Abstract
The radial growth of plant stem is based on the development of cribro-vascular cambium tissues. It affects the transport efficiency of water, mineral nutrients and photoassimilates and, ultimately, also plant height. The rate of cambial cell divisions for the assembly of new xylem and phloem tissue primordia and the rate of differentiation of the primordia into mature tissues determine the amount of biomass produced and, in the case of woody species, the wood quality. These complex physiological processes proceed at a rate which depends on several factors, acting at various levels: growth regulators, resource availability and environmental factors. Several hormonal signals and, more recently, further regulatory molecules, have been shown to be involved in the induction and maintenance of cambium and the formation of secondary vascular tissues. The control of xylem cell patterning is of particular interest, because it determines the diameter of xylem vessels, which is central to the efficiency of water and nutrient transport from roots to leaves through the stem and may strongly influence the growth in height of the tree. Increasing scientific evidence have proved the role of other hormones in cambial cell activities and the study of the hormonal signals and their crosstalking in cambial cells may foster our understanding of the dynamics of xylogenesis and of the mechanism of vessel size control along the stem. In this article, the role of the hormonal signals involved in the control of cambium and xylem development in trees and their crosstalking are reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trees are living organisms that can achieve extraordinary age and size during their ontogenesis. Potentially, trees can age indefinitely, whereas their growth in height is limited and the maximum height is a site- and species-specific characteristic that involves genetic potential and environmental constraints, thus it represents a very important object of study in forest ecology.
Investigating the processes of growth and water use in trees is gaining scientific interest, particularly to forecast the impact of global change on these functions. Development and biomass production are tightly correlated with the morphological and physiological traits of the conduit elements and, more widely, with the biological mechanisms underlying secondary growth. The radial growth of plant stem is based on the development of cribro-vascular cambium tissues, which allows for the characteristic perennial ontogenesis of tree species and directly affects the transport efficiency of water, mineral nutrients and photoassimilates that are responsible for the formation of the new xylem and phloem elements. The cambial zone includes the cambium, which is composed of meristematic cells called “initials” and the xylem and phloem mother cells, both of which are produced by the dividing cambial initials (Plomion et al. 2001). The number of cambial cell divisions and the rate of differentiation of the derivative cambial cells into mature xylem determine product yield and quality (Deslauriers et al. 2009). Cambial activity and xylogenesis are complex and dynamic processes, influenced by many factors and regulated by different signals, which operate at multiscale levels. Among these are the edaphic and climatic factors like temperature, water availability and nutrients (Arend and Fromm 2007; Escalante-Perez et al. 2009; Oribe et al. 2003) and several endogenous factors such as plant growth regulators, sugars (non-structural carbohydrates and starch) and regulatory mechanisms of perception and defense against oxidative stress (Berta et al. 2010; Deslauriers et al. 2009; Oribe et al. 2003; Sundberg et al. 1991; Wareing et al. 1964). Classical plant hormones, as well as further signal molecules, play a crucial role in the regulation of cell proliferation activity in the cambium and during primary and secondary vascular development, thus giving rise to a complex process of patterned growth, characterized by both radial and longitudinal components. Beyond the differentiation of phloem and xylem, which occurs radially on each side of the vascular cambium, a longitudinal component exists in temporal and spatial patterns of cambial cell division, as well as in relevant traits of the different types of xylem elements (Uggla et al. 1996). Below, we discuss the relationship between xylem patterning and growth in trees, and in the following sections we summarize current knowledge on the role of biochemical signals in cambial activity and xylogenesis.
Xylogenesis and hydraulic architecture
Xylem fulfills two important functions: mechanical support and transport of sap from roots to leaves. The photosynthetic activity depends on a continuous replenishment of water that is transpired through the stomata during gas exchange. It follows that maintaining a high metabolic rate in trees strongly depends on the efficiency of the hydraulic system that supports transpiration. In forest species the structure of the transport system is very complex and has some peculiarities that are not comparable to any hydraulic man-made system (Tyree 2003). A first peculiarity of the transport system of plants is that water is under tension. A second one is that the system is designed to ensure invariance of the hydraulic resistance despite the increase in the length of the distribution pathway (i.e. despite the plant grows in height) (Enquist 2003; Midgley 2003; West et al. 1999). According to recent advances, trees maintain good physiological performances until late ontogenesis, when the achieved height triggers physical limitations to water transport (Mencuccini et al. 2005; Vanderklein et al. 2007). However, how trees can compensate for the constraints due to the length and the anatomical structure of their xylem transport system still represents a challenge for plant ecologists. In the xylem, water flows through a complex network of empty cells and the tension–cohesion theory is widely accepted to explain the movement of sap from roots to leaves (Tyree 2003). According to physical laws of water transport in confined conduits, the resistance to water transport increases proportionally with tree height (McDowell et al. 2002a; Ryan and Yoder 1997). The rate of increase in resistance with tree height strongly depends on how conduits vary in size along the stem (Fig. 1a). When xylem cells increase in diameter from the stem apex to the base (configuration known as “conduit tapering”), most of the resistance is confined towards the apex. The higher is the degree of tapering, the greater is the magnitude of resistance confined to the apex. This implies that with further stem elongation, widening conduits towards the stem base would compensate for the increase of path length resistance. Consequently, transport efficiency is highest for a high degree of tapering (Becker et al. 2000; Petit and Anfodillo 2009). Therefore, the whole-tree xylem architecture plays a central role in the physiology of the plant. Besides some compensation mechanisms, such as the increase in the sapwood area to leaf area ratio (McDowell et al. 2002b), the increase in fine roots allocation (Magnani et al. 2000) or the increase in sapwood permeability (Pothier et al. 1989), conduit tapering has been supposed to be a universal strategy of evolution allowing plants to minimize the increase in hydraulic resistance due to increased height (WBE model) (Enquist 2003; West et al. 1999). Indeed, empirical measurements revealed that the diameter of xylem conduits increases from the stem apex to the base as predicted by the WBE model (Anfodillo et al. 2006; Coomes et al. 2007; Lintunen and Kalliokoski 2010; Petit et al. 2008, 2011; Petit and Anfodillo 2009), according to a power function relating the diameter of the vessel with the distance from the top of the plant, whose exponent is close to 0.2. However, examples of higher conduit tapering are also known (Mencuccini et al. 2007; Petit et al. 2010). Given the essential contribution of conduit tapering in maintaining an efficient water transport system (Petit et al. 2008), it has been argued that departures from optimal tapering can have negative effects on the hydraulic system, that would lead to an increase in hydraulic constraints (Fig. 1b) which, in turn, would hamper photosynthetic activity and stem elongation (Anfodillo et al. 2006; Petit et al. 2008, 2010). This ultimate and fundamental mechanism of convergence towards the maximum hydraulic constraint physiologically tolerable can be supposed to be universal in plants. The different scales of maximum tree height observed among terrestrial plants would be set by the restriction imposed by the environmental factor (temperature, water availability, etc.) that most heavily limits average cell size. Since conduit tapering is probably the most important mechanism of control for the increase in hydraulic resistance with increased height, it is likely that the plant can precisely control the dimension of xylem conduits along the stem. Despite the relevant role of cambium in plants, few studies have dealt with the molecular and structural mechanisms at the basis of its functionality (Berta et al. 2010; Deslauriers et al. 2009; Sundberg et al. 1991, 2000). According to a recent study, wider cells along the stem are those that undergo a longer distension stage during xylogenesis (Anfodillo et al. 2012). Therefore, it is likely that plants adopt a mechanism to modulate the duration of the cell enlargement step to precisely design an architecture of xylem conduits optimally tapered for hydraulic purposes.
