Abstract
Key message
The collapse of some cell types and the simultaneous growth or expansion of others hinder the estimation of the contribution of individual tissues to the variation of bark dimension over time.
Abstract
Information on the spatio-temporal pattern of secondary changes occurring in older bark, as well as the activity of phellogen and the development of periderm is still relatively scarce. Anatomical and histometrical investigations were carried out on the bark of mature Quercus petraea growing in Ljubljana, Slovenia. The bark of the oaks was on average 19 mm thick, with the inner bark and the rhytidome accounting for 39 and 61 %, respectively. A high correlation was found between the widths of the rhytidome and of the entire bark, but a fairly weak one between inner bark and entire bark. The youngest phloem increment on average represented around 5 % of the inner bark and around 2.1 % of the entire bark. Growth-ring boundaries were not distinguishable in the collapsed phloem; however, counting the phloem increments was possible due to the presence of phloem fibres at the transition from early to late phloem. We also followed the spatial–temporal secondary changes in collapsed phloem tissue. Phloem increment development in Q. petraea showed that patterns of phloem formation at one location remained practically unchanged in different years. The relationship between processes occurring in different bark tissues is not linear. In addition to the high variability in bark, the collapse of some cell types and the simultaneous growth or expansion of others hinder the estimation of the contribution of individual tissues to the variation of bark dimension over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In trees of temperate climatic regions, annual growth increments in the phloem are formed similarly as in the xylem (Holdheide 1951). Usually radial growth of trees is simplistically considered resulting from the activity of the vascular cambium, a bi-facial meristem that produces two complex tissues: secondary xylem (wood) in centripetal and secondary phloem (bast) in centrifugal direction (Larson 1994). However, the annual radial growth of trees includes also the activity of phellogen and numerous secondary changes in older bark tissue.
In Slovenia, oaks (Quercus robur L. and Q. sessiliflora Salisb.) are economically and ecologically very important tree species, representing 7 % of the entire wood stock (Gozdnogospodarski 2006). Oak is considered as one of the most important wood in European dendrochronology and oak tree-ring chronologies are amongst the longest in the world (Friedrichs et al. 2009). Furthermore, investigating wood formation at the intra-annual level has already been demonstrated to be a promising approach in tree biology and climate change research, because the period in which cells are developing is the time window in which environmental signals are perceived and encoded (e.g. Fonti and García-González 2004). For monitoring radial growth or wood formation dynamics, dendrometers have been widely used (e.g. Deslauriers et al. 2003; Zweifel et al. 2006, 2010). Amongst other factors, which need to be taken into account when processing the data, swelling and shrinking of the dead bark lead to changes in tree diameter. To minimise these effects, sensors are usually placed on the bark surface after the dead outermost layer of the bark was removed (Zweifel et al. 2011). However, in trees with thick bark, such as oaks, other secondary changes are occurring in older living bark, i.e. collapse of sieve elements, inflation of parenchyma cells, formation of dilatation tissue and of sclereids and the activity of a cork cambium, are likely to significantly contribute to stem diameter radius changes. Little is known about the contribution of each of these processes in the bark on the annual increase in tree girth although it would improve data accuracy and the interpretation of the results.
The structure of bark and its development are still fairly unknown, especially in comparison to wood. It is subject to substantial changes with time (age), which limits its usefulness for histometrical, dendrochronological or dendroecological analyses. With regards to the anatomy of bark of Quercus spp., it was investigated in stems and roots of Q. robur by Trockenbrodt (1991, 1994, 1995a, b). Furthermore, Pereira and her co-workers have published many papers on the structure of bark, cork and rhytidome in various Quercus species: Q. suber, Q. cerris var. cerris, Q. faginea and Q. variabilis (Pereira 1988; Graça and Pereira 2004; Sen et al. 2010, 2011a, b; Miranda et al. 2013; Quilhó et al. 2013). Due to its economic importance, Q. suber bark, with very thick cork layer, is particularly well studied and provides a model for cork in barks (Pereira 2013).
The main objective of our study was to investigate seasonal changes and age-related trends in bark tissues in mature Quercus petraea trees. Anatomical and histometrical investigations were carried out. The specific goals were (1) to determine main milestones of phloem formation (onset and end of phloem formation, transition from early to late phloem) in 2009, (2) to check whether it is possible to follow phellogen activity with repetitive sampling of trees during the growing season, and (3) to analyse the width and structure of bark tissues.
