nevertheless, the model of the synergy of multi-scale toughening mechanism explains the interaction of the different factors on all hierarchical levels, but does not describe in detail the fibre alignment within the tree. The knowledge of both could be probably used in future for a biomimetic approach to generate optimized fibre reinforced composites.
In this contribution, the microstructure and function of the branch–stem junction is described, based on highly spatially resolved experimental data. For this purpose, 200 consecutive micro slides of a branch–stem interface were prepared in radial and tangential directions (with respect to the stem axis). A 3D reconstruction of the fibre pattern in the region of interest was performed to understand the arrangement of sacrificial tissue between stem and branch cells as well as the fibre alignment. From this data, an extension of the existing classical model of Shigo (1985) was derived.
To verify the self-repair mechanism of these special cells in a living tree, branches of young Norway spruce wood trees were heavily stressed until crack opening were observed. After 1 year these trees were cut down and branches were tested in bending and compared to unaffected fresh branches. Additionally, micro-cuts were dissected to show resin deposition and overgrowing of the crack by cell division of the cambium activity.
Materials and methods
Wood material
Ten young spruce wood trees (Picea abies [L.] Karst) with a diameter of 10–15 cm and age of approx. 20 years were used for the experiments. Trees originated in a spruce forest (eastern Austria: 47°47′N, 15°54′O, sea level 750 m). Annual ring width of the trees ranged from 0.2 to 4 mm, depending on the position (branch, stem) and the ring age. Branches with a thickness of about 20 mm from four trees were heavily stressed in bending until crack opening was observed. For this purpose, the branch was manually loaded by an experimenter (approx. 900 N, load application at a distance from the stem basis of 500 mm). Branches were stress tested in September 2015. To observe the self-repair mechanism and covering of the crack, these trees were cut down exactly after 1 year (i.e. in September 2016). Additionally, four other young trees with similar diameters from the same site were cut at the same time to investigate unaffected branch–stem connections. Two samples per group (affected and pre-stressed branches) were used for micro-sectioning and microscopic image analysis. Another four samples (two pre-stressed and two unaffected branches) were used for mechanical experiments. For conservation of the material until the preparation of the micro-section was performed and material tests were conducted, the samples were stored in a cooling chamber at − 20 °C.
Preparation of branch–stem sections
Microtome sections were taken from the freshly cut spruce trees by means of a conventional sledge microtome with a thickness of 13 µm. On the upper side of the branch at the transition from the stem to the branch tissue small specimen (approx.: rad. × tan. × long. = 10 × 6 × 6 mm3) were dissected. 200 micro-sections were cut off layer-wise in radial and tangential directions. Figure 1 shows a schematic, presenting position and direction of the radial and tangential micro-sections. The sections were stained for better image contrast. Staining (methylene blue) and washing (distilled water) of the sections were performed on the object carriers. Microtome sections were covered with a cover glass, and a Zeiss Axioplan 2 Imaging microscope in transmission light mode was used to obtain micrographs with a magnification of 50×.
3D-reconstruction
Micrographs (12 × 10 mm) of the 200 microtome sections were stored and 3D-reconstruction of the volume was performed by means of Photoshop CS6. Each image was defined as a layer. The position in x- and y-direction of every single layer was corrected manually, using prominent structures (e.g. resin ducts, ray cells, ring boarder, etc.). For this step, the opacity of the layers was changed, which allows to observe two consecutive layers. The bottom layer was fixed and the following layer was adjusted by shifting and rotation. The whole stack of layers was adjusted in this way. All layers were exported as single images to generate an image series. Then the image series was imported as a DICOM picture series in Photoshop CS6 and virtual crosscuts were generated in any direction at different positions.