a Simplified representation of axial variation of xylem conduits diameter from tree top to stem base. Conduits diameter (D h) widens basipetally following a power law when plotted against distance from the tree top (h): D h ~ h ≈0.2. The physiological mechanism controlling the gradient of vessel diameters of this precise catena of xylem conduits is still unknown. b Variation of total hydraulic resistance as function of tree height and different degrees of tapering. With no tapering (cylindrical conduits) the resistance increases linearly with tree height. Under optimal tapering the exponent of the power law approaches 0.2 and the total hydraulic resistance becomes virtually constant with tree height
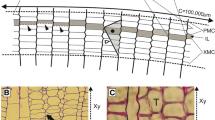
Summary of the main biological effects of IAA on cambium development and xylem and phloem differentiation processes. The diagram on the left shows the hypothesized relationship between the longitudinal auxin gradient concentration (decreasing from the top to the base of the stem) and the pattern of vessel development. [↓], decreasing concentration; [↑], increasing concentration; D h, conduits diameter. Light micrograph shows a transverse section of stem, close to the cambial region, collected in July from 3-years-old Populus alba. Cr cambial region, Ec enlarging xylem cells, MXy maturing xylem, Ph phloem
Hormonal factors involved in cambial activity and xylem differentiation
Auxin
The role of auxin in the differentiation of vascular tissue during both normal development and wounding is well documented (Sachs 1991). The auxin indoleacetic acid (IAA) is considered the primary hormonal signal among those involved in the regulation of cambium activity and cell differentiation (Fig. 2; Table 1). The continuity of vascular tissues along the plant, from the margin of leaves to the root apices, is induced by the steady polar flow of auxin from leaves to roots. The major path of auxin flow along the stems of pine and poplar trees is in the vascular cambium (Sundberg et al. 2000; Uggla et al. 1996, 1998). The continuous polar transport of auxin through these cells induces a complex series of events which finally result in the formation of a vascular strand. The canalization of auxin flux through meristematic, or parenchymatic cells, induces the orderly pattern of continuous vascular strands from leaves to roots. This suggests that vascular differentiation can be used as an anatomical expression of auxin flow (Sachs 1981, 2000). IAA is thought to induce cambium cell division and, at decreasing concentrations, the expansion of xylem derivative cambial cells (and probably also those of phloem) and later the onset of the maturation stage, when secondary wall apposition and lignification of cell wall take place. Recent researches have greatly increased the understanding of both auxin transport and signaling. Genetic and inhibitor studies have identified a clear role for polar auxin transport in developing vascular networks and these works were recently reviewed (Carlsbecker and Helariutta 2005; Sieburth and Deyholos 2006). In the Zinnia elegans Jacq. system, xylogenesis may be blocked by auxin efflux inhibitors, which appear to activate metabolism of intracellular auxin, resulting in its enhanced degradation. Consequently, the concentration of intracellular auxin is lower in these treated cells and this is presumed to be the cause of the low rates of differentiation (Yoshida et al. 2005). Mutants with impaired auxin signaling helped to define a role for auxin in the early stages of vascular patterning during the period when procambial cells are formed. Studies of secondary growth in Pinus species demonstrate that the highest levels of auxin are found in cambium initial mother cells, which is consistent with the role of auxin in maintaining cambial cell identity (Uggla et al. 1996). Instead, the role of auxin in promoting procambial cells to differentiate into xylem is unclear. Auxin is an absolute requirement in the Zinnia system, where its addition during a specific 10-min window is sufficient to induce differentiation (Milioni et al. 2001). The expression of some genes is specifically correlated with high levels of auxin in the dividing cells of the cambium and xylem mother cells. In contrast, at least one of the IAA signal transduction genes is expressed in mature xylem, where it responds to much lower levels of auxin (Moyle et al. 2002). One suggestion is that the hormone may act as a morphogen to define the fate of differentiating cells. In this model, auxin concentrations are translated into positional information via the IAA genes, whose expression level is regulated by different concentrations of the hormone. Consequently, the expression of IAA genes in the cambium, where auxin concentrations are high, would contribute to maintaining a population of cambial cells, whereas other IAA genes that are expressed at lower concentrations of auxin facilitate the later stages of xylem development (Bhalerao and Bennett 2003). The results of Nilsson et al. (2008) are in conflict with the above-mentioned model. They found that the expression of only a few auxin-responsive genes correlated well with the auxin concentration gradient and that the transcription of such genes responded dynamically to the changes in auxin levels, rather than being dependent on the steady state of auxin concentration. The authors hypothesize that auxin could regulate secondary xylem development through two mechanisms: (1) direct regulation of the expression of a few key genes, including some transcription factors; (2) posttranscriptional destabilization of transcriptional repressors.
The control of vessel size is an important trait for ensuring the ascent of water and minerals from roots to leaves and the adaptation of plants to the environment (Aloni 1987). Research in this direction is aimed at understanding the mechanisms that control vessel size and density (number of vessels per transverse-sectional area) along the plant in specific sites (e.g., top vs. base of the stem) and in different species (e.g., ring-porous vs. diffuse-porous trees). The narrow vessels differentiate near the leaves, while the widest vessels are formed in the roots. The increase in vessel diameter from leaves to roots is associated with a decrease in vessel density. Hence, vessel density is generally greater in branches, where the vessels are narrow, than in roots, where they are wide (West et al. 1999). To explain such general increase in vessel diameter and decrease in vessel density from leaves to roots, a six-point hypothesis (Aloni and Zimmermann 1983) has been proposed. The six-point hypothesis attributes the general increase in vessel size and decrease in vessel density along the plant axis to a gradient of decreasing auxin concentration from leaves to roots (Fig. 2). High auxin levels near the young leaves induce narrow vessels because of their rapid differentiation, allowing only limited time for cell growth. Conversely, low auxin concentrations further down result in slow differentiation, which permits more cell expansion before secondary wall deposition and therefore results in wide vessels. Consistently, vessel density is controlled by auxin concentration: high concentrations (near the sites of auxin synthesis) induce greater density, while low concentrations (further down, towards the roots) lower density. Therefore, the hypothesized auxin gradient would cause vessel density to decrease from leaves to roots (Aloni 2001). Although the detailed mechanisms of how IAA would regulate growth and differentiation are not fully understood, the aforesaid hypothesis is substantiated by several observations: (1) the major sources of IAA are developing buds and young shoots (Scarpella and Meijer 2004; Uggla et al. 1998); (2) IAA is transported basipetally from the leaves towards the roots (Aloni 2001); (3) the major path of the IAA flow in tree stems is in the vascular cambium (Sundberg et al. 2000); (4) there is a general decrease in the IAA concentration towards the stem base (i.e. far from the sites of IAA production) (Aloni 2001).