Materials and methods
Study site characteristics and sample collection and preparation
Sampling was performed in a forest site, Roznik, in Ljubljana (46°03′N, 14°28′E, 323 m a.s.l.), which belongs to the Blechno fagetum forest association. The predominant tree species are Fagus sylvatica and Quercus petraea. This privately owned forest is devoted to its natural development. Its predominant function is recreational and social, so only basic forest management, such as sanitary felling, is performed.
The climate is humid continental. According to the climate record from the nearby meteorological station of Ljubljana for the period 1900–2009, the mean annual temperature is 9.8 °C, with January being the coolest (−1.0 °C) and July the warmest month (20.0 °C). The annual sum of precipitation is 1,409 mm, of which 47 % is falling from May to September.
We selected 20 dominant, healthy sessile oaks (Quercus petraea (Matt.) Liebl.) at the beginning of the 2009 vegetation period. All trees were about 150 years old with diameters at breast height of approximately 60–80 cm and heights of 25–30 m. They are growing on the southern slope of Roznik (about 20°), on deep, slightly acidic, brown soils on sandstone. For bark-anatomical analysis, these 20 trees were sampled in autumn of 2009, whilst for monitoring the seasonal dynamics of changes in bark tissues, four trees were sampled at 10-day intervals during the growing season of 2009; i.e. from the end of March until the end of September. In all cases, we carried out sampling of blocks of intact tissue (10 × 10 × 30 mm3) containing the entire bark, cambium and outer xylem from living trees, 1.1–1.7 m above the ground. The distance between neighbouring samples was at least 10 cm in a horizontal direction to avoid the influence of wounding on tissues of the next sampling locations (Gričar 2007). These blocks of tissue were immediately fixed in FAA (formaldehyde–ethanol–acetic acid solution), after 1 week dehydrated in a graded series of ethanol (30, 50 and 70 %), and finally embedded in paraffin (for details see Rossi et al. 2006). For light microscopy, permanent cross-sections of 10 µm thickness were prepared on a Leica RM 2245 rotary microtome (Leica Microsystems, Wetzlar, Germany), using Leica 819 disposable blades. The sections were transferred to object glasses and stained with a water mixture of safranin (Merck, Darmstadt, Germany) (0.04 %) and astra blue (Sigma-Aldrich, Steinheim, Germany) (0.15 %) (van der Werf et al. 2007) and embedded in Euparal (Waldeck, Münster, Germany). An Olympus BX51 light microscope (Tokyo, Japan) and a Nikon NIS-Elements Basic Research v.2.3 image analysis system (Tokyo, Japan) were used for histometrical observations and measurements.
Anatomical and histometrical observations of cambium and phloem and xylem increments
We analysed the structure of bark along three radial files in each histological section by measuring including the widths of the (1) entire bark, (2) rhytidome, (3) inner bark, (4) individual periderms, (5) dead secondary phloem tissue between two successive periderms, (6) youngest phloem increments (i.e. phloem cells that were produced in the growing season of 2009), (7) early phloem in the youngest phloem increments, (8) late phloem in the youngest phloem increments, and (9) tangential diameter of early and late phloem sieve tube lumina in the youngest phloem increments.
The terminology for bark anatomy follows Trockenbrodt (1990), who defines bark as all tissue outside the vascular cambium (in brief, cambium), regardless of its specific structure. The term secondary phloem is applied to all bark tissues derived from the cambium. Living secondary phloem, also called the inner bark, is the part from the cambium up to the latest-formed, or innermost, periderm. Rhytidome includes all tissues outside the latest periderm. The periderm consists of a meristematic layer called phellogen and of phellem and phelloderm. The cells outward and inward from the phellogen form the phellem and the phelloderm, respectively (Trockenbrodt 1990).