Mechanical experiments
Bending stress applications were performed on green material at moisture content higher than 100%. For sample preparation, all branches were removed from the stem, excepting the branch which was selected for the mechanical tests. Then the stem was crosscut 15 cm above and below the branch. For better observation of crack opening during the experiment, the bark was carefully removed from the stem and the branch. On the opposite side of the branch, the stem was set parallel to the stem axis by means of a planning machine. On this new surface, the stem was mounted to plywood with a thickness of 38 mm by means of six screws (3.5 × 50 mm). The plywood board with the stem section was fixed with screws to a metal bracket mounted on a universal testing machine, in this manner that the stem axis was oriented in the direction of the moving crosshead. Exactly 100 mm apart from the stem basis the load was applied by a connecting rod, which was attached to the load cell at the centre of the moving crosshead. The connecting rod was mounted in such a way that free rotation at the fixing points (tip of the branch and load cell) was guaranteed. Therefore, only vertical load was applied at the tip of the branch. All mechanical experiments were performed by means of a universal testing machine (Zwick/Roell Z20, Germany). Two unaffected branches with a diameter of 25.5 and 21.6 mm as well as pre-stressed branches with a diameter of 25.0 and 17.6 mm were tested. The diameter of the branch was measured with a calliper at the basis (5 mm distance to the stem). The bending stresses at the basis of the branch (σb) were calculated as follows:
where F is the load at the branch tip, l corresponds to the length of the branch cantilever and d is the diameter at the basis of the branch.
Results and discussion
Extension of Shigo’s model
Figure 2 presents a typical branch–stem junction of the spruce wood. The formation of the annual rings shows that branch and stem tissues were formed in parallel by the active cambium, which differentiates into xylem cells, building the wood body, and phloem cells for the bark. The specific formation of the tracheids in the vicinity of the branch–stem interface can be explained by an adaptive feature of conifer trees in response to external displacement forces (Li et al. 2013). The cambial activity of cells in secondary xylem is regulated by hormone signals (Uggla et al. 1996; Sorce et al. 2013). It is assumed that specific stress conditions in the different regions at the branch collar during tree growth (i.e. shear-tension stresses at the branch–stem interface) led to cell differentiation. This mechanism influences length and orientation of the tracheids as well as the arrangement of the micro-fibrils within the secondary cell wall (Müller et al. 2015).
The existing physiological model of Shigo (1985) explains water and nutrient transportation. However, the model of Shigo does not include an explanation for the mechanical optimization of the junction. In particular, a description of the fibre morphology in the corner at the upper side of the branch of deviated stem tracheids is lacking. However, wood tissue within this corner is thought to have a fundamental function with respect to the failure mechanism of coniferous branch–stem connections (Müller et al. 2015).
Recent studies on tree forks and branches (Neely 1991, Slater and Harbison 2010; Slater and Ennos 2013, 2015a, b), which also consider mechanical aspects, were done on hardwood species. Norway spruce is a gymnosperm species which develops no buds. Branches are built during vertical growth and these trees do not develop new branches afterwards. Since the branches serve for photosynthesis, the tree has to pay special care to protect them. Fracture surfaces of branch–stem junction of softwoods (Böhlmann 1970a) and hardwoods (Böhlmann 1970b) show significant differences. Consequently, it is assumed that the mechanism and the structure on all hierarchical levels differ between hardwood and softwood species.
For the understanding of the failure mechanism of Norway spruce branch–stem connections, the full hierarchical nature of the junction has to be considered. In the vicinity of a branch, different wood tissue with various cell shapes and orientations can be observed. At a finer scale, varying orientation and variation of cellulose microfibrils within the cell walls can be seen (Müller et al. 2015). Based on X-ray computer tomography images, investigations of fracture surfaces at the branch–stem interface by means of scanning electronic microscope and optical micrographs, Müller et al. (2015) tried to find an explanation for the exceptional mechanical properties of this biological material. After overloading a branch–stem junction in bending, the cracks follow a distinctive zig–zag path. The insert in Fig. 2 clearly shows the formation of such a zig–zag crack path. The tissue around the crack along the branch–stem interface is thought to be a predetermined breaking zone and was therefore called sacrificial tissue. In the case of overloading the system, the sacrificial tissue guaranties material failure along the branch–stem interface and protects the rest of the branch and stem material. Therefore, the multi-scale synergetic concept of a sacrificial tissue represents a pre-determined breaking point between stiff stem and flexible branch, as well as mechanical tree integrity, intact physiological functions. Exceptional high fracture toughness of the structure can be explained by the interaction of tracheids with changing orientation and by cross-aligned ray cells, which are closely interwoven with surrounding and meandering tracheids (Müller et al. 2015). However, although the model of Müller et al. (2015) gives an explanation for the failure mechanism, a comprehensive understanding of the overall fibre arrangement around a branch–stem junction is still lacking.