Experiments on downwardly oriented grape vines have demonstrated that a common response to such a growth orientation is reduced shoot growth (Kliewer et al. 1989; Prasad and Cline 1985). Furthermore, the downward shoot orientation negatively affects the radial development of the vessels and thus the shoot hydraulic conductivity (Schubert et al. 1995, 1999). When forced into a downward orientation, the growing shoot is subjected to different perturbations of its metabolism: in particular, the downward position of the apex may affect auxin metabolism and distribution (Bandurski et al. 1984). The downwardly oriented shoot portion below the apex has lower hydraulic conductivity, and this can potentially limit the transport of water, nutrient and hormones to the growing internodes (Lovisolo and Schubert 2000). Auxin concentration increased in the apical internodes of downwardly oriented shoots compared with upwardly growing controls, thus providing direct evidence of a perturbation in auxin metabolism (Schubert et al. 1999). This observation confirms previous measurements on upward and downward shoots and, following the six-point hypothesis, agrees with the higher frequency and lower diameter of vessels in downward shoots (Lovisolo et al. 2002). Besides the polar transport, large amounts of auxin are translocated via the vascular tissues, promoting cambium activity and cell differentiation. Indeed, a positive correlation was found between the radial growth of the trunk and the concentration of IAA in the xylem sap extracted from two hybrid rootstocks of peach and from a peach cultivar grafted on them (Sorce et al. 2007).
The six-point hypothesis has been criticized by those authors who claim that auxin radial distribution is physiologically more relevant than its longitudinal gradient along the stem. The radial distribution pattern of IAA was determined across the developing tissues of the cambial region in the stem of hybrid aspen (Populus tremula L. × Populus tremuloides Michx). The peak level of IAA was within the cambial zone, where cell division takes place. Low levels were reached in the region where secondary wall formation was initiated. Transgenic trees (with an ectopic expression of Agrobacterium tumefaciens IAA-biosynthetic genes) displayed a lower peak level and a wider radial gradient of IAA compared with the wild type: this alteration was related to a lower rate of cambial cell division and a longer duration of xylem cell expansion, resulting in a decreased xylem production and a larger fiber lumen area. The results indicate that IAA has a role in regulating not only the rate of physiological processes such as cell division, but also the duration of developmental processes such as xylem fiber expansion, suggesting that IAA functions as a morphogen, conveying positional information during xylem development (Tuominen et al. 1997) and, as such, it contributes to regulate the production of new tissues and affects some essential anatomical traits of the xylem. Moreover, Uggla et al. (2001) demonstrated that in Scots pine (Pinus sylvestris L.) the total amount of IAA in the cambial region did not change with latewood initiation. Nevertheless, its radial distribution pattern was altered, resulting in an increased concentration in the cambium and its recent derivatives. Thus, initiation of latewood formation and cessation of cambial cell division is not a consequence of decreased IAA concentrations in dividing and expanding cells. Rather, IAA most likely has a role in defining the altered developmental pattern associated with latewood formation.
Auxin appears to play a primary role in vascular patterning, but the importance of additional factors must not be overlooked. For instance, novel biochemical signals such as peptide hormones and some polyamines are clearly involved in the processes of timing and spatial patterning of xylem differentiation. Their role is discussed in the following sections.
Cytokinins
Cytokinins (CKs) have been shown to stimulate plant cell divisions (Miller et al. 1955) and, when supplied exogenously, appear to be involved in the regulation of cambial activity (Table 1). Cytokinins occur in the cambial region (reviewed by Little and Pharis 1995; Little and Savidge 1987). However, until recently the role of CK in cambial activity remained unclear. The CRE1 gene of Arabidopsis thaliana codes for a CK receptor which is expressed in the vascular tissue in the procambium of the root, thus indicating the involvement of a specific CK signal transduction pathway in the regulation of vascular cell proliferation (Inoue et al. 2001; Ueguchi et al. 2001). Nieminen et al. (2008) reported that genes encoding CK receptors and a CK primary response gene (PtRR7) are expressed in the cambial zone of Populus stems. The same authors engineered transgenic Populus trees to express a CK catabolic gene, which reduced the concentration of a biologically active CK. The consequent impaired CK responsiveness showed that the stimulatory effect of CKs on cell divisions is required for normal vascular cambium function and the same conclusions were reported in Arabidopsis by Matsumoto-Kitano et al. (2008).
During primary vascular development, CKs appear to be required for both cell proliferation and cell differentiation, while during secondary development their major function is the regulation of cell proliferation. Thus, CKs appear to have diverse roles during vascular and meristem development, perhaps dependent on how they interact with other growth regulators (Nieminen et al. 2008).
Brassinosteroids
Although a great deal of attention has been focused on the role of brassinosteroids (BRs) in regulating cell expansion (Clouse and Sasse 1998), several independent experiments suggest that BRs are also involved in the regulation of xylem development (Turner et al. 2007) (Table 1). Brassinosteroids have been detected in developing pine cambium, and more direct evidence has come from the Zinnia system. The addition of uniconazole, a known inhibitor of BR and gibberellin biosynthesis, to the Zinnia system blocks the transdifferentiation of tracheary elements (TE) in a manner that can be overcome by the addition of exogenous BRs but not gibberellin (Iwasaki and Shibaoka 1991). Uniconazole appears to act in the later stages of TE differentiation, preventing the expression of genes associated with programmed cell death (PCD) and secondary cell wall (SCW) deposition, two processes that are induced by BRs (particularly brassinolide; Demura and Fukuda 1994; Fukuda 1997; Yamamoto et al. 1997). Consistent with the idea that BRs are required for the later stages of TE differentiation is the identification of five different BRs that accumulate both within the cells and in the TE culture medium when TEs are differentiating (Yamamoto et al. 2001). According to the hypothesis of Demura and Fukuda (1994) and Fukuda (1997), the process of differentiation of Zinnia mesophyll cells into TE is divided into three stages, among which stage III (48–96 h of culture) involves TE-specific events including secondary wall thickening and cell death. Brassinolide is a prerequisite for the expression of stage III-specific genes (Yamamoto et al. 1997) and five BRs were found to rapidly increase between 30 and 54 h of culture, in cultured cells differentiating into TE, while they were below detectable levels in freshly isolated cells. Brassinosteroids are synthesized in late stage II and direct the transition of cells from stage II to stage III in which secondary wall thickening and PCD occur (Yamamoto et al. 2001). In addition to promoting xylem development at the expense of phloem, BRs also promote cell divisions in the procambial cells to provide the precursors for vascular cells (Cano-Delgado et al. 2004). If the length of the expansion phase of the differentiating xylem cells may determine the final anatomical traits of xylem tissues, all signals affecting the duration of such phase could play a pivotal role in this regard.
Gibberellins
Gibberellins (GAs) have been implicated in promoting xylogenesis. Gibberellic acid (GA3) supplied exogenously promotes differentiation and lignification of TE in Zinnia cell cultures (Tokunaga et al. 2006). Xylem fiber elongation is enhanced both in transgenic trees overproducing GAs and in hormone-treated plants (Digby and Wareing 1966; Eriksson et al. 2000) (Table 1). Apparently, in Populus alba this role should be assigned to GA1, whose endogenous concentration largely exceeded that of GA4 (Luisi et al., submitted), while according to Israelsson et al. (2005) the reverse occurred in P. tremula: the discrepancy might be due to the species and/or to the ontogenetic stage of the saplings. Moreover, in P. alba a strong increase of endogenous GA1 concentration was recorded during the recovery from a water deficit: given the well-documented promoting effect of GA1 on wood growth, the hormone might have substantially enhanced the restoration of the activity and development of cambial tissues. Ragni et al. (2011) observed that the possibility that the enhanced xylem production might have been the result of the overall growth increase in height induced by the elevated GA signaling could not be ruled out. Their study, aimed at determining the cause for the acceleration of secondary growth and the shift toward xylogenesis in Arabidopsis hypocotyls, suggests that this developmental process is caused by a mobile signal, viz GA, which promotes the proliferation of xylem, whereas the occurrence of fibers appears to be a delayed secondary effect. The level of endogenous GAs in the dividing cells of the active cambium of poplar is markedly low and the greatest amount has been found in the differentiating xylem cells. The same pattern was observed for the expression of the genes coding for GA biosynthetic enzymes and GA signalling pathway (Israelsson et al. 2005), with the exception of GA receptor genes, whose expression peaked in the phloem and dividing cambial cells (Mauriat and Moritz 2009). This result calls for a comprehensive expression analysis of GA biosynthesis and signaling genes across the cambial zone.