The sieve tubes of the phloem were defined by thin, unlignified, blue-stained cell walls and a round to irregular shape (Fig. 1). The axial parenchyma is located between tangential bands of fibres and interspersed with sieve elements. Companion cells were difficult to recognise in transverse sections. Just as in xylem, the rays in phloem are of two sizes, uniseriate and multiseriate (more than 10 cells wide). At the end of the increment, a narrow layer of axial parenchyma is formed (Holdheide 1951). Phloem fibres have thick lignified cell walls that stain red and are part of the late phloem. The fibre walls exhibit birefringence in polarised light (Gričar 2010). Morphological characteristics and ontogenetic considerations were taken into account to distinguish between sclereids and phloem fibres. Sclereids are predominantly derived from enlarged axial and ray parenchyma cells in the collapsed phloem. Phloem fibres, in contrast, are long slender cells that develop from fusiform cells near the cambium and reach maturity in the non-collapsed phloem (Trockenbrodt 1990).
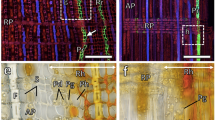
Transverse section of inner bark with non-collapsed and collapsed parts. The non-collapsed phloem consists of the most recently formed phloem increment (black line) and of a part of the previously formed increment. Cells of the unicellular rays are conspicuously wider in the collapsed than in the non-collapsed phloem, an aspect of dilatation growth. CC cambium, PR youngest phloem increment, EP early phloem, LP late phloem, EpSt early phloem sieve tube, LpSt late phloem sieve tube, PhF fibres, AxP axial parenchyma, R uniseriate ray. Sampling date: 4 September 2009. Scale bar 200 μm
Mean values for all bark parameters were first calculated for each tree. We used simple linear regression analysis to describe the relation between the widths of the different parts of the bark. A simple Pearson’s correlation was applied for the relations between pairs of variables. Box–Cox transformation was used when necessary to linearise these relationships.
Results
Widths of bark tissues
Bark tissue in oak consists of non-collapsed and collapsed inner bark and of rhytidome (Fig. 2). The entire bark was 18.3 ± 4.8 mm wide, of which about 39 % (6.7 ± 1.1 mm) was inner bark and 61 % (11.7 ± 4.6 mm) rhytidome. The widths of the bark were highly variable, ranging from 10.5 to 29.1 mm, mainly on account of the width of the rhytidome (from 3.7 to 23.3 mm).
Inner bark constituted more than half of the entire bark width only in oaks with bark narrower than 12 mm, whereas rhytidome part predominated in wider barks (Fig. 3). Nevertheless, the entire bark was narrower than 12 mm in only three oaks (15 % of the trees). The widths of the rhytidome and of the entire bark correlated highly significantly to one another (r = 0.975, p < 0.000, F = 459.17), whereas the correlation between the widths of the inner bark and the entire bark was rather weak though statistically significant (r = 0.339, p < 0.10, F = 3.00). No correlation was found between the widths of inner bark and the rhytidome (r = 0.088, p = 0.678, F = 0.18). The youngest phloem increment on average represented around 5 % of the inner bark and around 2.1 % of the entire bark. However, there was no significant correlation between the widths of the youngest phloem increment and the inner bark (r = −0.018, p = 0.943, F = 0.01) or between the widths of the youngest phloem increment and the entire bark (r = −0.033, p = 0.896, F = 0.02).
Spatio-temporal changes in structure of inner bark
The annual phloem increment in oak is composed of sieve tubes, companion cells and phloem fibres, as well as axial and ray parenchyma cells (Fig. 1). The development of early phloem cells occurred during April and May (Fig. 4a). Transition from early to late phloem was identified in the second half of May, when phloem fibres started to form (Fig. 4b). The fibres were arranged in small groups as discontinuous tangential bands, usually 2–6 cell layers wide and interrupted by uniseriate rays. Development of the phloem fibres continued in June and July. Fibres were thick walled, lignified and accompanied by chambered crystalliferous cells (Fig. 4b). Production of new cells in the cambium was completed by mid-August. Adjacent to the cambium, 1–3 layers of parenchyma cells were observed. Early phloem sieve tubes collapsed only a few weeks after the cessation of cambium cell production (Fig. 4c).