In Fig. 3a, b, selected cubes of the 3D reconstruction of the stem–branch junction are presented. The cubes show cross cuts in the region of interest at the upper side of the branch, where the branch cells merge into the stem tissue. On the x1–x2 plane of the cube, the network of ray cells and meandering pattern of short tracheids can be observed. Jenkel and Kaliske (2014) suggested a flow model to describe the fibre orientation around a knot. In general, the model explains fibre structure reasonably well. However, in the region of the sacrificial tissue, the fibres do not follow a flow pattern and are inconsistent with the meandering fibres observed. So it is thought that the development of a model describing fibre morphology should be trigged by other mechanisms then simple flow mechanics. Additionally, the micrographs show that tracheids of the sacrificial tissue are much shorter (ranging from ~ 0.5 to 2 mm) in comparison to normal tracheids of the branch and stem material (~ 2 to 6 mm as reported by Buksnowitz et al. 2010). Therefore, it is assumed that these short and malformed cells are also characterized by different mechanical properties.
The organisation of the meandering tracheids (i.e. sacrificial tissue) in this region results in clusters, where the cells are oriented perpendicular to the stem (i.e. x1-direction) and to the branch axis (i.e. x3-direction). These clusters are arranged with an offset from one annual ring to another, forming a channel with a zig–zag pattern (Fig. 2).
Meandering cells were only observed at the upper side of the branch in between the corner of deflected tracheids of the stem tissue and position where the branch cells merge into the stem body. However, at the lower side of the branch, cells follow in a direct smooth pathway into the stem tissue. Thus, for this branch section, fibre arrangement is in strong agreement with the model of Shigo (1985) and Jenkel and Kaliske (2014).
For a consistent explanation of the fibre pattern observed and presented in Fig. 3, the model of Shigo (1985) needs a small but significant modification regarding the upper region of the branch–stem interface, which includes the formation of the sacrificial tissue with meandering cells in the corner of deflected stem tracheids and branch cells (Figs. 4, 5). Figure 4 shows a micrograph of the x1–x3 plane with meandering cells in this corner. For a better understanding of the formation of the zig–zag pattern of cross-aligned tracheids, the microtome section in Fig. 5 presents a cross-cut through the branch–stem interface at the vertex of the branch. Tracheids of the branch and the stem are arranged in the x1–x3 plane and are oriented more or less at an angle of 90°, whereas tracheids of the sacrificial tissue in the corner are oriented perpendicular to the x1–x3 plane in the x2-direction. Ray cells of the branch which merge into the channel of sacrificial tissue are bending up (approx. 45° in the x1–x3 as well as in the x1–x2 plane) and are rotating around their own axis of about 90°. In combination with different orientations of the tracheids, the ray cells are contributing to a network of interwoven cells. This network explains the highly ductile behaviour of the tissue surrounding the branch.
Advanced analysis of the failure mechanism of wood on the microscale indicates brittle failure in tension and ductile failure in compression (Lukacevic et al. 2015, 2017). Increased ductility of the wood tissue in this region was also observed by Oscarsson et al. (2012). Own experiments of highly stressed branches show extreme ductile behaviour and gradual crack opening in the upper region of the branch, which cannot be explained by the failure mechanism of axially or transversal loaded stem wood in tension (i.e. wood with mainly axially oriented tracheids with low MFA). Moreover, the complex network of interwoven and meandering tracheids and ray cells within the channel of sacrificial tissue gives a conclusive explanation of this phenomenon.