Abscisic acid
Besides the promoting signals, others may exist which inhibit cambium development. Abscisic acid (ABA) probably represses secondary growth of woody species (Table 1). It is a primary signal mediating the response of plants to environmental stresses, such as drought, which commonly causes an increase in the biosynthesis and accumulation of the hormone (Zhu 2002). Changes in ABA levels deeply affect the physiology of the whole plant. The increase of the root:shoot ratio occurring under water stress is attributed, at least partly, to this hormonal signal (Creelman et al. 1990), which may take part in the decline of stem diameter as observed, for instance, in Populus euphratica undergoing soil water depletion (Bogeat-Triboulot et al. 2007). The response of trees to drought stress has been investigated in depth, but most of the experimental work deals with the molecular mechanisms operating in leaves and roots, while the biochemical signals involved in stem growth deserve further consideration. It is well known that soil water depletion enhances ABA biosynthesis and export from the roots, thus increasing the concentration of the molecule in the xylem stream and the leaves (Zhang et al. 1987). ABA-induced stomatal closure is an early step of a series of events leading to reduced leaf transpiration and net CO2 assimilation rates and, invariably, to impaired photosynthesis and restricted shoot growth (Nobel 2009). Furthermore, high ABA levels are responsible for the fast and strong decline of leaf cell expansion that occurs when the roots sense that soil water content is critical (Davies and Zhang 1991) and promote senescence and leaf abscission (Lindoo and Noodén 1978). The decrease of cambial growth rate observed under conditions of suboptimal water availability (Berta et al. 2010) might be mediated by ABA, at least partly, through the aforesaid chain of ABA-mediated events that repress photosynthesis. The concentration of the hormone strongly increases in P. alba under water stress and attains pre-stress values a few days after the resumption of water supply (Luisi et al., submitted). This correlation suggests that ABA may restrain cambial growth. Beyond its negative effect on photosynthesis, the hormone might operate by downregulating aquaporin genes, reducing the concentration of auxin in the stem, or interfering with auxin signal transduction (Popko et al. 2010). Moreover, ABA is known to repress several genes involved in the hydrolysis of various cell wall polymers: in this way, the hormone hinders cell wall loosening, which is a prerequisite for cell expansion (Gimeno-Gilles et al. 2009).
Ethylene
In many species, the application of this growth regulator has been shown to stimulate cambial activity (Biro et al. 1980; Little and Pharis 1995). However, the most relevant evidence of the involvement of ethylene in stem secondary growth emerges from investigations on Zinnia cell cultures. Pesquet and Tuominen (2011) demonstrated that TE differentiation is strictly dependent on ethylene biosynthesis and that the hormone promotes TE differentiation. The authors suggest that, in whole plants, ethylene would be synthesized in the apoplast of the xylem elements and would also contribute to the control of the pool size of cambial stem cells during secondary xylem formation. Much work has focused on the formation of tension wood in trees (Table 1). Ethylene biosynthesis increases during tension wood formation because of an asymmetric induction of the biosynthetic enzyme ACC oxidase (Andersson-Gunnerås et al. 2003; Du and Yamamoto 2003). Transgenic Populus trees, either ethylene-overproducers or ethylene-insensitive, have been used to probe the role of the hormone in the development of tension wood (Love et al. 2009). The results demonstrate that ethylene stimulates cambial activity by acting through ethylene receptors, and that endogenous ethylene produced in leaning trees is a key regulator for the asymmetrical cell growth in the tension wood response. This finding suggests that ethylene plays a common primary role in cambium activity responses induced by both leaning and mechanical loads and that its regulation of secondary growth entails both cell expansion and cell division.
Strigolactones
The most recently identified group of plant growth regulators, strigolactones (SLs), have been shown to be involved in many developmental processes, such as stem branching, photomorphogenesis, root growth and seed germination (Foo and Reid 2012). Studies on SL-deficient ccd8 pea and Arabidopsis mutants and applications of the synthetic SL, GR24, to Arabidopsis and Eucalyptus demonstrate that SLs have a direct effect on cell division in the cambium and induce cambium-specific reporter genes (Agusti et al. 2011) (Table 1). The question then arose as to whether this stimulation of cambium activity could be related to the known function of SLs as branching inhibitors: Agusti et al. (2011) showed that the two effects were independent, thus suggesting that SLs might decisively contribute to control plant architecture. The same authors also provided evidence that SLs function predominantly downstream of auxin in regulating the secondary growth of the stem.
Polyamines
Polyamines are small molecules with two or more primary amino groups that are involved in cell growth and development (Pegg 1986; Tabor and Tabor 1984). The cationic nature confers on these molecules a high binding affinity to proteins and nucleic acids and, accordingly, they may regulate translation by forming complexes with RNA (Igarashi and Kashiwagi 2010). Thermospermine, first detected in an extreme thermophile, Thermus thermophilus (Oshima 1979), is synthesized from spermidine in plant cells (Knott et al. 2007), where it suppresses xylem differentiation and promotes stem elongation, as demonstrated in Arabidopsis (Kakehi et al. 2008). Furthermore, in Zinnia mesophyll cell cultures the differentiation of TE is repressed by exogenously supplied thermospermine (Kakehi et al. 2010). Thermospermine appears essential for tuning the timing and spatial pattern of xylem differentiation (Yoshimoto et al. 2012).
Peptide hormones
An intercellular signaling system contributes to the determination of the fate of procambial cells. A small peptide molecule, which has been named TE differentiation inhibitory factor (TDIF), is secreted from the phloem and its neighboring cells and binds to the receptor kinase PXY/TDR (PHLOEM INTERCALATED WITH XYLEM/TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR RECEPTOR), located on the plasma membrane of procambial cells. The signal inhibits the differentiation of procambial cells into xylem cells and enhances their proliferation (Fisher and Turner 2007; Hirakawa et al. 2008; Ito et al. 2006). In Arabidopsis TDIF is encoded by members of the CLE (clavata3/embryo surrounding region-related) gene family, like CLE41 and CLE44 (Jun et al. 2008) and its transcriptional target belongs to the WUSCHEL-related HOMEOBOX (WOX) gene family. TDIF upregulates the expression of Arabidopsis WOX4 in a TDR-dependent manner, thus promoting vascular stem cell proliferation. Instead, TDIF inhibits the xylem commitment of these cells through a WOX4-independent pathway. Hence, this phloem-synthesized peptide signal acts as a positional clue for stem cell maintenance in the vascular meristem, ensuring a continuous supply of new cells to the developing vascular tissues (Hirakawa et al. 2010). Besides CLE41 and CLE44, further genes might encode peptide signals involved in vascular development. According to Whitford et al. (2008), Arabidopsis CLE6, CLV3 (CLAVATA3) and CLE19 peptides cooperate with TDIF to promote procambial cell proliferation. The emerging picture would be rather complex, because each CLE ligand might bind to a range of receptors, that in turn might recognize different CLE molecules (Ohyama et al. 2009).