Transverse sections of developing phloem increment in 2009. a Newly formed early phloem sieve tubes (red arrow) at the beginning of April; b development of phloem fibres (yellow arrow) accompanied by chambered crystalliferous cells (red arrow) in mid-May under polarised light; c collapse of the early phloem sieve tubes and companion cells (red arrow) after the cessation of cambial cell production. Sampling date: 14 September. Scale bars 100 μm (a), 200 μm (b) and 500 μm (c)
The average width of phloem increment in oaks was 322.7 ± 61.1 µm, of early phloem 206.2 ± 41.5 µm and of late phloem 116.2 ± 47.7 µm. The sieve tubes of early phloem were characterised by larger lumina (49.7 ± 7.1 μm) than those of late phloem, which were by about one-third smaller (32.4 ± 4.9 μm). Early phloem was generally wider than late phloem and constituted on average more than half of the entire increment (64.1 ± 9.7 %). However, the early phloem proportion was mostly lower in wider increments (Fig. 5). There was practically no relation between the widths of early and late phloem (r = 0.061, p = 0.805, F = 0.06).
The collapse of sieve tubes and companion cells marked the onset of secondary changes in the bark (Fig. 1). Enlargement and sclerification of parenchyma cells followed and were associated with dilatation growth (Fig. 6). Due to all these changes, recognition of growth-ring boundaries was difficult if not impossible in the collapsed phloem. However, we were able to count the phloem increments by the presence of phloem fibres that separate early and late phloem, to determine the year in which sclereid formation and expansion of tissue started (Fig. 8b).
Sclereids started to form in multiseriate rays in some trees even in the current year’s phloem increment (Fig. 6). Sporadic groups of sclereids were detected in 4- or 5-year-old phloem increments, whereas more abundant groups of sclereids were detected in 10-year-old increments. The frequency of sclereid groups increased especially in the vicinity of multiseriate rays and between tangential bands of phloem fibres. Sclerified multiseriate rays and groups of sclereids bordering them form radially aligned groups of sclereids, which Şen et al. (2011a) described as rays with a “palm” formation (Fig. 6). Solitary sclereids were also common. Due to dilatation and other secondary changes of phloem cells, uniseriate rays became indistinguishable and the structure disorganised in the older parts of the inner bark tissues (Fig. 3), as already mentioned by Trockenbrodt (1991). Solitary crystals were located in sclereids and parenchyma cells (Figs. 7, 10d). Druses were also present in other axial parenchyma cells (Fig. 7a).
a Transverse section of collapsed phloem with three tangential bands of fibres (F) with accompanying crystalliferous cells (yellow arrows), and a group of crystal-containing sclereids (S). Some axial parenchyma cells contain druses (blue arrows). b Radial section through non-collapsed phloem with most recently formed phloem on the right and part of the previous year’s phloem increment on the left, crystalliferous cells associated with a fibre band of previous year’s phloem increment (yellow arrow). Ep early phloem, Lp late phloem, R ray. Sampling date: 4 September. Scale bars 100 μm
Spatio-temporal changes in structure of rhytidome
The rhytidome in the bark of mature Q. petraea consists of alternating layers of periderm and dead phloem tissue (Fig. 8). Periderms are formed as layers more or less parallel to the cambium and sequential layers may anastomose (Fig. 8a). We observed high variability in the number of periderms, amongst trees and also within individuals (3–11 periderms). Since we sampled trees aged around 150 years, the first periderm was not preserved; only younger, sequential ones. The innermost periderm appeared in phloem increments of various age; on average 28 years old (the earliest 8-year-old and the latest 37-year-old increment).
Transverse sections. Structure of rhytidome: sequential layers of periderms (black arrows) with dead secondary phloem between them (black lines). a Innermost periderm (blue arrow) separating living (below) and dead (above) phloem tissue. Successive periderms may anastomose (white arrows). Sampling date: 8 August; b conspicuous tangential bands of phloem fibres (white arrows) enable counting the number of phloem increments. Sampling date: 4 September; c new phellem cells (black arrow) with thin walls. Sampling date: 10 May 2009. Scale bars 1,000 μm (a), 500 μm (b) and 100 μm (c)
Quercus petraea does not produce extensive phellem, or cork, and the phelloderm is poorly developed. Because of the presence of the tangential bands of fibres, we were able to count phloem increments between two neighbouring periderms, at least in younger parts of the rhytidome in which the tissue was not too decayed (Fig. 8b). On average, there were 6–15 layers of phloem increment between periderms and the width of dead phloem tissue decreased outwards (Fig. 9a). The youngest dead phloem layer was thus about 2.5 mm wide; the width of the intermediate bands of dead phloem layers then decreased rapidly below 1.5 mm in the sixth layer and below 1.0 mm after the tenth layer. In contrast, the thickness of the periderms gradually increased in the centrifugal direction; from 56.5 µm in the youngest periderm to 80–100 µm in the 10th–15th successive periderms (Fig. 9b).