Within the sacrificial tissue, we have observed a significant increase of resin ducts. The shape of these resin ducts corresponds to the shape of traumatic resin ducts induced by drought stress (Wimmer and Grabner 1997; Kohler et al. 2010). Fahn and Zamski (1970) performed experiments with young saplings and adult trees of Pinus halepensis and proved that the formation of resin ducts is induced by different factors such as wounding, mechanical pressure, wind and auxins. Therefore it is assumed that special stress conditions within the sacrificial tissue not only control the microstructure of the cell wall (i.e. microfibril angle) and the orientation, shape and length of the tracheids, but also the formation of resin ducts.
Micro-mechanical mechanism of the optimized structure
In case the branch is bent downwards, the tracheids of the branch are stressed in tension and compression parallel to the cell axis on the upper and lower sides, respectively. Microfibril angles (MFA) of these cells range from 30° to 40° on the upper side and from 40° to 45° on the lower side of the branch, whereas tracheids are close to the pith of the branch ranging from 20 to 25° (Färber et al. 2001; Jungnikl et al. 2009; Müller et al. 2015). However, the tracheids of the stem show low MFA from 10° to 20° (Färber et al. 2001; Müller et al. 2015). Since small and large MFAs are related to stiff and brittle as well as flexible and ductile wood properties, respectively, the MFA variation within the secondary cell wall gives high flexibility and fracture toughness to the branch as well as stiffness and stability to the stem. For the sacrificial tissue at the branch–stem interface, MFA ranging from 15° to 25° and with a high degree of scatter was observed (Müller et al. 2015), which also enables this tissue to be highly flexible. In combination with the meandering arrangement of these cells, sacrificial tissue acts as a spring element at the first stage of branch loading. Normal tracheids with small micro-fibril angles show brittle fracture behaviour (Lukacevic et al. 2015) and a low elongation at break in the range of approx. 1.5%. Due to large microfibril angles and special structure of these tracheids, sacrificial tissue is thought to be much tougher and characterized by much larger elongation at break than normal stem material.
Bending stresses on the branch are transferred to the stem basis mainly by loading stem tracheids in compression at the bottom side, where the branch tracheids merge directly into stem tissue. Stem tissue on the upper side of the branch is minimally stressed due to the meandering pattern of the transversally oriented tracheids within the channel of sacrificial tissue, allowing a high degree of deformation. In the case of overloading of the branch, primary cracks which propagate along the interface parallel to the branch axis (i.e. along the channel of sacrificial tissue) occur in form of a crack which develops in a zig–zag pattern (Fig. 2). Due to the arrangement of tracheids and rays within this special tissue, multiple crack deflection (at every annual ring) occurs, which causes high energy absorption during crack growth. Secondary cracks are deflected by about 90° at the high density ring borders. The zig–zag pattern of the crack propagation results in an increased fracture resistance.
The high energy absorption can be derived from the stress–strain curves of two loaded branches (Fig. 6). A maximum load of more than 2.0 kN was observed in the experiments, which is large compared to the maximum bending stress of 171 and 155 N/mm2 at the basis of the two unaffected branches at maximum load. No fracture occurred before bending stress reached about 50 N/mm2. After reaching the yield point, continuous crack opening was observed (Fig. 6), starting at the vertex where the branch merges into the stem body. At higher loads (approx. 80 N/mm2), compression failure (buckling) of the wood tissue below the branch and further crack opening on the upper side of the branch increasingly occurs. However, even if the branch was twisted approx. 80° downwards; the structural integrity was not destroyed. Thus, it is assumed that physiological functionality of heavily stressed branches is still intact.
Repair mechanism
The preservation of the physiological functionality could be derived from the fact that all overloaded branches did not show any adverse effects after 1 year, i.e. these branches did not lose their needles, no fungal attack was observed, and no damage (i.e. open crack) was visible within the upper corner of the branch–stem junction. This means that the cambium has produced new xylem and phloem cells, closing the crack. Figure 7 shows a micrograph of the interface of a branch overloaded in September 2015 after 1 year cambial activity. It is clearly visible that the cambium needs time to close the crack opening, but before latewood formation of the annual ring of 2016 the gap was already closed. Additionally, a high number of traumatic resin ducts can be observed in the new annual ring. Injury of the sacrificial tissue, which contains an increased density of resin ducts, results also in resin deposition, which fills the crack. After the load removal, stress relaxation of the material and closing of the crack opening was observed. The zig–zag crack propagation path (Fig. 2) results in a mechanical interlock closing after the bending moment is removed. Resin ducts and epithelial cells are responsible for the resin deposition on the crack surface. Resin is acting as an antimicrobial barrier to protect the tree against the penetration of spores and bacteria (Müller et al. 2015). It is supposed that resin deposition and interlocking of the zig–zag crack surface after stress relaxation give the affected branch a minimum stability, which supports the self-repairing mechanism of the tree.