Hormonal crosstalking
Plant hormones control cambial growth and differentiation through a complex regulatory network (Fig. 3). The signal triggered by a single hormone may be enhanced or dampened by other molecules, thus yielding additive/synergistic or competitive effects, respectively, depending also on how the response pathways to these molecules are integrated and on how their biosynthesis and metabolism are related. Much work must be done to achieve a better understanding of the hormonal crosstalk operating in the control of cambium development and xylogenesis. Our knowledge on this subject is limited and we tried to summarize the most relevant evidence on this topic.
Simplified model of hormone crosstalking operating in cambial cell division and identity and xylem differentiation. Evidence of peptides crosstalking has been shown only in roots. Black arrowhead lines, positive regulation of cellular processes; black inhibition lines, negative regulation of cellular processes; cyan arrowhead lines, positive interactions with signaling pathways; red arrowhead lines, positive regulation on hormone metabolism; red dotted arrowhead lines, hypothesized regulation on hormone metabolism. ABA, abscisic acid; CK, cytokinins; GA, gibberellins
Cambial cell divisions in diverse plant organs and species are enhanced following treatments with exogenous CK and auxin, as shown in excised Raphanus roots (Torrey and Loomis 1967), during regeneration of wound internodes in Coleus (Baum et al. 1991) and during secondary development in Helianthus (Saks et al. 1984). Moreover, treatments with both CKs and auxin may induce TE differentiation, while exposure to either auxin or CKs alone failed to reproduce this effect (Fukuda and Komamine 1980; Milioni et al. 2001). This suggests that high levels of both hormones are required for a commitment step, after which point they are no longer required for TE formation.
Gibberellins stimulate cambial activity when they are administered to tree stems, particularly when applied together with auxin (Björklund et al. 2007; Dayan et al. 2010; Digby and Wareing 1966; Wang et al. 1997). Auxin is able to induce cell divisions in the vascular cambium of decapitated tree stems (Little and Bonga 1974). The application of GAs to decapitated and auxin-depleted Populus stems stimulates cell divisions in the cambial zone, but the identity of the newly formed cells was reported to be relatively obscure, thus indicating that GA alone is not sufficient to maintain and stimulate cambial activity (Björklund et al. 2007). Conversely, the application of IAA together with GA enhances cambial cell divisions more than either hormone alone; therefore, they will function synergistically to regulate cambial development (Björklund et al. 2007; Digby and Wareing 1966). While GAs are thought to act as promoters of auxin transport into the cambial cells, auxin may stimulate the expression of GA biosynthesis genes and inhibit the expression of GA catabolism genes, suggesting a feedforward loop that would promote cambial activity. Additionally, these hormonal classes may interact by sharing part of their transcriptome (Björklund et al. 2007).
The expression of ethylene biosynthesis and signaling genes increases during the development of tension wood in poplar and the same occurs to auxin signaling genes (Andersson-Gunnerås et al. 2003, 2006). Furthermore, the well-known induction of ethylene biosynthesis by auxin has been shown to function also in the wood-forming tissues of poplar (Nilsson et al. 2008). Hence, these hormones might interact in regulating cambial activity, but their relationship appears to be complex and still awaits to be elucidated: for example, it has been shown that tension wood formation does not lead to a rise of IAA concentration in the upper side of a bent poplar shoot, whereas in the lower side of the shoot the IAA level decreases (Hellgren et al. 2004).
Although its precise mode of action has not yet been fully elucidated, thermospermine enhances the translation of Suppressor of acl5 (SAC51), a transcription factor that restricts proliferation of xylem cells. As suggested by Yoshimoto et al. (2012), the auxin signaling that promotes xylem differentiation is normally limited by SAC51 signaling. Consequently, thermospermine would counteract auxin by promoting SAC51 translation, which is responsible for the suppression of the auxin-inducible xylem vessel differentiation program.
In general, CLE peptides may regulate the fate of stem cells through crosstalk with signaling pathways mediated by other plant hormones, such as auxin and CKs. The aforesaid combined action of several peptide signals may be effective in enhancing the proliferation of procambial cells uniquely when the auxin signaling is functioning. A model has been proposed to explain this process: the peptide signals would act synergistically to prime specific vascular cell types, whose auxin level or auxin sensitivity would then rise, thus enhancing their proliferation and delaying their differentiation into phloem and xylem (Whitford et al. 2008). This model agrees with the radial concentration gradient of auxin observed by Uggla et al. (1998) in the cambium and neighboring tissues of Scots pine. Some CLE peptides, including CLE10, which is preferentially expressed in the root vascular system, are involved in the regulation of xylem development in Arabidopsis roots. CLE10 peptides repress specifically the expression of two type-A Arabidopsis Response Regulators (ARRs), ARR5 and ARR6, whose products act as negative regulators of CK signaling (Ren et al. 2009) and this leads to the inhibition of protoxylem vessel formation (Kondo et al. 2011). Moreover, adenylate isopentenyltransferase 7 (IPT7) and cytokinin oxidase 3 (CKX3), that act in CK synthesis and degradation, respectively (Takei et al. 2001), are downregulated by CLE10 treatment. Thus, a crosstalk between CLE signaling and CK signaling functions in protoxylem vessel formation in roots. Nevertheless, the mechanisms operating in vessel development might differ between the roots and the stem: TDIF, which represses vessel differentiation in leaves and hypocotyls, does not affect protoxylem vessel formation in roots (Kondo et al. 2011).
Even more limited evidence is available for other hormonal groups. Brassinosteroids and auxin control a number of similar processes, such as cell elongation and vascular development. The induction of several auxin-inducible IAA genes by BRs (Nakamura et al. 2003) suggests a common link in the signaling of these two hormones; however, how they act together to regulate vascular development remains unclear. As to ABA, some authors have proposed an interaction between this growth regulator and auxin (Dumbroff et al. 1979; Jenkins and Shepherd 1974; Little and Wareing 1981).
Future directions
Understanding the biological mechanisms that regulate the activity of vascular cambium is still a challenging task to accomplish. As previously underlined, a feature of central importance is the control of xylem cell patterning, which may give rise to xylem conduit tapering. According to the aforesaid hypothesis, xylem tapering is a prerequisite for the transport of water to the top of tall trees, but it may also restrain the growth in height of the tree. The study of the biochemical signaling which determines the maximum height in trees and the variability of this trait in different terrestrial biomes is particularly attractive. Special emphasis should be laid on the key role played by xylem architecture in compensating for the negative effect of path length (i.e. tree height) on total hydraulic resistance, which represents the effective limitation to stem elongation. A better comprehension of the dynamics of cambial activities (xylogenesis) and of the mechanism of cell size control along the stem may be achieved through the biochemical and genetic study of hormone signals in the cells of the cambial zone, to elucidate the role played by these molecules in the control of the length of the expansion stage of xylem cells along the stem. Populus has been acknowledged as the model system for physiological and genomic studies, as well as for secondary wall development and wood production, and morpho-physiological modifications due to climatic changes and stress conditions studies. In order to clarify the molecular mechanisms, several studies investigated the response of the cribro-vascular cambium to abiotic stresses (drought, salinity, low and high temperature, heavy metals, Blaudez et al. 2003; Nanjo et al. 2004; Ralph et al. 2008) and how they modify the dynamics of wood formation and stem growth. In these studies, microarray analysis and gene transcription profile have proved useful for the identification of potential regulators of cambial initials in wood-forming tissues (Druart et al. 2007; Schrader et al. 2004).