In cross-section, the phellogen cells were difficult to distinguish in the periderm (Figs. 8c, 10). A narrow phelloderm formed by 2–4 cell layers of rectangular to round cells develops, which often cannot be distinguished from the neighbouring parenchyma cells except by their radial alignment (Fig. 8c). Each phellem layer comprised 4–10 layers of isodiametric or sometimes radially flattened cells arranged into a more or less distinct radial pattern (Figs. 8, 10). Different cell types can be present in phellem: thin walled and wide lumened or thick walled and narrow lumened (Fig. 10b, d). The phellem may contain sclerified cells arranged in tangential bands alternating with tangential bands of non-sclerified cells (Fig. 8a). The phelloderm was composed of 2–3 thin-walled cells in radial rows and morphologically resembling the adjacent parenchyma cells, except for their radial alignment. Dark-stained material was observed in the cells of phellem and phelloderm, especially in older periderms.
a–c Transverse sections; d radial section. Detailed structure of rhytidome: successive layers of periderm with dead secondary phloem between them. a Sclerified portion of multiseriate ray (blue arrow) interrupted by periderm at site of anastomosis between two periderms. b, c Different cell types in phellem: thin walled and wide lumened (white arrows) or thick walled and narrow lumened (black arrows). d Tangential bands of phloem fibres (green arrow) interrupted by periderm with accompanying crystalliferous cells (yellow arrows). Scale bars 100 μm
During the formation of a new periderm, the outer parts of the inner bark were isolated from the inner tissue. In samples taken in April, the formation of new phellem cells with thin walls (Fig. 8c) was detected and thick-walled cells appeared later in June (Fig. 10c). However, it was difficult to set any milestones (onset, dynamic, end) in phellogen activity/inactivity or new cell formation because some innermost periderms seemed inactive at the time of sampling; others looked variously old according to their thickness. Development of the newly formed cells was also not possible to follow. In addition, it was not possible to estimate whether the development of the periderm cells continued in the next growing season(s). We did not observe the formation of new phellogen in any of the samples.
Discussion
Widths of bark tissues
We found high variability in the widths of the entire bark, mainly on account of rhytidome. Bark thickness is known to vary greatly in different species; with oaks, periderms are formed quickly one after another (Holdheide 1951). Trockenbrodt (1994) observed that, in addition to a general age-related increase in bark thickness, there are also differences in its thickness between trees of the same age, and even around the stem circumference of one individual. However, little is known about its cause; it may be related to site conditions, tree vitality or even to the bark exposition (south or north) (Trockenbrodt 1994).
A high correlation was found only between the widths of the rhytidome and the entire bark, but a rather weak between the widths of the inner bark and the entire bark. Lack of association between the widths of other tissues in the bark (i.e. the inner bark and the rhytidome, the youngest phloem increment and the inner bark or the youngest phloem increment and the entire bark) may be partly explained by the continuous secondary changes occurring in the older phloem tissue, as well as by the formation and activity of numerous phellogens in the rhytidome. Finally, older tissues of bark are usually crushed and the external, distorted and collapsed phloem tissues eventually exfoliate (Whitmore 1963).
Phloem increment
Due to differences in the characteristics of early and late phloem cells, it was possible to recognise growth-ring boundaries in oak. Since phloem cells collapse within a few years after their formation, cellular structure can only be studied in a narrow zone close to the cambium (Esau 1939; Alfieri and Evert 1968; Prislan et al. 2012).
Comparison of the seasonal formation of the phloem growth ring in Q. petraea at the same location in 2007 and 2009 showed that the timing and dynamics of its formation were similar in the two vegetation periods (Gričar 2010). This is in line with observations in F. sylvatica, in which patterns of phloem formation at a single location remained practically unchanged in different years (Prislan et al. 2013). The formation and structure of the phloem increment are known to be relatively consistent if trees of a similar age, position in stand, vigour, vitality and growing in similar environments are observed (e.g. Larson 1994).