The micrograph in Fig. 7 shows that cell division of the cambium around the crack opening is able to overgrow the wound within only 1 year, forming a sound annual ring.
Loading a pre-loaded branch again results in a reduction of maximum load. After 1 year, cambium activity and overgrowing of the crack pre-loaded branches can endure a maximum load of about 50% in comparison to the unaffected branches at the same deformation level (Fig. 6). Due to the formation of new annual rings, it is expected that a pre-stressed branch will perform the same mechanical resistance as unstressed branches within a few years.
Author contribution statement
Ulrich Müller prepared all wood sections and performed optical as well as mechanical tests. Moreover, the author designed different figures and contributed significantly to the data interpretation and manuscript preparation. Wolfgang Gindl-Altmutter contributed valuable discussion and gave support for data interpretation. Jozef Keckes contributed to the data interpretation and manuscript preparation.
References
Böhlmann D (1970a) Anatomisch-histologische Untersuchungen im Bereich der Astabzweigung bei Nadel- und Laubbäumen. I. Die Verhältnisse im Abzweigungsbereich der Langtriebe von Nadelbäumen. Allg For Jagdztg 141(7):134–140
Böhlmann D (1970b) Anatomisch-histologische Untersuchungen im Bereich der Astabzweigung bei Nadel- und Laubbäumen. IV. Die Abzweigungsverhältnisse bei Juglans, Fraxinus, Betula und Fagus und ihre Zuordnung zu Abzweigungstypen. Allg For Jagdztg 141(12):245–250
Buksnowitz C, Teischinger A, Grabner M, Müller U, Mahn L (2010) Tracheid length in Norway spruce (Picea abies (L.) Karst.) analysis of three databases regarding tree age, cambial age, tree height, inter-annual variation, radial distance to pith and log qualities. Wood Res 55(4):1–13
Eberhardsteiner J (2002) Mechanisches Verhalten von Fichtenholz—Experimentelle Bestimmung der biaxialen Festigkeitseigenschaften. Springer, New York
Fahn A, Zamski E (1970) The influence of pressure, wind, wounding and growth substances on the rate of resin duct formation in Pinus halepensis wood. Isr J Bot 19:429–446
Färber J, Lichtenegger HC, Reiterer A, Stanzl-Tschegg S, Fratzl P (2001) Cellulose microfibril angles in a spruce branch and mechanical implications. J Mater Sci 36:5087–5092
Fink G, Kohler J (2011) Multiscale variability of stiffness properties of timber boards. In: Applications of statistics and probability in civil engineering—ICASP 11. Taylor & Francis, London, pp 1369–1376
Hu M, Briggert A, Olsson A, Johansson M, Oscarsson J, Sa H (2018) Growth layer and fibre orientation around knots in Norway spruce: a laboratory investigation. Wood Sci Technol 52:7–27
Jenkel C, Kaliske M (2014) Finite element analysis of timber containing branches—an approach to model the grain course and the influence on the structural behaviour. Eng Struct 75(15):237–247
Jungnikl K, Goebbels J, Burgert I, Fratzl P (2009) The role of material properties for the mechanical adaptation at branch junctions. Trees Struct Funct 23:605–610
Kandler G, Lukacevic M, Füssl J (2016) An algorithm for the geometric reconstruction of knots within timber boards based on fibre angle measurements. Constr Build Mater 124(15):945–960
Kohler M, Sohn J, Nägele G, Bauhus J (2010) Can drought tolerance of Norway spruce (Picea abies (L.) Karst.) be increased through thinning? Eur J For Res 129(6):1109–1118
Li X, Yang X, Wu HX (2013) Transcriptome profiling of radiata pine branches reveals new insights into reaction wood formation with implications in plant gravitropism. BMC Genom 14:768