Biochemical analyses may probe the existence of longitudinal gradients of auxin throughout the growing season, thus eventually lending support to the hypothesis put forward to explain the observed phenomenon of increasing duration of cell expansion towards the stem base, which may lead to the development of the tapered architecture of the xylem transport system. The existence of a longitudinal gradient of IAA would be a relevant evidence in favor of a control of xylem cells size operated by a hormonal network. Notably, if correlation between tapered pipeline structure and potential tree height can be demonstrated, a new physiological tool for predicting variations in maximum tree height in different sites might become available, thus opening up new perspectives for understanding ecosystem functionality, forest management planning and potential applications in forecasting future ecosystem scenarios in the context of climate change.
References
Agusti J, Herold S, Schmarz M, Sanchez P, Ljung K, Dun EA, Brewer PB, Beveridge CA, Sieberer T, Sehr EM, Greb T (2011) Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc Natl Acad Sci USA 108:20242–20247
Aloni R (1987) Differentiation of vascular tissues. Annu Rev Plant Physiol 38:179–204
Aloni R (2001) Foliar and axial aspects of vascular differentiation: hypotheses and evidence. J Plant Growth Regul 20:22–34
Aloni R, Zimmermann MH (1983) The control of vessel size and density along the plant axis—a new hypothesis. Differentiation 24:203–208
Andersson-Gunnerås S, Hellgrent JM, Björklund S et al (2003) Asymmetric expression of a poplar ACC oxidase controls ethylene production during gravitational induction of tension wood. Plant J 34:339–349
Andersson-Gunnerås S, Mellerowicz EJ, Love J, Segerman B, Ohmiya Y, Coutinho PM, Nilsson P, Henrissat B, Moritz T, Sundberg B (2006) Biosynthesis of cellulose-enriched tension wood in Populus: global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. Plant J 45:144–165
Anfodillo T, Carraro V, Carrer M et al (2006) Convergent tapering of xylem conduits in different woody species. New Phytol 169:279–290
Anfodillo T, Deslauriers A, Menardi R et al (2012) Widening of xylem conduits in a conifer tree depends on the longer time of cell expansion downwards along the stem. J Exp Bot 63:837–845
Arend M, Fromm J (2007) Seasonal change in the drought response of wood cell development in poplar. Tree Physiol 27:985–992
Bandurski RS, Schulze A, Dayanandan P et al (1984) Response to gravity of Zea mays seedlings. Time course of the response. Plant Physiol 74:284–288
Baum SF, Aloni R, Peterson CA (1991) Role of cytokinin in vessel regeneration in wounded Coleus internodes. Ann Bot 67:543–548
Becker P, Gribben RJ, Lim CM (2000) Tapered conduits can buffer hydraulic conductance from path-length effects. Tree Physiol 20:965–967
Berta M, Giovannelli A, Sebastiani F et al (2010) Transcriptome changes in the cambial region of poplar (Populus alba L.) in response to water deficit. Plant Biol 12:341–354
Bhalerao RP, Bennett MJ (2003) The case for morphogens in plants. Nat Cell Biol 5:939–943
Biro RL, Hunt ER, Erner Y et al (1980) Thigmomorphogenesis: changes in cell division and elongation in the internodes of mechanically-perturbed or ethrel-treated bean plants. Ann Bot 45:655–664
Björklund S, Antti H, Uddestrand I, Moritz T, Sundberg B (2007) Cross-talk between gibberellin and auxin in development of Populus wood: gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. Plant J 52:499–511
Blaudez D, Kohler A, Martin F et al (2003) Poplar metal tolerance protein 1 confers zinc tolerance and is an oligomeric vacuolar zinc transporter with an essential leucine zipper motif. Plant Cell 15:2911–2928
Bogeat-Triboulot MB, Brosché M, Renaut J et al (2007) Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol 143:876–892
Cano-Delgado A, Yin YH, Yu C et al (2004) BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 131:5341–5351
Carlsbecker A, Helariutta Y (2005) Phloem and xylem specification: pieces of the puzzle emerge. Curr Opin Plant Biol 8:512–517
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol 49:427–451
Coomes DA, Jenkins KL, Cole LES (2007) Scaling of tree vascular transport systems along gradients of nutrient supply and altitude. Biol Lett 3:86–89
Creelman RA, Mason HS, Bensen RJ et al (1990) Water deficit and abscisic acid cause differential inhibition of shoot versus root growth in soybean seedlings. Plant Physiol 92:205–214
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
Dayan J, Schwarzkopf M, Avni A et al (2010) Enhancing plant growth and fiber production by silencing GA 2-oxidase. Plant Biotechnol J 8:425–435
Demura T, Fukuda H (1994) Novel vascular cell-specific genes whose expression is regulated temporally and spatially during vascular system development. Plant Cell 6:967–981
Deslauriers A, Giovannelli A, Rossi S et al (2009) Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiol 29:1223–1235
Digby J, Wareing PF (1966) The effect of applied growth hormones on cambial division and the differentiation of the cambial derivates. Ann Bot 30:539–548
Druart N, Johansson A, Baba K et al (2007) Environmental and hormonal regulation of the activity-dormancy cycle in the cambial meristem involves stage-specific modulation of transcriptional and metabolic networks. Plant J 50:557–573
Du S, Yamamoto F (2003) Ethylene evolution changes in the stems of Metasequoia glyptostroboides and Aesculus turbinata seedlings in relation to gravity-induced reaction wood formation. Trees Struct Funct 17:522–528
Dumbroff EB, Cohen DB, Webb DP (1979) Seasonal levels of abscisic acid in buds and stems of Acer saccharum. Physiol Plant 45:211–214
Enquist BJ (2003) Cope’s rule and the evolution of long-distance transport in vascular plants: allometric scaling, biomass partitioning and optimization. Plant Cell Environ 26:151–161
Eriksson ME, Israelsson M, Olsson O et al (2000) Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat Biotechnol 18:784–788
Escalante-Perez M, Lautner S, Nehls U et al (2009) Salt stress affects xylem differentiation of grey poplar (Populus × canescens). Planta 229:299–309
Fisher K, Turner S (2007) PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr Biol 17:1061–1066
Foo E, Reid JB (2012) Strigolactones: new physiological roles for an ancient signal. J Plant Growth Regul. doi:10.1007/s0034401293046
Fukuda H (1997) Tracheary element differentiation. Plant Cell 9:1147–1156
Fukuda H, Komamine A (1980) Establishment of an experimental system for the study of tracheary element differentiation from single cells isolated from the mesophyll of Zinnia elegans. Plant Physiol 65:57–60
Gimeno-Gilles C, Lelièvre E, Viau L et al (2009) ABA-mediated inhibition of germination is related to the inhibition of genes encoding cell-wall biosynthetic and architecture: modifying enzymes and structural proteins in Medicago truncatula embryo axis. Mol Plant 2:108–119