Early phloem sieve tubes collapsed only a few weeks after the cessation of cambium cell production, indicating that they function for only a single growing season (Alfieri and Evert 1968). The detailed structure of the youngest phloem increment, also in relation to the xylem, was investigated in depth in our recent study in Q. robur (Gričar et al. 2014). According to Holdheide (1951), there are no differences between the two species in the phloem anatomical structure. Comparing the widths of phloem increment of Q. petraea and Q. robur in the two studies, it is evident that average values are similar in the two oaks. We noted that Q. petraea has a substantially higher proportion of early phloem than Q. robur (Gričar et al. 2014). However, based on only these, relatively limited data, it is difficult to speculate whether the proportions of early and late phloem are characteristics that are species specific or site specific, or maybe both.
Secondary changes in inner bark
Age-related structural changes in older bark tissues in oak have already been described in detail in previous papers (e.g. Holdheide 1951; Whitmore 1963; Evert 2006). Our observations were consistent with their findings. In Q. petraea, sclereids started to form in multiseriate rays in some trees even in the current year’s phloem increment, which is in line with the observations of Şen et al. (2011a) in Q. cerris var. cerris and Quilhó et al. (2013) in Q. faginea. A large variation in the share and distribution of sclereids was observed amongst the sampled trees. Sclereids increased in abundance in older parts of the bark, similar as in Q. robur (Trockenbrodt 1991).
Solitary crystals in sclereids and parenchyma cells were also observed by others (Howard 1977; Trockenbrodt 1995a, b; Sen et al. 2011a, b; Quilhó et al. 2013). In Q. petraea, the overall quantity of crystals varied considerably in different parts of the bark, with no regular trends in their distribution. Calcium oxalate crystals are generally more common in bark than in wood (Trockenbrodt 1995a). As in xylem, their presence, type and location in bark could serve as a reliable feature for diagnostic description. In contrast, their dimensions and quantity are highly variable and are inappropriate for such purposes (Trockenbrodt 1995a).
Rhytidome
We observed high variability in the number of periderms, amongst trees and also within individuals, which is in line with the observations of Holdheide (1951). The innermost periderm appeared in phloem increments of various age; on average 28 years old. In most oak species, phellogen has a short lifespan of a few years and is periodically renewed in internal regions of the phloem (Whitmore 1963; Şen et al. 2010). Şen et al. (2011a) estimated a phellogen lifespan in Q. cerris var. cerris of about 25 years, which is in accordance with rhytidome formation at 25–35 years reported for oaks (Trockenbrodt 1994).
Despite the relatively constant number of phloem increment layers between two periderms, the width of dead phloem tissue decreased outwards, indicating continuous changes in the tissue. Conversely, the thickness of the periderms gradually increased in the centrifugal direction suggesting that the extent of the contribution of each of the processes affecting the annual increase in tree girth is not easy to estimate.
In contrast to studies on the seasonal dynamics of cambial activity, only a few references are available on the development of the periderm and the seasonal dynamics of phellogen activity (e.g. Srivastava 1964; Lev-Yadun and Liphschitz 1989; Graça and Pereira 2004). Due to huge variations in rhytidome structure, the number and spatial distribution of periderms and seasonal phellogen activity within an individual, it is not possible to monitor the seasonal dynamics of phellogen activity, in which only a few cells are produced, by adopting the methodology for cambial activity observations. A different sampling approach is needed. The extraction of more samples around the stem circumference might be more appropriate. In addition, proper fixation of samples with preservation of the living content of phellogen and newly formed cells, in combination with observation of cells at different resolution is necessary. Nevertheless, we observed that the formation of new phellem cells with thin walls started already in April, whereas thick-walled cells appeared later in June. However, it was difficult to set any milestones (onset, dynamic, end) in phellogen activity or new cell formation. We did not observe the formation of new phellogen in any of the samples.
In Quercus ithaburensis and Q. infectoria similar seasons of activity were recorded for the vascular cambium and phellogen (Arzee et al. 1978). Srivastava (1964) commented that phellem cells produced in the early part of the growing season have thinner walls and wider lumina than those produced later in the season. The activity of phellogen in trees of the temperate regions is considered to start sometime in June and to continue through July. In Aesculus hippocastanum, it is reported to start in May, whereas in Tilia not until the end of July (Srivastava 1964). However, observations of phellogen activity have focused mainly on the first periderm (Lev-Yadun and Liphschitz 1989; Graça and Pereira 2004) and the pattern of development of sequential periderms is assumed to be similar (Srivastava 1964).