Lukacevic M, Füssl J, Lampert R (2015) Failure mechanisms of clear wood identified at wood cell level by an approach based on the extended finite element method. Eng Fract Mech 144:158–175
Lukacevic M, Lederer W, Füssl J (2017) A microstructure-based multisurface failure criterion for the description of brittle and ductile failure mechanisms of clear-wood. Eng Fract Mech 176:83–99
Mattheck C (1998) Design in nature—learning from trees. Springer, Berlin
Mattheck C, Bethge K (1998) The structural optimization of trees. Naturwissenschaften 85:1–10
Mattheck C, Kubler H (1997) Wood—the internal optimization of trees. Springer, Berlin
Meierhofer U (1976) Der Ast als qualitätsbeeinflussendes Strukturmerkmal. Bulletin 4/2. Schweizer Arbeitsgemeinschaft für Holz, Lignum, Zürich
Müller U, Gindl W, Jeronimidis G (2006) Biomechanics of a branch–Stem junction in softwood. Trees Struct Funct 20:643–648
Müller U, Gindl-Altmutter W, Konnerth J, Maier GA, Keckes J (2015) Synergy of multi-scale toughening and protective mechanisms at hierarchical branch–stem interfaces. Sci Rep. https://doi.org/10.1038/srep14522
Neely D (1991) Water transport at stem–branch junctures in woody angiosperms. J Arboric 17(11):285–290
Oscarsson J, Olsson A, Enquist B (2012) Strain fields around knots in Norway spruce specimens exposed to tensile forces. Wood Sci Technol 46:593–610
Reuschel JD (1999) Untersuchungen der Faserandordnung natürlicher Faserverbunde und Übertragung der Ergebnisse auf technische Bauteile mit Hilfe der Finite-Elemente-Methode. Dissertation. Forschungszentrum Karlsruhe GmbH, Karlsruhe
Shigo AL (1985) How tree branches are attached to trunks. Can J Bot 63:1391–1401
Shigo AL (1990) A new tree biology. Thalacker, Braunschweig
Slater D, Ennos R (2013) Determining the mechanical properties of hazel forks by testing their component parts. Trees 27:1515–1524
Slater D, Ennos R (2015a) The level of occlusion of included bark affects the strength of bifurcations in Hazel (Corylus avellana L.). Arboric Urban For 41(4):194–207
Slater D, Ennos R (2015b) Interlocking wood grain patterns provide improved wood strength properties in forks of hazel (Corylus avellana L.). Arboric J 37(1):21–32
Slater D, Harbinson C (2010) Towards a new model of branch attachment. Arboric J 33(2):95–105
Sorce C, Giovannelli A, Sebastiani L, Anfodillo T (2013) Hormonal signals involved in the regulation of cambial activity, xylogenesis and vessel patterning in trees. Plant Cell Rep 32:885–898
Timell TE (1986) Compression wood in gymnosperms. Springer, Berlin
Trendelenburg R (1955) Das Holz als Rohstoff. Carl Hanser Verlag, München
Uggla C, Moritz T, Sandberg G, Sundberg B (1996) Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci USA 93:9282–9286
Weinkamer R, Fratzl P (2011) Mechanical adaptation of biological materials—the examples of bone and wood. Mater Sci Eng C 31(6):1164–1173
Wimmer R, Grabner M (1997) Effects of climate on vertical resin duct density and radial growth of Norway spruce (Picea abies (L.) Karst.). Trees 11:271–276
Acknowledgements
The authors sincerely thank Lukas Graf for microscopy laboratory work and Alexander Stadlmann for providing young spruce wood trees.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by R. Grote.
Rights and permissions
About this article
Cite this article
Müller, U., Gindl-Altmutter, W. & Keckes, J. How softwood tree branches are attached to stems: hierarchical extension of Shigo’s stem–branch model. Trees 32, 1113–1121 (2018). https://doi.org/10.1007/s00468-018-1699-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-018-1699-7