Hellgren JM, Olofsson K, Sundberg B (2004) Patterns of auxin distribution during gravitational induction of reaction wood in poplar and pine. Plant Physiol 135:212–220
Hirakawa Y, Shinohara H, Kondo Y, Inoue A, Nakanomyo I, Ogawa M, Sawa S, Ohashi-Ito K, Matsubayashi Y, Fukuda H (2008) Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc Natl Acad Sci USA 105:15208–15213
Hirakawa Y, Kondo Y, Fukuda H (2010) TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 22:2618–2629
Igarashi K, Kashiwagi K (2010) Modulation of cellular function by polyamines. Int J Biochem Cell Biol 42:39–51
Inoue T, Higuchi M, Hashimoto Y et al (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409:1060–1063
Israelsson M, Sundberg B, Moritz T (2005) Tissue-specific localization of gibberellins and expression of gibberellin-biosynthetic and signaling genes in wood-forming tissues in aspen. Plant J 44:494–504
Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H (2006) Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313:842–845
Iwasaki T, Shibaoka H (1991) Brassinosteroids act as regulators of tracheary-element differentiation in isolated Zinnia mesophyll-cells. Plant Cell Physiol 32:1007–1014
Jenkins PA, Shepherd KR (1974) Seasonal changes in levels of indole-acetic acid and abscisic acid in stem tissues of Pinus radiata. N Z J Bot 4:511–519
Jun JH, Fiume E, Fletcher JC (2008) The CLE family of plant polypeptide signaling molecules. Cell Mol Life Sci 65:743–755
Kakehi J, Kuwashiro Y, Niitsu M, Takahashi T (2008) Thermospermine is required for stem elongation in Arabidopsis thaliana. Plant Cell Physiol 49:1342–1349
Kakehi J, Kuwashiro Y, Motose H, Igarashi K, Takahashi T (2010) Norspermine substitutes for thermospermine in the control of stem elongation in Arabidopsis thaliana. FEBS Lett 584:3042–3046
Kliewer WM, Bowen P, Benz M (1989) Influence of shoot orientation on growth and yield development in Cabernet Sauvignon. Am J Enol Vitic 40:259–263
Knott JM, Römer P, Sumper M (2007) Putative spermine synthases from Thalassiosira pseudonana and Arabidopsis thaliana synthesize thermospermine rather than spermine. FEBS Lett 581:3081–3086
Kondo Y, Hirakawa Y, Kieber JJ, Fukuda H (2011) CLE peptides can negatively regulate protoxylem vessel formation via cytokinin signaling. Plant Cell Physiol 52:37–48
Lindoo SJ, Noodén LD (1978) Correlation of cytokinins and abscisic acid with monocarpic senescence in soybeans. Plant Cell Physiol 19:997–1006
Lintunen A, Kalliokoski T (2010) The effect of tree architecture on conduit diameter and frequency from small distal roots to branch tips in Betula pendula, Picea abies and Pinus sylvestris. Tree Physiol 30:1433–1447
Little CHA, Bonga JM (1974) Rest in the cambium of Abies balsamea. Can J Bot 52:1723–1730
Little CHA, Pharis RP (1995) Hormonal control of radial and longitudinal growth in the tree stem. In: Gardner BL (ed) Plant stems: physiology and functional morphology. Academic Press, San Diego, pp 281–319
Little CHA, Savidge RA (1987) The role of plant growth regulators in forest tree cambial growth. Plant Growth Regul 6:137–169
Little CHA, Wareing PF (1981) Control of cambial activity and dormancy in Picea sitchensis by indol-3-yl acetic acid and abscisic acid. Can J Bot 59:1480–1493
Love J, Björklund S, Vahalab J et al (2009) Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc Natl Acad Sci USA 106:5984–5989
Lovisolo C, Schubert A (2000) Downward shoot positioning affects water transport in field-grown grapevines. Vitis 39:49–53
Lovisolo C, Schubert A, Sorce C (2002) Are xylem radial development and hydraulic conductivity in downwardly-growing grapevine shoots influenced by perturbed auxin metabolism? New Phytol 156:65–74
Magnani F, Mencuccini M, Grace J (2000) Age-related decline in stand productivity: the role of structural acclimation under hydraulic constraints. Plant Cell Environ 23:251–263
Matsumoto-Kitano M, Kusumoto T, Tarkowski P et al (2008) Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA 105:20027–20031
Mauriat M, Moritz T (2009) Analyses of GA20ox- and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. Plant J 58:989–1003
McDowell NG, Phillips N, Lunch C et al (2002a) An investigation of hydraulic limitation and compensation in large, old Douglas-fir trees. Tree Physiol 22:763–774
McDowell NG, Barnard H, Bond BJ et al (2002b) The relationship between tree height and leaf area: sapwood area ratio. Oecologia 132:12–20
Mencuccini M, Martinez-Vilalta J, Vanderklein D et al (2005) Size-mediated ageing reduces vigour in trees. Ecol Lett 8:1183–1190
Mencuccini M, Holtta T, Petit G et al (2007) Sanio’s laws revisited. Size-dependent changes in the xylem architecture of trees. Ecol Lett 10:1084–1093
Midgley JJ (2003) Is bigger better in plants? The hydraulic costs of increasing size in trees. Trends Ecol Evol 18:5–6
Milioni D, Sado PE, Stacey NJ et al (2001) Differential expression of cell-wall-related genes during the formation of tracheary elements in the Zinnia mesophyll cell system. Plant Mol Biol 47:221–238
Miller CO, Skoog F, Von Saltza MH et al (1955) A cell division factor from deoxyribonucleic acid. J Am Chem Soc 77:1392
Moyle R, Schrader J, Stenberg A et al (2002) Environmental and auxin regulation of wood formation involves members of the Aux/IAA gene family in hybrid Aspen. Plant J 31:675–685
Nakamura A, Higuchi K, Goda H et al (2003) Brassinolide induces IAA5, IAA19, and DR5, a synthetic auxin response element in arabidopsis, implying a cross talk point of brassinosteroid and auxin signaling. Plant Physiol 133:1843–1853
Nanjo T, Futamura N, Nishiguchi M et al (2004) Characterization of full-length enriched expressed sequence tags of stress-treated poplar leaves. Plant Cell Physiol 45:1738–1748
Nieminen K, Immanen J, Laxell M et al (2008) Cytokinin signalling regulates cambial development in poplar. Proc Natl Acad Sci USA 105:20032–20037
Nilsson J, Karlberg A, Antti H, Lopez-Vernaza M, Mellerowicz E, Perrot-Rechenmann C, Sandberg G, Bhalerao RP (2008) Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell 20:843–855
Nobel PS (2009) Physicochemical and environmental plant physiology, 4th edn. Elsevier, Oxford
Ohyama K, Shinohara H, Ogawa-Ohnishi M, Matsubayashi Y (2009) A glycopeptide regulating stem cell fate in Arabidopsis thaliana. Nat Chem Biol 5:578–580
Oribe Y, Funada R, Kubo T (2003) Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees Struct Funct 17:185–192
Oshima T (1979) A new polyamine, thermospermine, 1,12-diamino-4,8-diazadodecane, from an extreme thermophile. J Biol Chem 254:8720–8722
Pegg AE (1986) Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J 234:249–262
Pesquet E, Tuominen H (2011) Ethylene stimulates tracheary element differentiation in Zinnia elegans cell cultures. New Phytol 190:138–149
Petit G, Anfodillo T (2009) Plant physiology in theory and practice: an analysis of the WBE model for vascular plants. J Theor Biol 259:1–4
Petit G, Anfodillo T, Mencuccini M (2008) Tapering of xylem conduits and hydraulic limitations in sycamore (Acer pseudoplatanus) trees. New Phytol 177:653–664