Conclusions
Because of limited knowledge of the anatomy, chemistry, and physiology of bark, its more efficient use remains a challenge for future studies. Phloem increment development in Q. petraea showed that patterns of phloem formation at one location remained practically unchanged in different years. Due to huge variations in rhytidome structure, the number and spatial distribution of periderms and seasonal phellogen activity within an individual, it is not possible to monitor the seasonal dynamics of phellogen activity by adopting the methodology for cambial activity observations.
In terms of the annual radial growth of trees, which includes in addition to cambial activity on the xylem and phloem sides also phellogen activity and numerous secondary changes in older bark tissue, the extent of the contribution of each of the processes affecting the annual increase in tree girth is not easy to estimate. Awareness that the relationship between all these processes is not linear could be particularly useful for improving data accuracy and interpretation for studies in which monitoring of wood production is performed using dendrometers, especially in trees with thick bark and numerous periderms.
References
Alfieri FJ, Evert RF (1968) Seasonal development of the secondary phloem in Pinus. Am J Bot 55:518–528
Arzee T, Kamir D, Cohen L (1978) On the relationships of hairs to periderm development in Quercus ithaburensis and Q. infectoria. Bot Gaz 139:95–101
Deslauriers A, Morin H, Urbinati C, Carrer M (2003) Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Quebec (Canada). Trees Struct Funct 17:477–484
Esau K (1939) Development and structure of the phloem tissue. Bot Rev 5(7):373–432. doi:10.1007/BF02878295
Evert RF (2006) Esau’s plant anatomy meristems, cells, and tissues of the plant body: their structure, function, and development. Wiley, Hoboken
Fonti P, García-González I (2004) Suitability of chestnut earlywood vessel chronologies for ecological studies. New Phytol 163:77–86
Friedrichs DA, Neuwirth B, Winiger M, Löffler J (2009) Methodologically induced differences in oak site classifications in a homogeneous tree-ring network. Dendrochronologia 27(1):21–30
Gozdnogospodarski načrt gozdnogospodarske enote Krakovo 2006–2015 (Forest management plan for Forest Management Unit Krakovo 2006–2015) (2006) Zavod za gozdove Slovenije, območna enota Brežice. Brežice
Graça J, Pereira H (2004) The periderm development in Quercus suber. IAWA J 25(3):325–335
Gričar J (2007) Xylo- and phloemogenesis in silver fir (Abies alba Mill.) and Norway spruce (Picea abies (L.) Karst.), vol 132. Studia Forestalia Slovenica, Slovenian Forestry Institute, Ljubljana
Gričar J (2010) Xylem and phloem formation in sessile oak from Slovenia in 2007. Wood Res 55(4):15–22
Gričar J, Jagodic Š, Šefc B, Trajković J, Eler K (2014) Can the structure of dormant cambium and the widths of phloem and xylem increments be used as indicators for tree vitality? Eur J Forest Res 133:551–562. doi:10.1007/s10342-014-0784-8
Holdheide W (1951) Anatomie mitteleuropäischer Gehölzrinden. In: Freud H (ed) Handbuch der Mikroskopie in der Technik. Umschau Verlag, Frankfurt am Main, pp 193–367
Howard ET (1977) Bark structure of southern upland oaks. Wood Fiber Sci 9(3):172–183
Larson PR (1994) The vascular cambium: development and structure. Springer, Berlin
Lev-Yadun S, Liphschitz N (1989) Sites of first phellogen initiation in conifers. IAWA Bull 10(1):43–52
Miranda I, Gominho J, Pereira H (2013) Cellular structure and chemical composition of cork from the Chinese cork oak (Quercus variabilis). J Wood Sci 59(1):1–9. doi:10.1007/s10086-012-1300-8
Pereira H (1988) Chemical composition and variability of cork from Quercus suber L. Wood Sci Technol 22:211–218
Pereira H (2013) Variability of the chemical composition of cork. BioResources 8(2):2246–2256