Petit G, Pfautsch S, Anfodillo T et al (2010) The challenge of tree height in Eucalyptus regnans: when xylem tapering overcomes hydraulic resistance. New Phytol 187:1146–1153
Petit G, Anfodillo T, Carraro V et al (2011) Hydraulic constraints limit height growth in trees at high altitude. New Phytol 189:241–252
Plomion C, Leprovost G, Sokes A (2001) Wood formation in trees. Plant Physiol 127:1513–1523
Popko J, Hänsch R, Mendel RR et al (2010) The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol 12:242–258
Pothier D, Margolis HA, Waring RH (1989) Patterns of change of saturated sapwood permeability and sapwood conductance with stand development. Can J For Res 19:432–439
Prasad TK, Cline MG (1985) Shoot inversion-induced ethylene in Pharbitis nil induces the release of apical dominance by restricting shoot elongation. Plant Sci 38:163–172
Ragni L, Nieminen K, Pacheco-Villalobos D et al (2011) Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. Plant Cell 23:1322–1336
Ralph SG, Chun HJE, Cooper D (2008) Analysis of 4,664 high-quality sequence-finished poplar full-length cDNA clones and their utility for the discovery of genes responding to insect feeding. BMC Genomics 9:57
Ren B, Liang Y, Deng Y, Chen Q, Zhang J, Yang X, Zuo J (2009) Genome-wide comparative analysis of type-A Arabidopsis response regulator genes by overexpression studies reveals their diverse roles and regulatory mechanisms in cytokinin signaling. Cell Res 19:1178–1190
Ryan MG, Yoder BJ (1997) Hydraulic limits to tree height and tree growth. Bioscience 47:235–242
Sachs T (1981) The control of patterned differentiation of vascular tissues. Adv Bot Res 9:151–262
Sachs T (1991) Cell polarity and tissue patterning in plants. Development 91(Suppl):83–93
Sachs T (2000) Integrating cellular and organismal aspects of vascular differentiation. Plant Cell Physiol 41:649–656
Saks Y, Feigenbaum P, Aloni R (1984) Regulatory effect of cytokinin on secondary xylem fiber formation in an in vivo system. Plant Physiol 76:638–642
Scarpella E, Meijer AH (2004) Pattern formation in the vascular system of monocot and dicot plant species. New Phytol 164:209–242
Schrader J, Nilsson J, Mellerowicz EJ et al (2004) A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. Plant Cell 16:2278–2292
Schubert A, Restagno M, Novello V et al (1995) Effects of shoot orientation on growth, net photosynthesis, and hydraulic conductivity of Vitis vinifera L. cv. Cortese. Am J Enol Vitic 46:324–328
Schubert A, Lovisolo C, Peterlunger E (1999) Shoot orientation affects vessel size, shoot hydraulic conductivity and shoot growth rate in Vitis vinifera L. Plant Cell Environ 22:197–204
Sieburth LE, Deyholos MK (2006) Vascular development: the long and winding road. Curr Opin Plant Biol 9:48–54
Sorce C, Mariotti L, Lorenzi R et al (2007) Hormonal factors involved in the control of vigour of grafted peach (Prunus persica (L.) Batsch) trees and hybrid rootstocks. Adv Hortic Sci 21:68–74
Sundberg B, Little CHA, Cui K et al (1991) Level of endogenous indole-3-acetic acid in the stem of Pinus sylvestris in relation to the seasonal variation of cambial activity. Plant Cell Environ 14:241–246
Sundberg B, Uggla C, Tuominen H (2000) Cambial growth and auxin gradients. In: Savidge RA, Barnett JR, Napir R (eds) Cell and molecular biology of wood formation. BIOS Scientific Publishers, Oxford, pp 169–188
Tabor CW, Tabor H (1984) Polyamines. Annu Rev Biochem 53:749–790
Takei K, Sakakibara H, Sugiyama T (2001) Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J Biol Chem 276:26405–26410
Tokunaga N, Uchimura N, Sato Y (2006) Involvement of gibberellin in tracheary element differentiation and lignification in Zinnia elegans xylogenic culture. Protoplasma 228:179–187
Torrey G, Loomis RS (1967) Auxin–cytokinin control of secondary vascular tissue formation in isolated roots of Raphanus. Am J Bot 54:1098–1106
Tuominen H, Puech L, Fink S et al (1997) A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiol 115:577–585
Turner S, Gallois P, Brown D (2007) Tracheary element differentiation. Annu Rev Plant Biol 58:407–433
Tyree MT (2003) The ascent of water. Nature 423:923
Ueguchi C, Sato S, Kato T et al (2001) The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant Cell Physiol 42:751–755
Uggla C, Moritz T, Sandberg G et al (1996) Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci USA 93:9282–9286
Uggla C, Mellerowicz EJ, Sundberg B (1998) Indole-3-acetic acid controls cambial growth in Scots pine by positional signaling. Plant Physiol 117:113–121
Uggla C, Magel E, Moritz T et al (2001) Function and dynamics of auxin and carbohydrates during earlywood/latewood transitions in Scots pine. Plant Physiol 125:2029–2039
Vanderklein D, Martinez-Vilalta J, Lee S et al (2007) Plant size, not age, regulates growth and gas exchange in grafted Scots pine trees. Tree Physiol 27:71–79
Wang Q, Little CH, Odén PC (1997) Control of longitudinal and cambial growth by gibberellins and indole-3-acetic acid in current-year shoots of Pinus sylvestris. Tree Physiol 17:715–721
Wareing PF, Hanney CEA, Digby J (1964) The role of endogenous hormones in cambial activity and xylem differentiation. In: Zimmermann MH (ed) The formation of wood in forest trees. Academic Press, New York, pp 323–345
West GB, Brown JH, Enquist BJ (1999) A general model for the structure and allometry of plant vascular systems. Nature 400:664–667
Whitford R, Fernandez A, De Groodt R, Ortega E, Hilson P (2008) Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells. Proc Natl Acad Sci USA 105:18625–18630
Yamamoto R, Demura T, Fukuda H (1997) Brassinosteroids induce entry into the final stage of tracheary element differentiation in cultured Zinnia cells. Plant Cell Physiol 38:980–983
Yamamoto R, Fujioka S, Demura T et al (2001) Brassinosteroid levels increase drastically prior to morphogenesis of tracheary elements. Plant Physiol 125:556–563
Yoshida S, Kuriyama H, Fukuda H (2005) Inhibition of transdifferentiation into tracheary elements by polar auxin transport inhibitors through intracellular auxin depletion. Plant Cell Physiol 46:2019–2028
Yoshimoto K, Noutoshi Y, Hayashi K, Shirasu K, Takahashi T, Motose H (2012) A chemical biology approach reveals an opposite action between thermospermine and auxin in xylem development in Arabidopsis thaliana. Plant Cell Physiol 53:635–645
Zhang J, Schurr U, Davies WJ (1987) Control of stomatal behaviour by abscisic acid which apparently originates in the roots. J Exp Bot 38:1174–1181
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kumar.
A contribution to the Special Issue: Plant Hormone Signaling.
Rights and permissions
About this article
Cite this article
Sorce, C., Giovannelli, A., Sebastiani, L. et al. Hormonal signals involved in the regulation of cambial activity, xylogenesis and vessel patterning in trees. Plant Cell Rep 32, 885–898 (2013). https://doi.org/10.1007/s00299-013-1431-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1431-4