Prislan P, Koch G, Schmitt U, Gričar J, Čufar K (2012) Cellular and topochemical characteristics of secondary changes in bark tissues of beech (Fagus sylvatica). Holzforschung 66(1):131–138. doi:10.1515/HF.2011.119
Prislan P, Gričar J, de Luis M, Smith KT, Čufar K (2013) Phenological variation in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agr Forest Meteorol 180:142–151. doi:10.1016/j.agrformet.2013.06.001
Quilhó T, Sousa V, Tavares F, Pereira H (2013) Bark anatomy and cell size variation in Quercus faginea. Turk J Bot 37:561–570. doi:10.3906/bot-1201-54
Rossi S, Anfodillo T, Menardi R (2006) Trephor: a new tool for sampling microcores from tree stems. IAWA J 27:89–97
Şen A, Miranda I, Santos S, Graça J, Pereira H (2010) The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind Crop Prod 31:417–422
Şen A, Quilhó T, Pereira H (2011a) Bark anatomy of Quercus cerris L. var. cerris from Turkey. Turk J Bot 35:45–55. doi:10.3906/bot-1002-33
Şen A, Quilhó T, Pereira H (2011b) The cellular structure of cork from Quercus cerris var. cerris bark in a materials’ perspective. Ind Crop Prod 34:929–936
Srivastava LM (ed) (1964) Anatomy, chemistry, and physiology of bark. Academic Press, New York
Trockenbrodt M (1990) Survey and discussion of the terminology used in bark anatomy. IAWA Bull 11(2):141–166
Trockenbrodt M (1991) Qualitative structural changes during bark development in Quercus robur, Ulmus glabra, Populus tremula and Betula pendula. IAWA Bull 12(1):5–22
Trockenbrodt M (1994) Quantitative changes of some anatomical characters during bark development in Quercus robur, Ulmus glabra, Populus tremula and Betula pendula. IAWA Bull 15:387–398
Trockenbrodt M (1995a) Calcium oxalate crystals in the bark of Quercus robur, Ulmus glabra, Populus tremula and Betula pendula. Ann Bot 75:281–284
Trockenbrodt M (1995b) Structure and identification of root bark of Quercus robur L. Trees Struct Funct 9:341–347
van der Werf GW, Sass-Klaassen U, Mohren GMJ (2007) The impact of the 2003 summer drought on the intra-annual growth pattern of beech (Fagus sylvatica L.) and oak (Quercus robur L.) on a dry site in the Netherlands. Dendrochronologia 25:103–112
Whitmore TC (1963) Studies in systematic bark morphology. IV. The bark of beech, oak and sweet chestnut. New Phytol 62(2):161–169
Zweifel R, PrometheusWikicontributors (2011) Stem radius fluctuations of trees. Prometheus Wiki. Accessed 14 Aug 2014
Zweifel R, Zimmermann L, Zeugin F, Newbery DM (2006) Intra-annual radial growth and water relations of trees: implications towards a growth mechanism. J Exp Bot 57(6):1445–1459. doi:10.1093/jxb/erj125
Zweifel R, Eugster W, Etzold S, Dobbertin M, Buchmann N, Häsler R (2010) Link between continuous stem radius changes and net ecosystem productivity of a subalpine Norway spruce forest in the Swiss Alps. New Phytol 187:819–830
Author contribution statement
J.G. together with P.P. developed the concept of the paper, wrote the paper, prepared the cross-sections and performed the wood-anatomical analysis, Š.J. prepared the figures, performed the wood-anatomical measurements, P.P. helped to develop the concept of the paper, helped to prepare the figures and measurements, wrote some parts of the results and discussion.
Acknowledgments
The authors would like to thank the anonymous reviewers for their valuable comments and suggestions to improve the quality of the paper. The work was supported by the Slovenian Research Agency, program P4-0107 and project Z4-9662 and by EUFORINNO (RegPot No. 315982) of the FP7 Infrastructures programme. The authors would like to acknowledge the contribution of the COST Action FP1106, STReESS. We thank Martin Cregeen for language editing.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Shane.
Rights and permissions
About this article
Cite this article
Gričar, J., Jagodic, Š. & Prislan, P. Structure and subsequent seasonal changes in the bark of sessile oak (Quercus petraea). Trees 29, 747–757 (2015). https://doi.org/10.1007/s00468-015-1153-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1153-z